Introduction
The landscape of clinical trials is intricately woven with the choice of control groups, a decision that significantly influences the integrity and interpretation of study outcomes. Active control groups, in particular, are paramount as they provide a robust foundation for comparing new treatments against established therapies, thereby offering insights into their efficacy and safety. This article delves into the nuanced distinctions between active and passive control groups, highlighting their respective roles in enhancing the reliability of clinical trial results.
It explores various types of control groups, ethical considerations in trial design, and the implications of using active controls in advancing medical knowledge. By examining case studies and meta-analyses, the discussion underscores the critical importance of thoughtful control group selection in clinical research, ultimately aiming to improve patient care and treatment methodologies.
Understanding Active and Passive Control Groups
Active participant ensembles serve a crucial function in the realm of clinical studies by receiving an intervention that is expected to provide a healing impact. This enables a robust comparison with the experimental procedure under investigation. In contrast to inactive groups, which may receive no intervention or a placebo, active comparison groups offer a more dynamic framework for assessing effectiveness and safety, greatly assisting in the analysis of study outcomes.
The integrity of trial outcomes hinges on the clear understanding of these distinctions. For instance, active controls can help reduce biases that may arise from using placebos, especially in studies where withholding care could pose ethical concerns. Such ethical considerations are underscored by recent discussions surrounding safety concerns in medical product development, emphasizing the need for diverse intervention comparisons.
A statistical evaluation indicates that many trials suffer from lower statistical power than the widely accepted benchmarks of 80% or 90%. This frequently results in overestimated effects of the interventions in reported outcomes. By utilizing active participant sets, researchers can better showcase the real-world effectiveness of new therapies, aiding in counteracting the common pitfalls of statistical misinterpretation. As emphasized in a recent study, a thorough examination of clinical research designs can uncover potential warning signs, assisting researchers in the choice of suitable comparisons while tackling the intricacies of treatment distribution.
Additionally, FDA recommendations regarding the use of historical data highlight the significance of employing clearly defined active comparison sets in studies. Such guidelines aim to streamline the process of incorporating complex interactive designs, ultimately enhancing the quality and reliability of clinical research outcomes. Consequently, comprehending the dynamics of active participant sets not only enhances the information gathered but also guarantees that the results add significant value to medical knowledge and patient care.
The Role of Active Control Groups in Clinical Trials
Active control groups are essential in clinical studies, particularly for evaluating the efficacy and safety of new interventions. Their significance lies in providing a comparative framework that demonstrates not only the effectiveness of a novel treatment but also its superiority over existing standard therapies. This approach is particularly crucial in therapeutic areas characterized by pronounced placebo effects, ensuring that any observed benefits can be reliably attributed to the intervention being studied.
Recent advancements in the regulatory landscape, as highlighted by the FDA's evolving guidelines, reflect a growing recognition of the value of historical data in clinical study design. For example, the FDA is currently investigating how patient-level historical data can be effectively employed alongside active comparison groups to enhance study results. This shift aligns with the need for innovative methodologies in drug development, especially in rare diseases where patient populations are limited. By utilizing historical benchmarks, researchers can reduce both the length and scale of clinical studies, thus enabling quicker access to potentially life-saving therapies.
Furthermore, the incorporation of machine learning and artificial intelligence into clinical research enables the possible sharing of data across studies. This capability is particularly advantageous in studies involving pediatric populations, where low patient numbers can significantly hinder research progress. 'Historical or synthetic data has already proven beneficial in instances such as the FDA's approval of AstraZeneca and Merck's Koselugo for treating neurofibromatosis type 1, which relied on historical reference data from earlier studies.'.
Ultimately, the use of active control teams not only improves the dependability of clinical study outcomes but also tackles ethical considerations in research design. By ensuring that experiments are carried out with strong comparisons, researchers can uphold the social objectives of enhancing patient results, thereby advancing the collective understanding of therapeutic effectiveness in various medical fields.
