Overview
The main focus of the article is on the best practices for utilizing Latin America medical trial expertise in clinical research, highlighting the region's advantages and strategies for successful trials. The article supports this by detailing the diverse patient population, cost-effectiveness, and the role of Contract Research Organizations (CROs) in enhancing efficiency, while also emphasizing the importance of local collaborations and community engagement to improve recruitment and study outcomes.
Introduction
Latin America is rapidly emerging as a preferred hub for clinical trials, captivating researchers and sponsors alike with its unique advantages. The region boasts a diverse patient population, essential for generating reliable trial outcomes, while offering significant cost benefits compared to North America and Europe.
As regulatory frameworks evolve and investments surge, countries like Colombia and Brazil are becoming increasingly attractive for conducting clinical research. However, navigating the complexities of local regulations and cultural nuances poses challenges that require strategic planning and local expertise.
This article delves into the factors making Latin America a prime location for clinical trials, the vital role of Contract Research Organizations (CROs), and the importance of inclusivity and local collaboration in optimizing trial success.
Why Latin America is a Prime Location for Clinical Trials
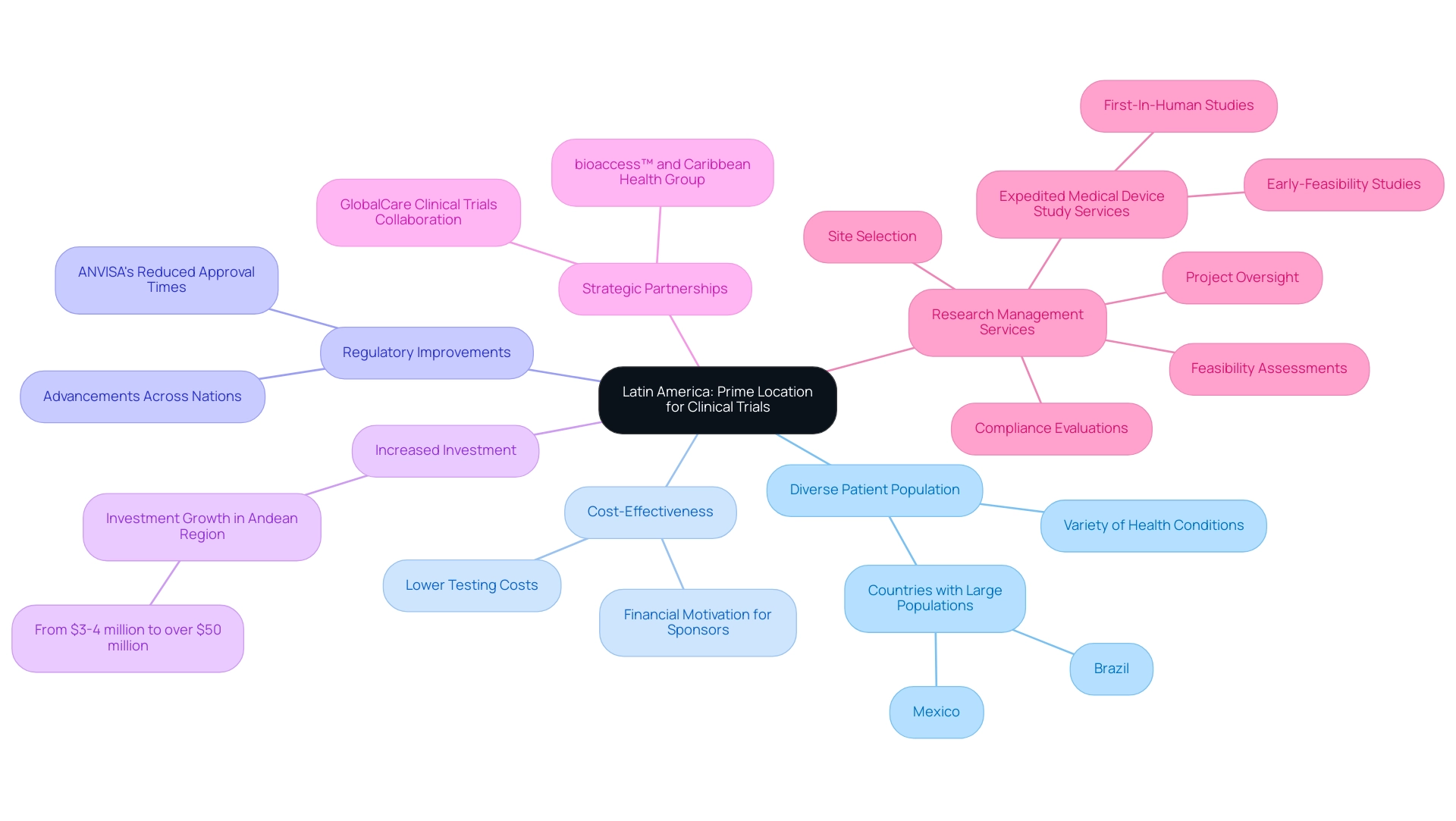
Latin America medical trial expertise has led to Latin regions emerging as a highly appealing location for clinical studies, driven by several compelling factors. A significant benefit of Latin America medical trial expertise is the region's varied patient population, which is crucial for guaranteeing the generalizability of research outcomes. Researchers can utilize their Latin America medical trial expertise to achieve robust recruitment for their studies, given that countries like Brazil and Mexico host large populations with a wide array of health conditions.
