Overview
Building partnerships for medtech trials in Paraguay is essential for enhancing the quality and efficiency of medical research. Collaborating with local institutions and stakeholders not only improves patient recruitment but also ensures compliance with regulations. Such alliances leverage diverse expertise and resources, reduce risks, and foster innovation in medical technologies. Ultimately, these collaborations are vital for achieving successful clinical outcomes in the region.
Introduction
In the rapidly evolving world of Medtech, the importance of strategic partnerships in clinical trials is paramount. These collaborations not only enhance research quality and efficiency but also grant access to a wealth of expertise and resources essential for navigating the complexities of regulatory landscapes and patient recruitment.
As the industry faces rising costs and an increasing demand for innovative solutions, organizations are recognizing that successful partnerships can lead to transformative outcomes. The dynamics of these alliances, from aligning objectives to fostering open communication, are crucial for driving advancements in medical technology and improving patient care.
It is vital for anyone looking to thrive in the competitive Medtech arena to understand the key qualities of effective partners and the steps necessary to initiate and nurture these relationships.
Understand the Importance of Partnerships in Medtech Trials
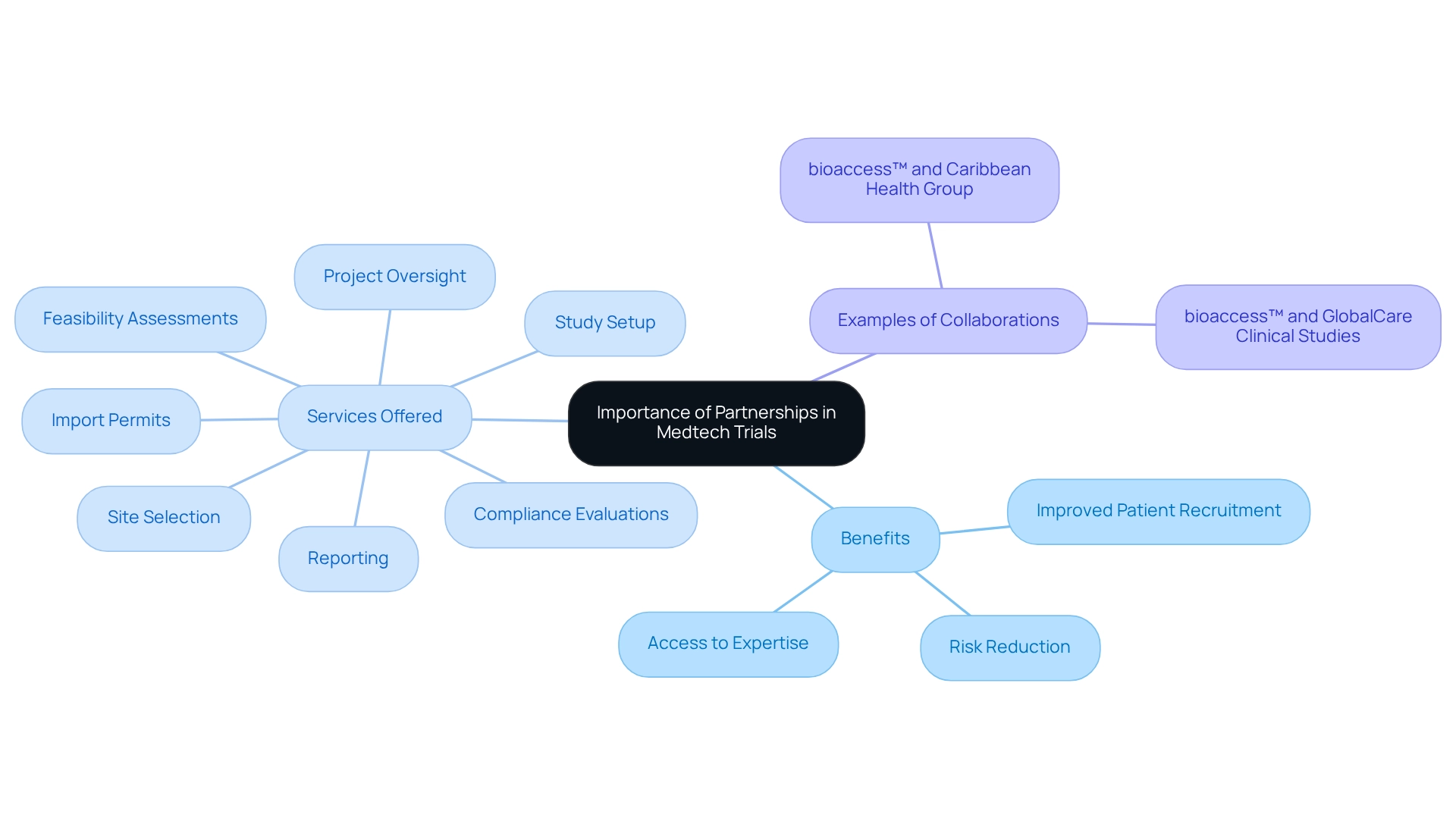
Building partnerships for medtech trials in Paraguay is essential for enhancing the quality and efficiency of medical research through collaborations in Medtech studies. They grant access to a diverse array of expertise, resources, and networks, significantly accelerating the research process. Building partnerships for medtech trials in Paraguay involves collaborating with local institutions, regulatory bodies, and other stakeholders, which not only improves patient recruitment but also streamlines processes to ensure compliance with local regulations. This cooperative approach reduces risks by sharing responsibilities and leveraging the unique advantages of each collaborator, ultimately leading to more favorable study outcomes.