Types of Control Groups: Active, Passive, Placebo, and Sham
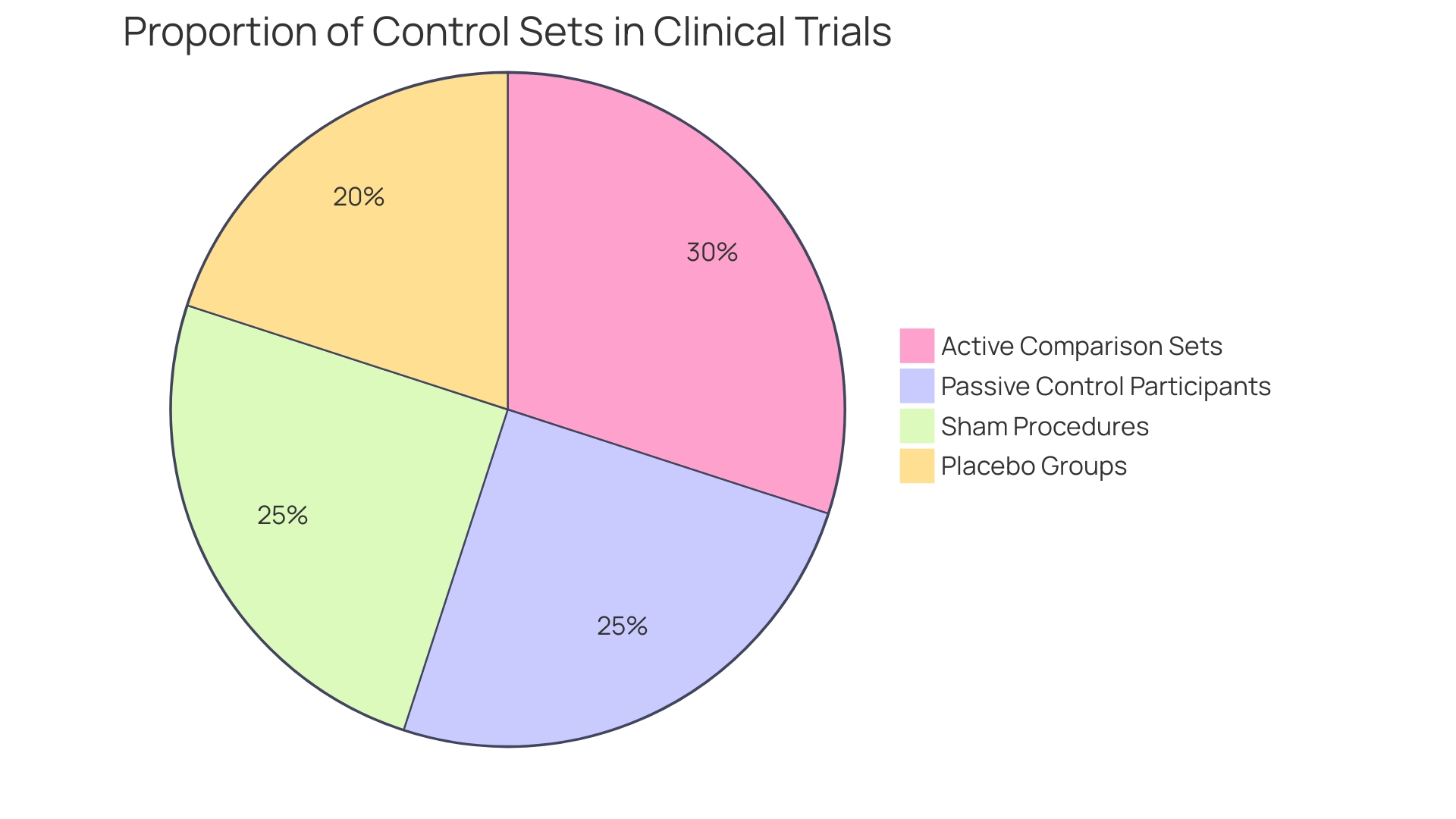
Control sets in clinical trials play a vital role in assessing the effectiveness of new therapies and can be categorized into several unique types: active, passive, placebo, and sham.