From a financial perspective, the expense of carrying out tests in Latin regions offers a considerable monetary motivation for sponsors, frequently being significantly lower than that in North and South regions. This cost-effectiveness is complemented by advancements in regulatory frameworks, reflecting Latin America medical trial expertise across many nations. For example, Brazil's National Health Surveillance Agency (ANVISA) has made significant strides in reducing approval times, thereby facilitating a more efficient initiation of studies.
Additionally, investment in the research sector in the Andean Region has increased from $3-4 million to over $50 million each year, showcasing rising interest in this field. A significant advancement is the partnership between bioaccess™ and Caribbean Health Group, intended to establish Barranquilla as a premier location for medical studies in Latin regions, backed by Colombia’s Minister of Health. This partnership, alongside GlobalCare Clinical Trials' collaboration with bioaccess™, has resulted in over a 50% reduction in recruitment time and impressive 95% retention rates, providing concrete evidence of success.
Furthermore, bioaccess® provides extensive research management services, including feasibility assessments, site selection, compliance evaluations, and project oversight, while focusing on expedited medical device study services like Early-Feasibility and First-In-Human studies, utilizing its Latin America medical trial expertise. Claudia Yvette Cravioto Guzman, Sr. Director of FSP Clinical Operations, emphasizes that these initiatives better position customers for success as opportunities in the region continue to emerge. Consequently, this blend of benefits not only establishes Latin regions as an ideal site for research studies, leveraging Latin America medical trial expertise, but also draws both domestic and global sponsors keen to optimize their investment in research.
In 2023, the area represented 3.5% of the worldwide research matching software market, highlighting its increasing significance in the sector.
The Role of CROs in Enhancing Clinical Trial Efficiency
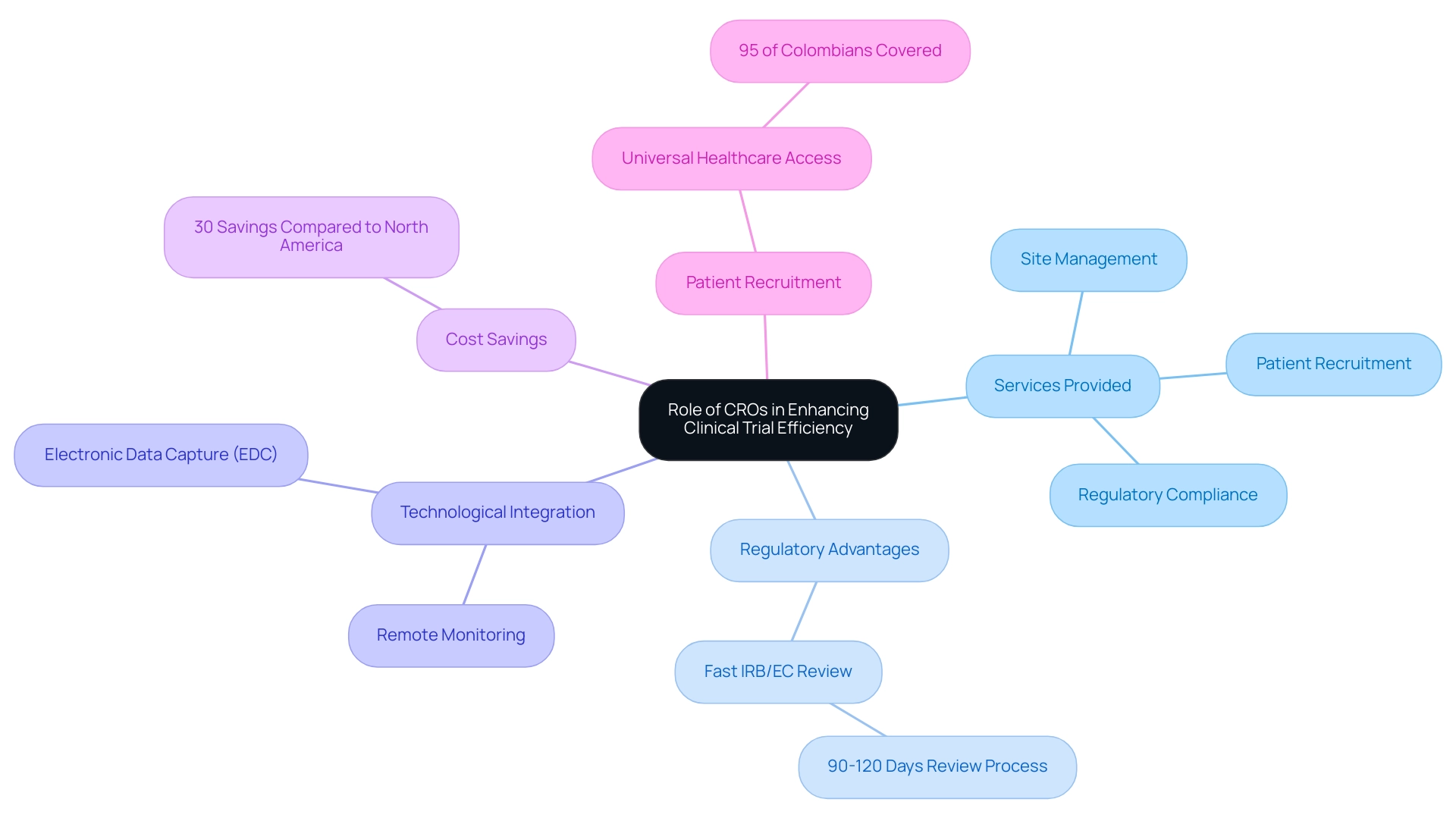