For instance, bioaccess™ specializes in comprehensive research management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Reporting
Their partnership with Caribbean Health Group aims to position Barranquilla as a leading hub for medical studies in Latin America, supported by Colombia's Minister of Health. Building partnerships for medtech trials in Paraguay yields valuable insights into patient demographics and regulatory requirements unique to the region, which are crucial for designing studies that are both effective and compliant from the outset. The significance of these alliances is underscored by the projected 7.7 percent compound annual growth rate (CAGR) for the contract research outsourcing market, indicating a growing recognition of the benefits of collaboration in clinical research.
Moreover, the rising costs associated with drug development, averaging around $2.6 billion in the US, highlight the financial challenges faced by medical technology firms. Building partnerships for medtech trials in Paraguay, such as those between bioaccess™ and GlobalCare Clinical Studies, can mitigate these costs by pooling resources and expertise, allowing collaborators to navigate the complexities of medical technology studies more effectively. As the medical technology landscape continues to evolve in 2025, emphasizing strategic partnerships will be essential for fostering innovation and ensuring the successful advancement of medical technologies.
Identify Key Qualities of Effective Partners
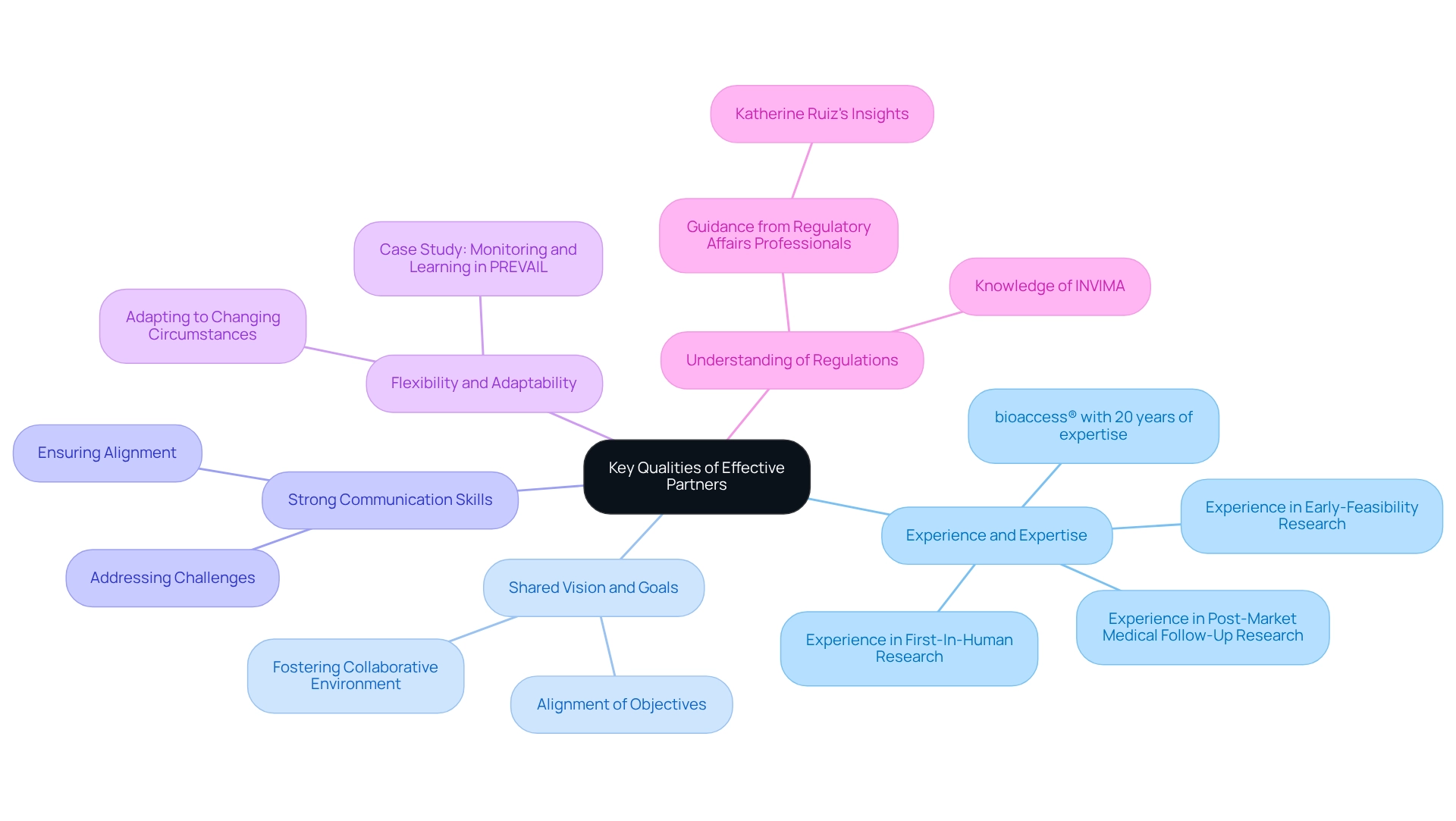
Building partnerships for medtech trials in Paraguay requires careful evaluation of key qualities when selecting partners.
-