-
Active comparison sets receive recognized interventions that are already known to be effective. This approach allows researchers to compare the new therapy directly against a standard, providing a clear measure of its relative effectiveness. Active measures are especially important in showing that a novel intervention is better than current alternatives.
-
Passive control participants, in contrast, may receive no intervention at all. This category is often used in studies where the goal is to assess the natural progression of a condition without any therapeutic intervention, thereby providing a baseline for comparison.
-
Placebo groups are administered inert substances that have no therapeutic effect. The use of placebos is essential in managing the placebo effect, where participants may experience improvements simply because they believe they are receiving care. This assists in isolating the true effect of the experimental intervention.
-
Deceptive organizations undergo procedures that imitate the experimental intervention but lack any therapeutic intent. This method is especially pertinent in surgical studies, where the process of having surgery itself may influence results, therefore necessitating a comparison to individuals who undergo similar procedures without the direct intervention.
Every category of experimental cohort fulfills unique research aims, and the selection of which to utilize is influenced by the particular hypotheses and objectives of the study. For instance, integrating active measures can provide stronger evidence of efficacy, while using placebo or sham methods can help clarify the psychological impact of receiving treatment. As pointed out in recent publications, including the focus on ethical factors in clinical study design, meticulous choice of comparison sets is essential for guaranteeing strong and trustworthy results in medical research.
Ethical and Design Considerations for Active Control Trials
The ethical factors related to the use of active control teams in clinical studies are crucial. Researchers are tasked with the dual responsibility of providing optimal care for participants while ensuring the scientific integrity of the study. Key design elements include the careful selection of active comparators that are both relevant and ethically justifiable, which plays a critical role in maintaining the balance between patient welfare and the need for rigorous data.
Blinding is an essential strategy that can enhance the validity of the results; it minimizes bias and helps ensure that outcomes are not influenced by participants' or researchers' expectations. Furthermore, maintaining equipoise—where there is genuine uncertainty within the expert community regarding the comparative therapeutic merits of each intervention—is crucial to ethically justify the trial.
Effective randomization techniques, such as Equal Randomisation (ER), which divides participants into categories in a 1:1 ratio, are commonly used to strengthen the reliability of findings. Although ER is intuitive and relatively straightforward to implement, it is important to recognize that it does not necessarily maximize statistical power. Alternative methods, such as Thompson Sampling (TS), dynamically assign treatments based on the perceived likelihood of efficacy, potentially leading to more insightful outcomes while also addressing ethical considerations.
In the end, the design and implementation of clinical trials with active comparison participants must emphasize both ethical integrity and scientific rigor to promote knowledge while protecting participant interests.