Contract Research Organizations (CROs) play a crucial role in the success of research studies throughout Latin America, particularly in Colombia, which showcases Latin America medical trial expertise and is a prime location for first-in-human investigations. They deliver essential services that encompass site management, patient recruitment, and regulatory compliance, allowing sponsors to concentrate on their primary objectives. By leveraging in-depth local knowledge and expertise, CROs adeptly navigate the region's regulatory landscape, which benefits from Colombia's rapid IRB/EC and MoH (INVIMA) review process of just 90-120 days.
Many local CROs have established strong relationships with regulatory authorities, expediting approval processes and streamlining operations.
Furthermore, Colombia's healthcare system ranks among the best globally, with the World Health Organization placing it at #22. The integration of innovative technologies, such as electronic data capture (EDC) and remote monitoring, significantly enhances data accuracy while reducing the time required for data collection and analysis. Partnering with a respected CRO can result in enhanced timelines and results, establishing them as an essential ally in the medical study environment.
Notably, Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, attests to the effectiveness of bioaccess® during its first human study in Colombia, highlighting the region's cost savings of over 30% compared to studies in North America or Western Europe.
Pharmaceutical companies emphasize that it typically takes 10 to 15 years and about $2.6 billion to bring a new medicine to market, underscoring the importance of CROs in improving study efficiency. Additionally, a case study titled 'Impact of Personalized Follow-Up in Brazil' demonstrated that personalized follow-up communications and educational resources significantly improved participant retention, resulting in a 25% increase compared to traditional methods. As Dr. John B. Simpson observes about Avinger's OCT-guided atherectomy research in Cali, Colombia, the success of research studies relies on cooperation with specialists who possess Latin America medical trial expertise.
Recent advancements, such as Lindus Health's successful acquisition of USD 55 million in funding to improve its research technology platform, further reflect the growing sophistication and efficiency of CROs in this region. Moreover, Colombia offers significant R&D tax incentives, including a 100% tax deduction, a 25% tax discount, and a 50% future tax credit, making it an attractive option for companies. With a population exceeding 50 million and approximately 95% of Colombians having access to universal healthcare, patient recruitment is also supported, offering a strong participant base for research studies.