Experience and Expertise: Seek partners with a robust background in medical research and a comprehensive understanding of the Medtech landscape. Their experience significantly impacts the success of experiments, especially as 69% of healthcare organizations are testing or adopting AI, highlighting the need for partners who can navigate evolving technologies. For instance, bioaccess® boasts over 20 years of expertise in overseeing medical studies, including Early-Feasibility Research, First-In-Human Research, and Post-Market Medical Follow-Up Research, providing a solid foundation for successful outcomes. Their extensive service capabilities encompass setup, project management, and reporting—essential for efficient execution.
-
Shared Vision and Goals: Aligning objectives and values with potential partners fosters a collaborative environment, which is essential for navigating the complexities of medical research.
-
Strong Communication Skills: Effective communication is vital for addressing challenges and ensuring that all parties remain aligned throughout the testing process.
-
Flexibility and Adaptability: The dynamic nature of clinical research necessitates partners who can swiftly adapt to changing circumstances and requirements. For example, the case study titled "Monitoring and Learning in PREVAIL" illustrates how operational reviews can diminish over time, emphasizing the need for partners to maintain strategic focus and adaptability.
A solid grasp of local regulations and compliance requirements is essential for partners involved in building partnerships for medtech trials in Paraguay to successfully navigate the complexities of Medtech studies. Understanding the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, as a Level 4 health authority by PAHO/WHO, is crucial for ensuring compliance and successful execution of the study. Additionally, insights from professionals like Katherine Ruiz in Regulatory Affairs for Medical Devices and In Vitro Diagnostics can provide invaluable guidance in navigating these regulations.
Integrating these attributes can lead to fruitful collaborations that enhance the effectiveness of trials and contribute to the overall advancement of medical technologies in the region. As noted by Sally Wang Liang, trends indicate waning investor interest in oncology, underscoring the importance of selecting partners aligned with current market dynamics.
