Introduction
In the intricate landscape of clinical trials, the definitions and management of endpoints are critical to ensuring that research outcomes genuinely reflect the efficacy and safety of new treatments. Primary endpoints serve as the cornerstone of these trials, providing clear metrics for evaluating treatment effectiveness, while secondary endpoints enrich the understanding of broader implications, including quality of life and adverse events. The hierarchical relationship between these endpoints is essential, as it shapes the interpretation of results and influences clinical decision-making.
Furthermore, the statistical rigor required to navigate the complexities of multiple endpoints cannot be overstated, as improper handling can lead to misleading conclusions. This article delves into the nuanced roles of primary and secondary endpoints, the importance of establishing a clear hierarchy, and the statistical considerations necessary for credible trial outcomes, ultimately underscoring their significance in the advancement of patient care and medical research.
Understanding Primary Endpoints
Main objectives are crucial measures in clinical studies, serving as the main standards for assessing the efficacy of a therapy. These targets are thoroughly specified in the research framework and correspond directly with the study's aims, enabling clear, evidence-based conclusions regarding the intervention's effectiveness. For example, in a Phase III study for a new cancer medication, typical main objectives may include overall survival (OS) or progression-free survival (PFS). The accuracy in specifying these limits is essential, as regulatory organizations such as the FDA require clear definitions to ensure uniformity and transparency in study results.
'The importance of key outcomes goes beyond simple measurements; they are essential to the ethical and scientific integrity of medical studies.'. A recent analysis of 387 oncology randomized controlled studies (RCTs) published from 2017 to 2021 revealed that 11% had suboptimal controls, which can lead to misleading conclusions about treatment efficacy. This emphasizes the significance of choosing suitable primary endpoints that represent optimal therapies in present medical practices.
Moreover, the study design may differ based on the chosen statistical approach—be it frequentist or Bayesian. Bayesian methods offer a more adaptable framework, allowing for interim analyses that can identify ineffective treatments earlier, thereby optimizing resource allocation. This flexibility can enhance the overall quality of medical research and its alignment with real-world patient care.
Integrating electronic health records (EHRs) into study designs can further streamline data collection and enhance the generalizability of study outcomes. 'This integration is one of the practical elements being discussed in initiatives like the FDA's Project Pragmatica, which aims to harmonize research with patient care.'. By promoting global partnerships and early involvement with regulatory bodies, the research community can tackle the inherent challenges of medical studies more effectively.
In summary, a clearly specified main goal is not merely a regulatory necessity; it is an essential aspect that guarantees the trustworthiness and relevance of research findings, ultimately impacting patient care and results.
Understanding Secondary Endpoints
'Secondary targets play a crucial role in clinical trials by providing additional insights into the effects of a treatment beyond the primary objectives.'. These targets may encompass various measures, such as quality of life assessments, incidence of adverse events, and responses in biomarkers. 'Although secondary measures are not the principal focus of the study, they are instrumental in understanding the broader implications of a treatment's efficacy and safety.'.
For example, in oncology trials, secondary measures might assess the percentage of patients who report significant improvements in their quality of life after receiving a new cancer therapy. This approach aligns with the growing emphasis on composite patient outcomes, which incorporate multiple facets of treatment benefit, safety, and patient well-being.
Experts from academia and the pharmaceutical sector are increasingly promoting the inclusion of these secondary objectives in clinical research. As mentioned in conversations at recent conferences, a detailed assessment of secondary outcomes can lead to a more thorough understanding of treatment effects. "When time and resources to demonstrate benefit in overall survival (OS) are too substantial, substitute metrics may be used to capture the treatment's effect efficiently," highlighting the importance of secondary measures in various contexts.
Moreover, the regulatory landscape is evolving to support this comprehensive approach. Regulatory agencies stress the importance of trustworthy information in submissions, especially regarding both primary and secondary objectives. The integration of these additional measures not only enriches the data collected but also aids in addressing health disparities and improving patient care outcomes. This aligns with the societal goals of medical research, focusing on enhancing human health through informed decision-making.