Navigating Challenges in Latin American Clinical Trials
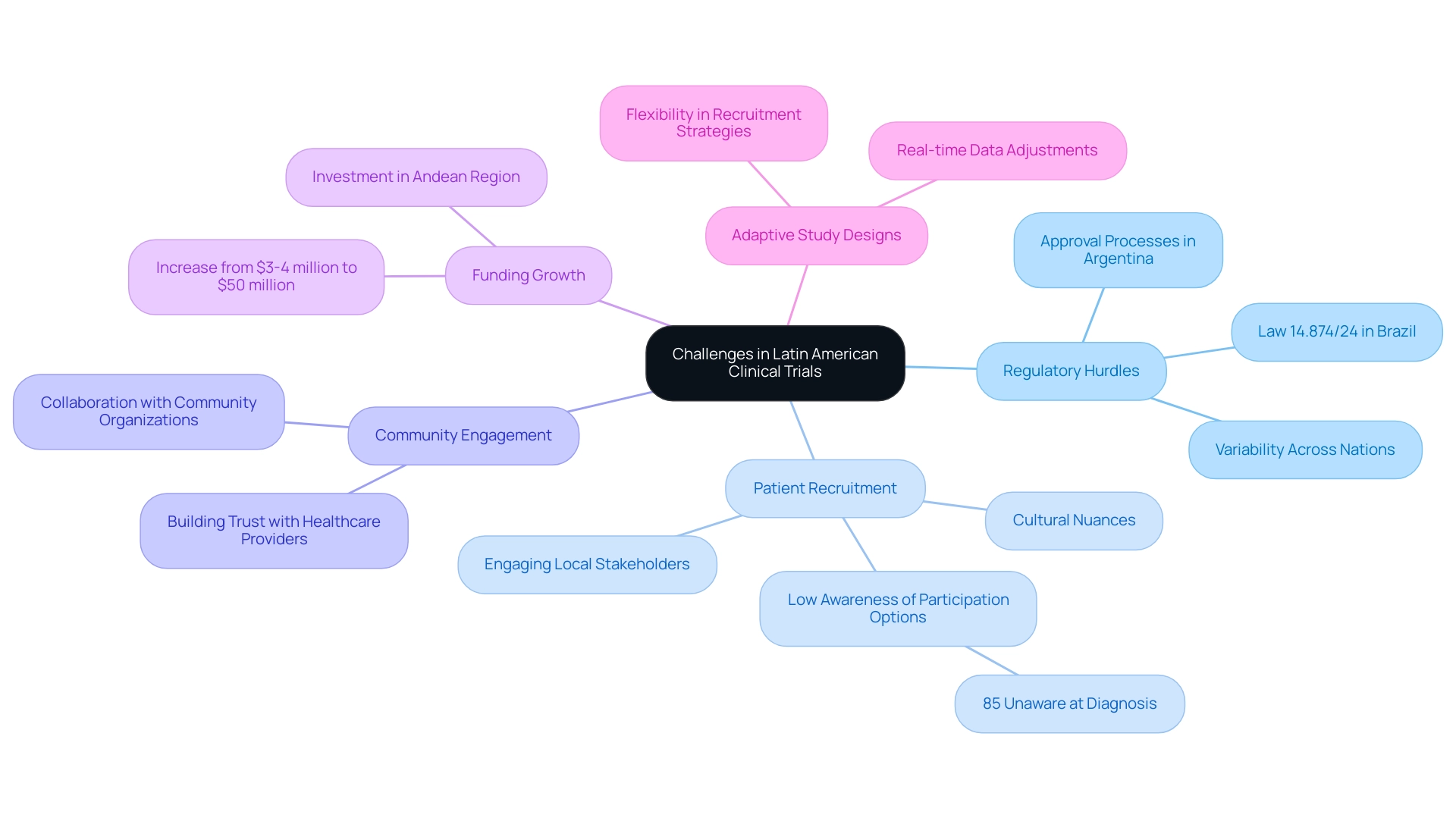
The Latin America medical trial expertise allows for carrying out research studies that provide substantial benefits, but it also introduces a variety of obstacles that need to be addressed with caution. As a prominent Contract Research Organization (CRO), bioaccess® is well-suited to expedite medical device studies in this region, drawing on its Latin America medical trial expertise along with its knowledge of local regulations and market access strategies. Regulatory hurdles can differ significantly across nations, often resulting in delays in study initiation.