Initiate Contact and Establish Communication
To effectively initiate contact with potential partners in clinical research, consider the following steps:
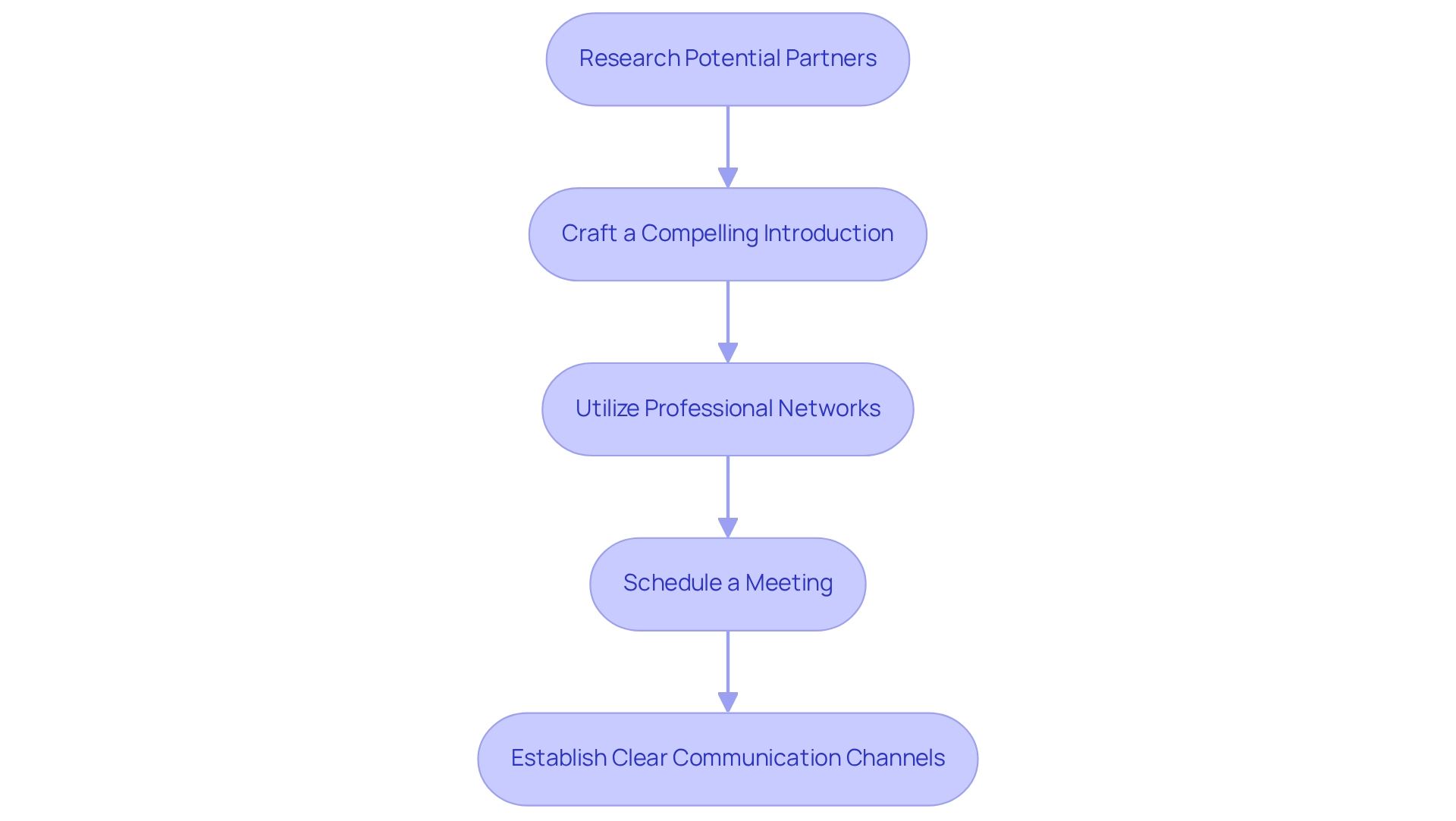
- Research Potential Partners: Identify organizations or individuals whose expertise aligns with your objectives. This essential step guarantees that your outreach is focused and pertinent, particularly in the intricate environment of Latin American healthcare technology, where grasping local dynamics is vital.