Hierarchy of Endpoints in Clinical Trials
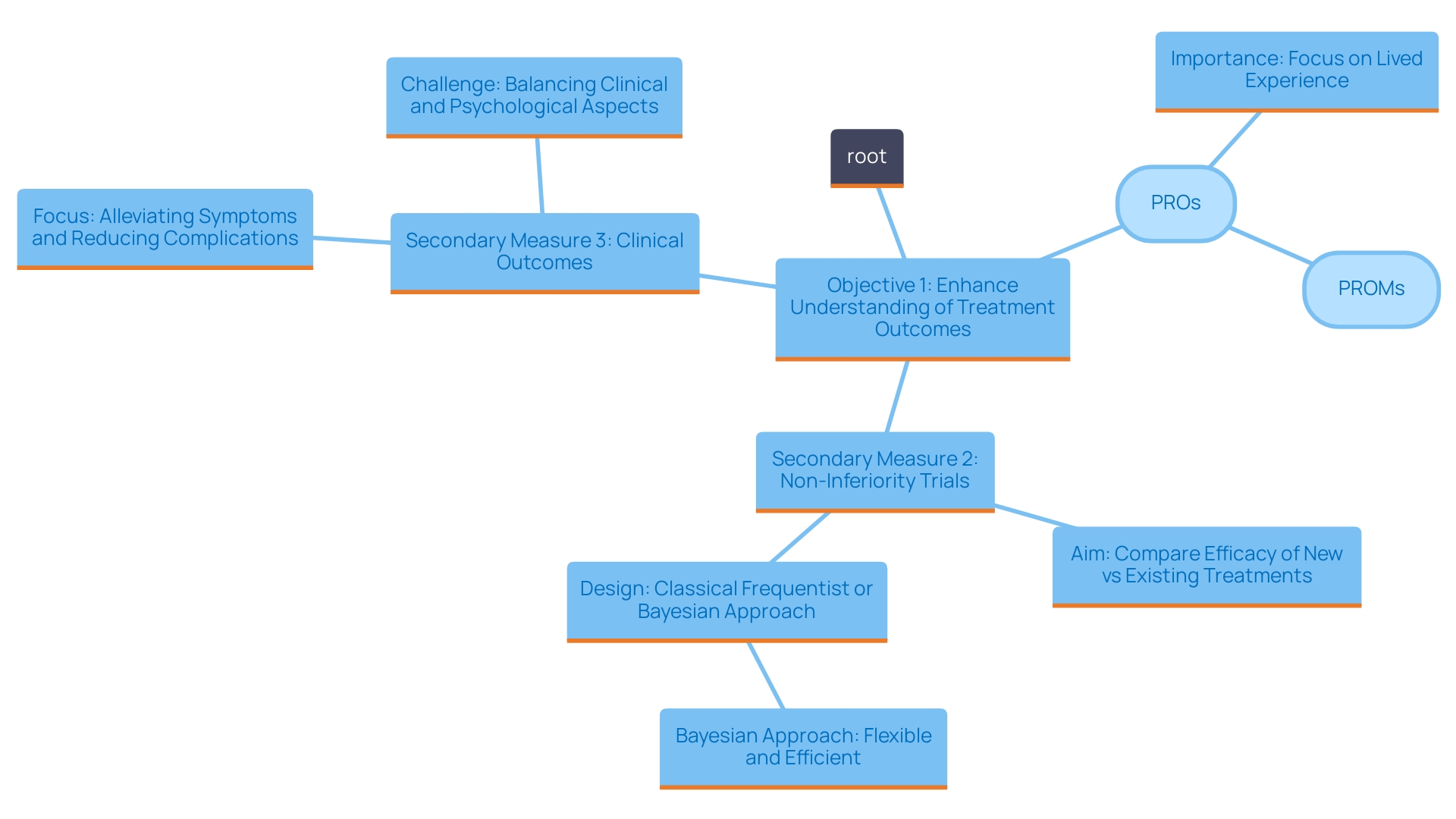
Creating a distinct ranking of objectives is essential in clinical studies, especially for managing several results efficiently. 'Main objectives are the focal point of the study; they dictate the main question the trial seeks to answer.'. Additional targets, although not the main emphasis, provide valuable extra details that can improve comprehension of the treatment's overall effect.