Case Study: Evaluating the Impact of Active Control Groups in Cognitive Training Trials
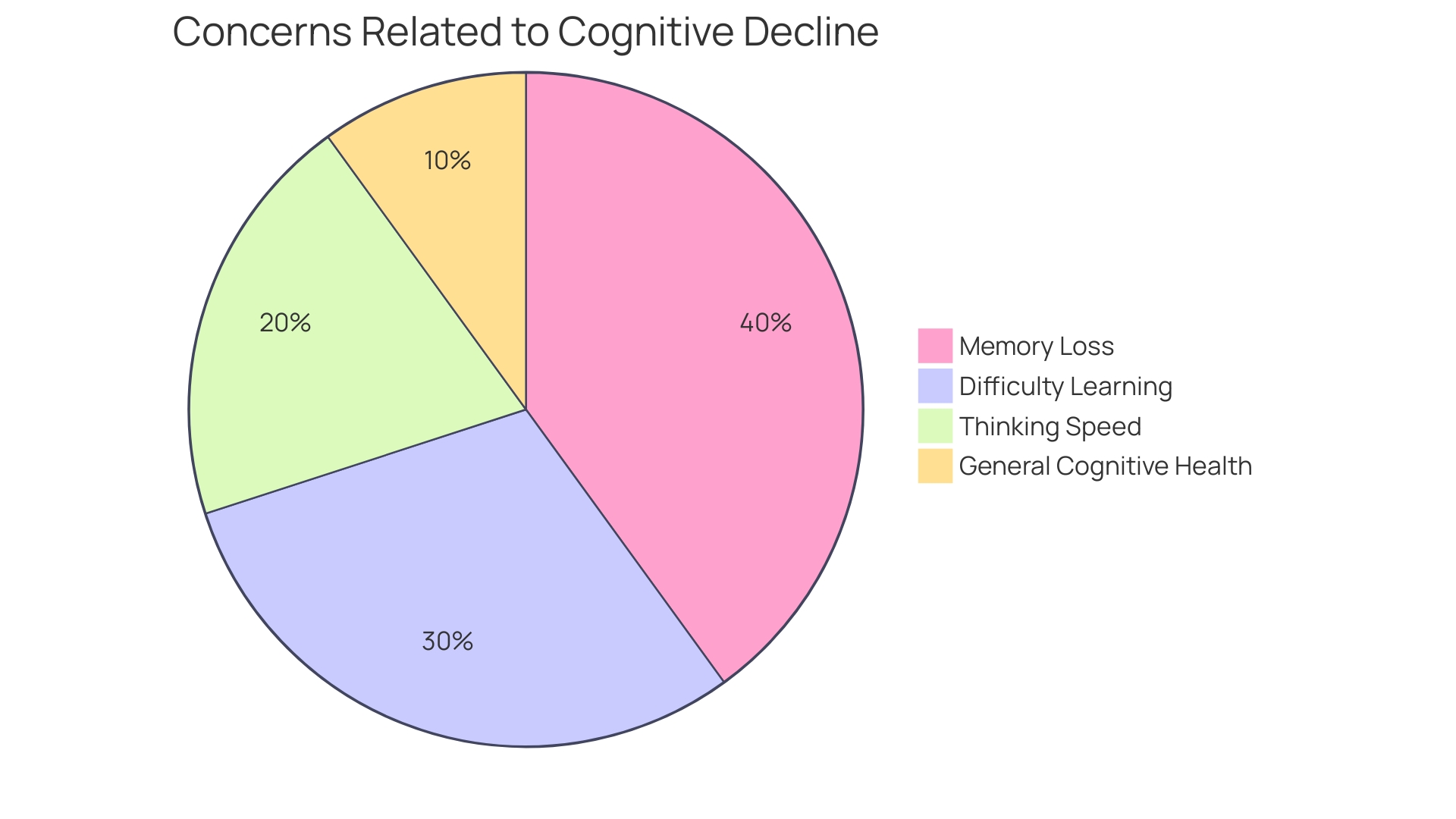
A significant case study examined cognitive training interventions aimed at older adults, utilizing an active comparison set that participated in established cognitive exercises. This study aimed to determine whether the innovative cognitive training program could yield superior improvements in cognitive performance compared to traditional methods. The findings revealed that participants in the experimental group exhibited statistically significant enhancements across various cognitive functions when contrasted with the active comparison group. This outcome highlights the essential function of active measures in the validation process of new interventions.
Research indicates that cognitive aging is a natural aspect of the aging process, often accompanied by changes in thinking, learning, and memory abilities. According to a survey, a considerable percentage of older adults express concerns about age-related cognitive decline, and many demonstrate a strong motivation to adopt lifestyle changes that could mitigate risks associated with dementia. As noted by experts, such as Dr. Lyketsos, improving the methodological quality of cognitive studies is essential for drawing reliable conclusions. 'This case study not only highlights the efficacy of the new training program but also emphasizes the importance of strict oversight measures in clinical research, particularly when addressing cognitive health in aging populations.'.