- Craft a Compelling Introduction: Develop a concise introduction that clearly outlines your goals and the mutual benefits of collaboration. As Corrina Thurston aptly states, "Get to the point quickly to prove that what you’re saying is worth reading or listening to, otherwise, people will lose interest." A clearly expressed message can engage attention and prepare the ground for additional conversations, especially when emphasizing how your collaboration can utilize bioaccess®'s proficiency in study management, including setup and project oversight.
- Utilize Professional Networks: Leverage platforms such as LinkedIn and attend industry conferences to connect with potential partners. Engaging in these networks can facilitate introductions and foster relationships, which is essential for building partnerships for medtech trials in Paraguay as well as navigating the Medtech landscape in Latin America.
- Schedule a Meeting: Propose a meeting to discuss shared interests and explore collaboration opportunities. This direct approach demonstrates your commitment and eagerness to engage, particularly in the context of bioaccess®'s comprehensive services, including feasibility studies, site selection, and compliance reviews.
- Establish Clear Communication Channels: Agree on preferred communication methods and frequency to maintain ongoing dialogue and transparency. Effective communication is essential for establishing trust and ensuring a successful collaboration, particularly when coordinating intricate medical studies that can greatly influence local economies through job creation and healthcare enhancement.
In the changing environment of medical research, building partnerships for medtech trials in Paraguay is essential. For instance, recent case studies highlight how international collaborations have improved health outcomes by including underserved populations, thereby advancing personalized medicine. Notably, only 0.1% of clinical trial participants in cancer treatment studies were Native American, underscoring the importance of understanding patient demographics in recruitment strategies. By following these steps, you can build a robust network that supports the success of your medical technology initiatives, reinforcing the key point that the way a team collaborates determines its overall success.
Negotiate Terms and Align Goals