For example, navigating the approval processes in Argentina tends to be more intricate compared to the relatively streamlined procedures in Colombia, necessitating that sponsors possess a deep understanding of local regulations. Recent modifications, including the endorsement of Law 14.874/24 in Brazil, seek to simplify the evaluation process for studies, possibly enhancing the environment for sponsors. Moreover, patient recruitment poses its own set of difficulties, influenced by cultural nuances and varying levels of health literacy across the region.
Alarmingly, awareness of clinical study participation options is low—85% of patients remain unaware at the time of diagnosis—indicating considerable potential for increasing enrollment by addressing these gaps. As researchers note, 'Explicar que fatos científicos não 'caem do céu', mas que são fruto de uma complexa construção do conhecimento, precisa ser parte do combate ao negacionismo,' emphasizing the importance of education in combating misinformation. To effectively alleviate recruitment challenges, it is crucial to engage local stakeholders early in the process.
Establishing relationships with local healthcare providers and community organizations not only enhances recruitment efforts but also fosters trust among prospective participants. Additionally, employing adaptive study designs can offer the necessary flexibility to respond to recruitment challenges dynamically, enabling sponsors to make adjustments based on real-time data and increasing the likelihood of successful study outcomes. Significantly, funding in the research sector in the Andean Region has increased from $3-4 million to over $50 million each year, suggesting a rising acknowledgment of the possibilities for studies leveraging Latin America medical trial expertise.
Furthermore, bioaccess® is committed to maintaining operational transparency and ensuring that patient information is handled with care, as highlighted in our FAQs regarding data protection and grievance handling. As a reliable ally, bioaccess® is committed to managing these challenges and discovering possibilities for successful studies in this promising market.
Emphasizing Diversity and Inclusivity in Clinical Research
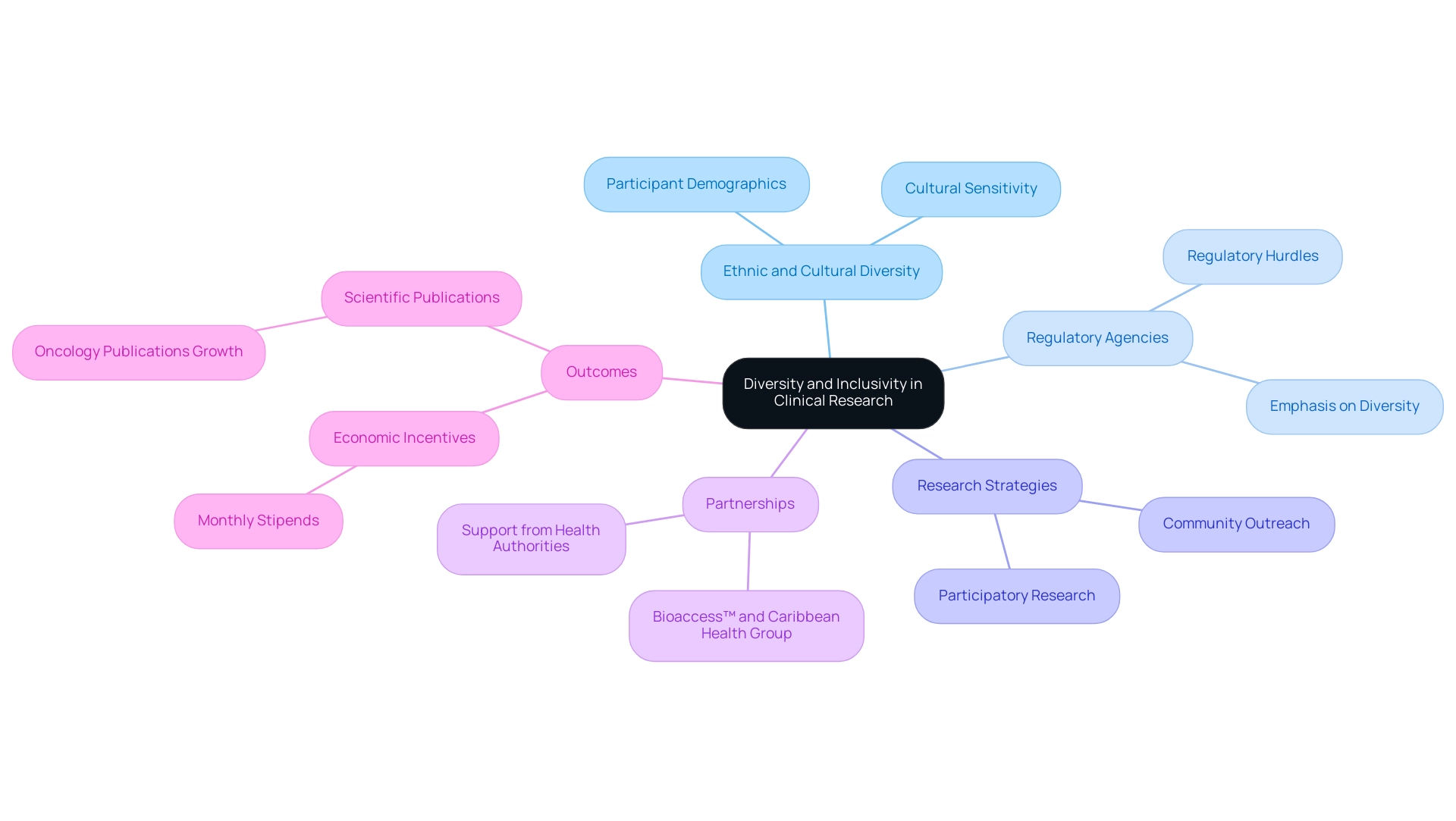