The connection between these points is significant. For example, the understanding of secondary measures can be affected by the outcomes of main measures. If a trial shows a statistically significant enhancement in overall survival (OS) as the main goal, the following assessment of quality of life-related secondary measures may indicate a more positive interpretation. This suggests that the treatment not only extends life but may also improve its quality.
Furthermore, the structure for assessing these targets has advanced, resembling the rigorous processes established for bringing medical devices to market. Just as manufacturers must validate the safety and efficacy of devices, researchers must ensure that the outcomes they select are robust and meaningful. In this context, the challenge lies in navigating regulatory requirements while embracing innovative methodologies that may include digital measures.
The focus on patient-reported outcomes (PROs) is especially significant in modern research. PROs have gained traction as they provide insights into the patient experience, which can be pivotal in understanding treatment benefits. Employing Pros effectively within the structure of primary and secondary goals enables researchers to obtain a thorough understanding of therapeutic efficacy.
As the setting of medical studies keeps evolving, keeping an emphasis on the ranking and interplay of outcomes will be crucial. 'This approach not only fosters clarity in research objectives but also aligns with the growing demand for patient-centered outcomes in medical development.'.
Statistical Considerations for Multiple Endpoints
In clinical study design, the inclusion of multiple outcomes requires thorough statistical evaluations to guarantee reliable and trustworthy results. The challenge of multiplicity arises when assessing various outcomes, which can inadvertently inflate Type I error rates if not addressed properly. To maintain the integrity of the study's findings, researchers often utilize strategies such as hierarchical testing procedures or adjustments to significance levels.
For instance, when evaluating a primary endpoint like survival alongside secondary endpoints related to quality of life, it is essential to pre-specify an analysis plan. This proactive approach minimizes the risks associated with multiple comparisons and reinforces the validity of the conclusions drawn from the data.
A significant instance showcasing the importance of thorough statistical techniques can be found in recent writings that underscore the role of Bayesian statistics in medical studies. This method enables the incorporation of previous information and can adjust more readily to the intricacies of contemporary study designs. As discussed in a recent paper, Bayesian frameworks have gained traction in regulatory submissions, demonstrating their utility in providing insights that traditional frequentist methods might overlook.
Moreover, the credibility of statistical analysis is underscored by the necessity for collaboration between authors and statisticians. As Tathagata Banerjee notes, a transparent and rigorous approach to data analysis is vital for advancing medical research. 'Mistakes in statistical interpretation can have far-reaching consequences, making it essential for researchers in the medical field to engage with statistical experts to validate their methodologies and findings.'.
Ultimately, the thoughtful design of clinical trials, with a particular focus on statistical integrity and endpoint management, is crucial for the advancement of medical knowledge and ensuring patient safety.
Conclusion
In the realm of clinical trials, the delineation and management of primary and secondary endpoints are paramount for yielding credible and impactful research outcomes. Primary endpoints serve as the main metrics for assessing the effectiveness of treatments, while secondary endpoints provide valuable insights into broader implications, such as quality of life and safety considerations. The precise definition and rigorous selection of these endpoints not only uphold the integrity of clinical trials but also significantly influence clinical decision-making and patient care.
Understanding the hierarchical relationship between these endpoints is essential. Primary endpoints guide the main research questions, while secondary endpoints enrich the narrative surrounding treatment efficacy and patient experience. The interplay between these endpoints can lead to a more comprehensive understanding of a treatment's overall impact, thus reinforcing the necessity for a well-structured endpoint hierarchy in study designs.
Furthermore, the statistical handling of multiple endpoints cannot be overlooked. Proper methodologies are required to mitigate risks associated with multiplicity and ensure valid conclusions. The adoption of innovative statistical frameworks, such as Bayesian approaches, enhances the analysis and interpretation of trial data, thereby fostering the advancement of medical research.
In conclusion, a meticulous approach to defining, managing, and analyzing endpoints in clinical trials is crucial. This not only ensures the reliability of trial outcomes but also aligns with the broader objectives of enhancing patient care and advancing medical knowledge. As clinical research continues to evolve, prioritizing these elements will be vital for meeting the growing demands for transparency, credibility, and patient-centered outcomes in the field.