In the negotiation stage of Medtech alliances, adopting a systematic method is crucial to guarantee effective cooperation.
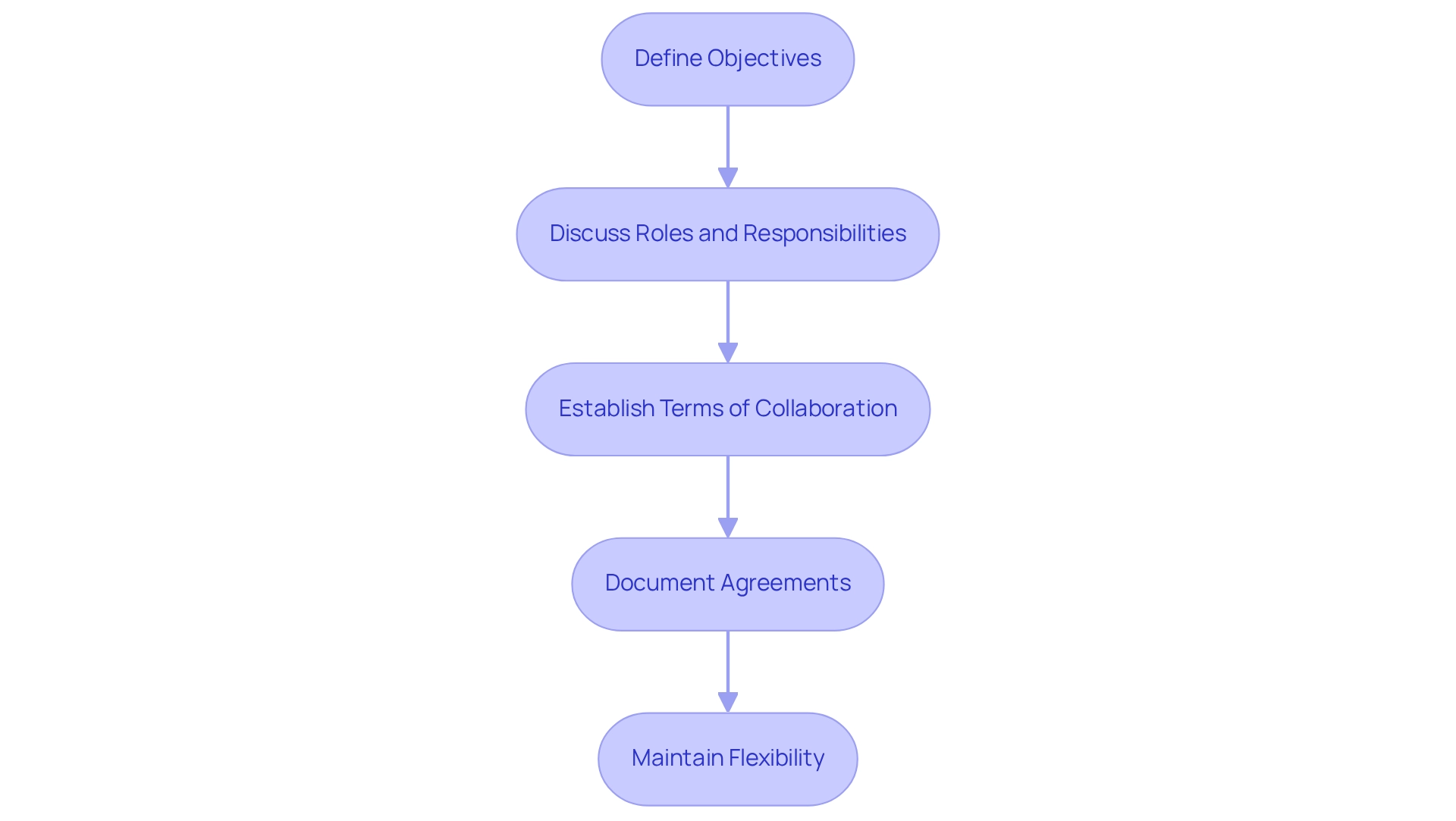
- Define Objectives: Clearly articulate the goals each party aims to achieve through the collaboration. This alignment is essential, as successful collaborations often hinge on shared objectives.
- Discuss Roles and Responsibilities: Ensure that all partners have a clear understanding of their specific contributions to the research study. This clarity fosters accountability and enhances team unity.
- Establish Terms of Collaboration: Negotiate critical terms related to funding, resource allocation, and timelines. Successful negotiation tactics can significantly influence the outcomes of research collaborations. Incorporating extensive research study management services from bioaccess—such as feasibility analyses, site selection, compliance evaluations, study setup, import permits, project management, and reporting—can enhance these negotiations by providing a clear framework and support for each aspect of the research.
- Document Agreements: Create a formal agreement that encapsulates all negotiated terms. This documentation is vital for preventing misunderstandings and ensuring that all parties are aligned.
- Maintain Flexibility: Be ready to revisit and modify terms as the collaboration evolves and new challenges emerge. Adaptability can be a crucial element in managing the intricacies of clinical research, and building partnerships for medtech trials in Paraguay allows medical technology firms to establish robust alliances that not only improve cooperation but also promote successful clinical study results.
Engaging diverse communities in observational trials can serve as a 'gateway' to broader research participation, highlighting the importance of understanding patient preferences in recruitment strategies. Significantly, with 80% of internet users seeking health information online, it is essential for medical technology firms to capitalize on this trend to enhance their understanding and meet patient needs during discussions. Moreover, the influence of medical technology clinical studies on local economies—through job creation, economic growth, healthcare enhancement, and international cooperation—should not be overlooked as a substantial advantage of these collaborations.