Diversity and inclusivity are essential components of clinical research in Latin America, highlighting the region's Latin America medical trial expertise, which is characterized by its rich ethnic, cultural, and socioeconomic diversity. By involving participants from various backgrounds—ethnicities, genders, and socioeconomic statuses—researchers can gather more nuanced data that accurately reflects the health needs of the population. This inclusivity not only enhances the validity and applicability of study outcomes but also fosters greater patient trust and engagement.
For instance, studies that prioritize cultural sensitivity and accessibility are statistically more successful in attracting diverse participants. Regulatory agencies throughout Latin America have progressively emphasized the significance of diversity in research studies, making it essential for sponsors to adjust their strategies accordingly. The partnership between bioaccess™ and Caribbean Health Group to position Barranquilla as a premier location for research studies is a prime example of how local initiatives can enhance participant outreach and involvement.
Moreover, with support from Colombia's Minister of Health, such efforts are essential to closing gaps in medical studies. However, Medtech companies still face significant challenges, including regulatory hurdles and language barriers, which can impede progress. Strategies like community outreach programs are vital for encouraging participation from underrepresented groups, supported by organizations like Pharm-Olam, which specializes in global patient recruitment.
This method greatly enhances study results, positioning Latin America medical trial expertise as a vital participant in the worldwide trial landscape. Additionally, researchers in Mexico receiving monthly stipends ranging from USD 380 to 1790 provide both economic incentives and a strong environment for impactful clinical studies. A significant case study is the rise in scientific publications in oncology from Latin countries, which increased from 1978 documents in 2015 to 3410 in 2020, demonstrating the expanding scholarly output in the region.
Manuela Fernandez Pinto, an assistant professor specializing in the philosophy of science, emphasizes the social dimensions of scientific knowledge, stating, 'Inclusivity in studies not only improves outcomes but also reflects the ethical responsibility of researchers to represent the populations they investigate.' Current trends indicate that CROs are increasingly utilizing participatory research and outsourcing to improve patient recruitment efficiency, further emphasizing the changing environment of medical studies and highlighting the importance of Latin America medical trial expertise.