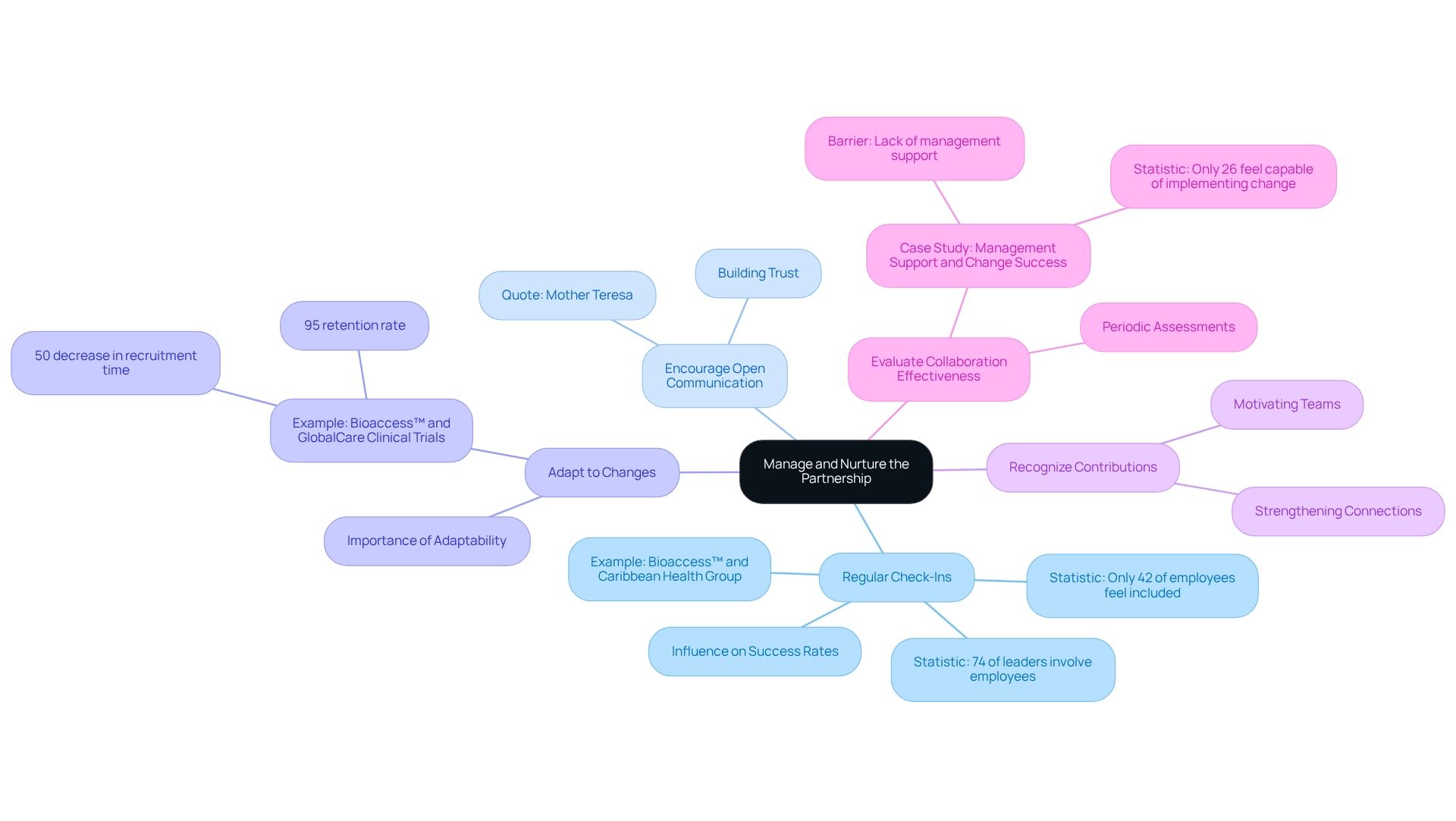
Manage and Nurture the Partnership
To effectively manage and nurture your partnership in the Medtech sector, consider implementing the following strategies:
- Regular Check-Ins: Schedule consistent meetings to review progress, tackle challenges, and celebrate milestones. Regular check-ins significantly influence success rates in experiments, promoting a proactive approach to problem-solving. In fact, 74% of leaders claim to involve employees in creating change strategies, but only 42% of employees feel included, highlighting the importance of these interactions. For instance, bioaccess™'s partnership with Caribbean Health Group underscores the necessity of building partnerships for medtech trials in Paraguay to secure Barranquilla's status as a prominent location for medical studies in Latin America, a goal backed by Colombia's Minister of Health.
- Encourage Open Communication: Create an environment where all partners feel empowered to share feedback and innovative ideas. Open communication is crucial for building trust and ensuring that all voices are heard, which can lead to more effective collaboration. As Mother Teresa wisely stated, "None of us, including me, ever do great things. But we can all do small things, with great love, and together we can do something wonderful."
- Adapt to Changes: Remain adaptable and prepared to modify strategies and goals as the experiment progresses and new insights are acquired. This adaptability is essential for building partnerships for medtech trials in Paraguay while navigating the complexities of clinical research. For example, the collaboration between bioaccess™ and GlobalCare Clinical Trials has led to over a 50% decrease in recruitment time and a 95% retention rate, highlighting the advantages of being adaptable to trial dynamics.
- Recognize Contributions: Acknowledge and appreciate the efforts of all partners. Acknowledgment not only strengthens connections but also motivates teams, improving overall collaboration effectiveness.
- Evaluate Collaboration Effectiveness: Conduct periodic assessments of the alliance's performance. This evaluation allows for necessary adjustments to improve collaboration and ensures that all partners are aligned with the project's goals. A case study titled "Management Support and Change Success" illustrates that lack of management support is a significant barrier to successful change, leading to employee distrust and disengagement. Organizations must prioritize management support to build trust and facilitate smoother transitions during change initiatives.
Insufficient planning and resources can obstruct change management, making these strategies essential for successful collaborations. By prioritizing management support and encouraging a culture of collaboration, organizations can build trust and enable smoother transitions during research initiatives, which includes building partnerships for medtech trials in Paraguay. Additionally, engaging diverse communities in observational trials may serve as a gateway to greater participation in clinical research overall, further emphasizing the importance of effective partnership management.
Conclusion
Strategic partnerships in the Medtech sector are vital for enhancing the quality and efficiency of clinical trials. Through collaboration, organizations gain access to valuable expertise, streamline processes, and navigate local regulatory landscapes more effectively. By aligning objectives and fostering open communication, these partnerships can lead to successful trial outcomes, as evidenced by collaborations such as that of bioaccess™ and Caribbean Health Group.
Identifying key qualities in potential partners—such as experience, shared vision, strong communication skills, and adaptability—significantly influences the success of clinical trials. By initiating contact thoughtfully and establishing clear communication channels, organizations can build robust networks that support their Medtech initiatives. Furthermore, negotiating terms and managing partnerships with flexibility and regular check-ins ensures all parties remain aligned and engaged throughout the research process.
Ultimately, the emphasis on strategic partnerships will play a pivotal role in driving innovation and improving patient care in the evolving Medtech landscape. As the industry faces increasing costs and demands for new solutions, organizations that prioritize collaboration will be better positioned to navigate challenges and achieve transformative outcomes. The future of Medtech relies on these alliances, underscoring the necessity for organizations to invest in building and nurturing effective partnerships.
Frequently Asked Questions
Why are partnerships important for medtech trials in Paraguay?
Partnerships enhance the quality and efficiency of medical research by providing access to diverse expertise, resources, and networks, which significantly accelerates the research process.
What stakeholders should be involved in building partnerships for medtech trials in Paraguay?
Collaborating with local institutions, regulatory bodies, and other stakeholders is essential for improving patient recruitment and streamlining processes to ensure compliance with local regulations.
What services does bioaccess™ provide in the context of medtech trials?
Bioaccess™ offers comprehensive research management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting.
How does collaboration in medtech trials contribute to favorable study outcomes?
Collaborations reduce risks by sharing responsibilities and leveraging the unique advantages of each partner, ultimately leading to more favorable study outcomes.
What is the significance of the projected growth rate for the contract research outsourcing market?
The projected 7.7 percent compound annual growth rate (CAGR) indicates a growing recognition of the benefits of collaboration in clinical research, underscoring the importance of partnerships.
What financial challenges do medical technology firms face?
The rising costs associated with drug development, averaging around $2.6 billion in the US, present significant financial challenges for medical technology firms.
How can partnerships help mitigate the costs of medtech trials?
Partnerships can pool resources and expertise, allowing collaborators to navigate the complexities of medical technology studies more effectively and reduce overall costs.
What key qualities should be evaluated when selecting partners for medtech trials?
Important qualities include experience and expertise, shared vision and goals, strong communication skills, and flexibility and adaptability.
Why is understanding local regulations important for partners in medtech trials?
A solid grasp of local regulations and compliance requirements is essential for successfully navigating the complexities of Medtech studies and ensuring compliance.
What role does INVIMA play in the context of medtech trials in Paraguay?
INVIMA, Colombia's National Food and Drug Surveillance Institute, serves as a Level 4 health authority by PAHO/WHO, making it crucial for ensuring compliance and successful execution of medical studies.