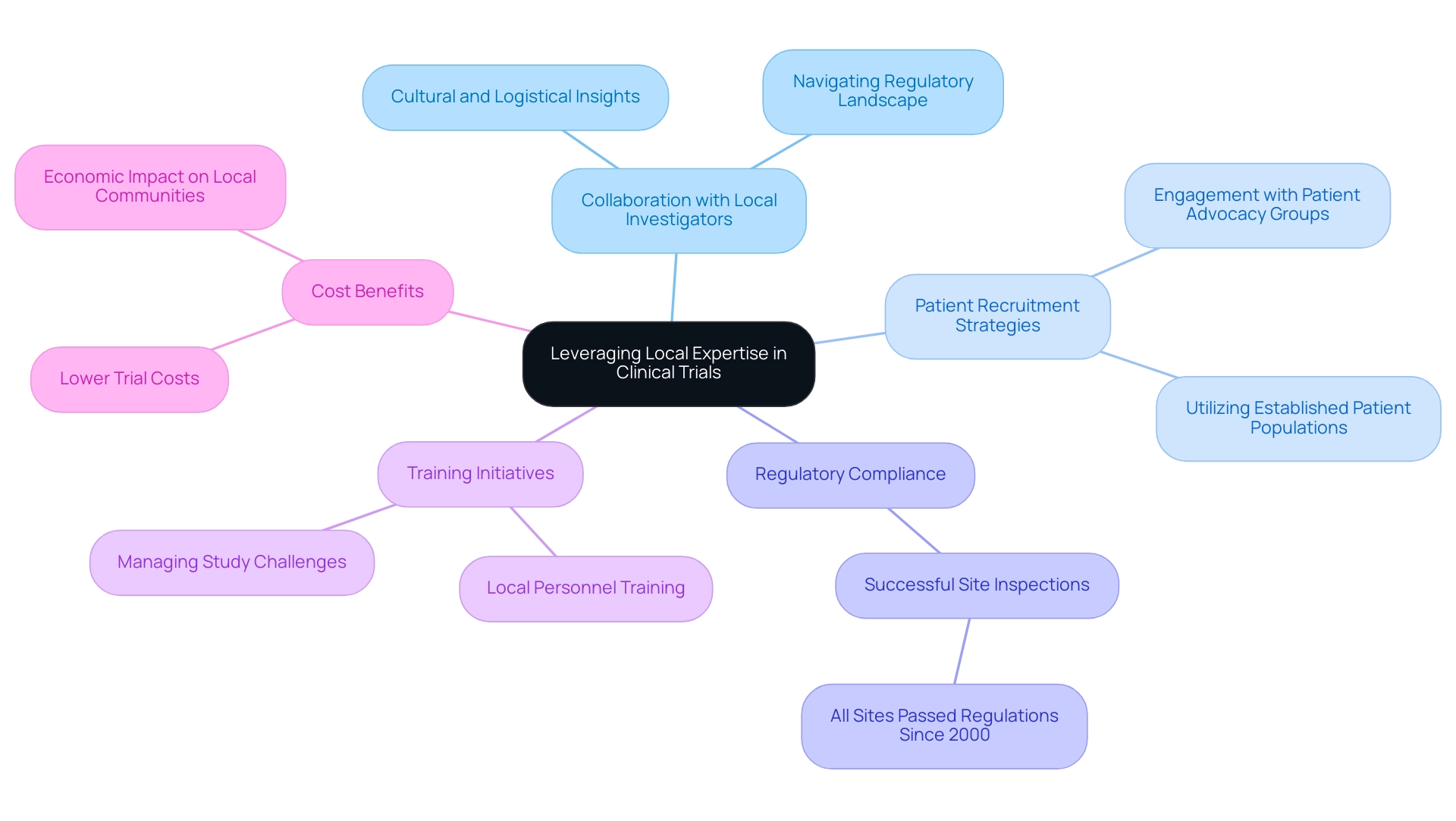
Best Practices for Leveraging Local Expertise in Clinical Trials
To enhance research results, leveraging Latin America medical trial expertise along with local knowledge and resources is essential. Collaborating with local investigators who have Latin America medical trial expertise and a profound understanding of the cultural and logistical nuances of the region can significantly enhance success. These investigators not only provide valuable insights into effective patient recruitment strategies but also assist in navigating the regulatory landscape, which highlights Latin America medical trial expertise as all inspected clinical research sites in Peru have successfully met regulations since 2000, demonstrating the effectiveness of local expertise in ensuring compliance.
Moreover, utilizing local research sites with established patient populations can benefit from Latin America medical trial expertise, streamlining recruitment efforts and shortening timelines. Considering that 85 percent of patients in Latin regions are unaware of their participation options at diagnosis, engaging with local patient advocacy groups becomes essential in facilitating outreach and education, thereby improving awareness and involvement in studies. Furthermore, investing in local training initiatives for personnel prepares teams to manage the distinct challenges of conducting studies, which enhances their Latin America medical trial expertise in this varied region.
Julio Martinez-Clark, CEO of bioaccess, has been a crucial supporter of Medtech clinical studies in Latin America, utilizing his Latin America medical trial expertise to implement successful clinical experiments for over 100 Medtech companies while concentrating on regional possibilities. His efforts include fostering partnerships with local stakeholders and enhancing the understanding of market dynamics, which are essential for successful execution. With the Latin America medical trial expertise of Monica Mora, Chief Operating Officer focusing on operations and regulatory strategies, bioaccess stands out as a prominent contract organization facilitating essential studies.
As Karen Politis Virk, director of biotech and pharmaceutical development at Language Connections, states, 'Proper management of studies requires that expert translation and localization strategies be implemented at all stages of medical exploration.' Moreover, the notably reduced expenses in Latin America medical trial expertise offer a persuasive benefit for sponsors, making it a desirable area for medical studies. By adopting these best practices, sponsors can maximize the benefits of conducting clinical trials in the region, utilizing Latin America medical trial expertise to position clinical research as a catalyst for social and economic development, as evidenced by its positive impact on access to innovative treatments and job creation in Colombia and beyond.
Conclusion
The emergence of Latin America as a prime location for clinical trials is underscored by its diverse patient population, cost-effectiveness, and evolving regulatory frameworks. With countries like Colombia and Brazil leading the way, the region offers significant advantages for sponsors seeking to optimize their research investments. The collaboration between local stakeholders and Contract Research Organizations (CROs) is vital in navigating the complexities of clinical trials, ensuring that both regulatory compliance and patient recruitment are effectively managed.
While challenges such as cultural nuances and regulatory differences exist, strategic planning and local expertise can mitigate these hurdles. The commitment to inclusivity and diversity in clinical research not only enhances the validity of trial outcomes but also fosters trust among participants, making it essential for successful engagement. As investment in the sector continues to grow, so does the potential for Latin America to play a pivotal role in the global clinical trial landscape.
Ultimately, leveraging local resources and expertise will be key to maximizing the benefits of conducting clinical trials in this promising region. By embracing best practices and fostering collaborative efforts, sponsors can not only achieve successful trial outcomes but also contribute to the broader goal of advancing healthcare solutions that are culturally relevant and accessible to diverse populations. As Latin America continues to evolve as a hub for clinical research, the opportunities for innovation and growth are boundless, positioning the region as a crucial player in the future of clinical trials.
Frequently Asked Questions
Why is Latin America considered an appealing location for clinical studies?
Latin America is appealing for clinical studies due to its varied patient population, cost-effectiveness, advancements in regulatory frameworks, and significant investment in the research sector.
How does the patient population in Latin America benefit clinical studies?
The diverse patient population in Latin America ensures the generalizability of research outcomes, allowing researchers to achieve robust recruitment for their studies.
What financial advantages does Latin America offer for conducting clinical trials?
Conducting trials in Latin America is often significantly cheaper than in North America or Europe, providing a strong monetary incentive for sponsors.
What advancements have been made in regulatory frameworks in Latin America?
Countries like Brazil have made strides in reducing approval times for clinical studies, with Brazil's National Health Surveillance Agency (ANVISA) facilitating more efficient study initiation.
What recent investments have been made in the research sector in the Andean Region?
Investment in the research sector in the Andean Region has increased from $3-4 million to over $50 million annually, indicating rising interest and growth in medical research.
What are the benefits of the partnership between bioaccess™ and Caribbean Health Group?
This partnership aims to establish Barranquilla as a premier site for medical studies, leading to over a 50% reduction in recruitment time and a 95% retention rate in clinical trials.
What services does bioaccess® provide for clinical trials?
Bioaccess® offers extensive research management services, including feasibility assessments, site selection, compliance evaluations, and project oversight, particularly focusing on expedited medical device studies.
How do Contract Research Organizations (CROs) contribute to clinical studies in Latin America?
CROs provide essential services such as site management, patient recruitment, and regulatory compliance, helping sponsors focus on their primary objectives while navigating local regulations.
What is the significance of Colombia’s healthcare system in clinical research?
Colombia's healthcare system ranks highly globally and has a rapid IRB/EC and MoH (INVIMA) review process, which aids in expediting approval processes for clinical trials.
What are the benefits of partnering with a CRO for clinical studies?
Partnering with a CRO can enhance timelines, improve data accuracy through innovative technologies, and ultimately lead to more efficient study results.
What financial incentives does Colombia offer for research and development?
Colombia provides significant R&D tax incentives, including a 100% tax deduction, a 25% tax discount, and a 50% future tax credit, making it an attractive option for companies conducting research.
How does patient access in Colombia support clinical trials?
With a population exceeding 50 million and about 95% having access to universal healthcare, Colombia offers a strong participant base for research studies, facilitating patient recruitment.