Meta-Analysis Findings: Comparing Active and Passive Control Groups
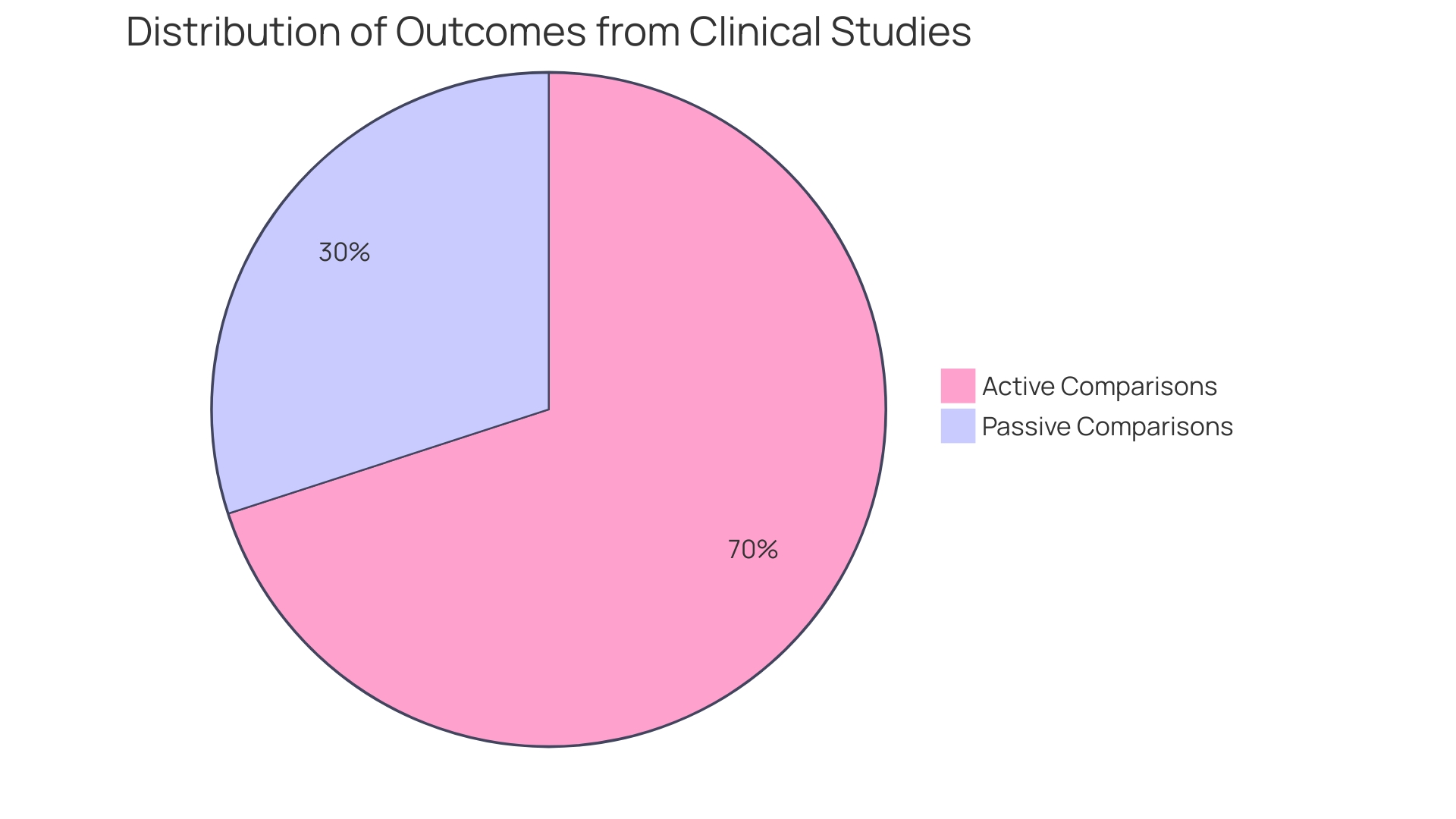
Meta-analyses suggest that clinical studies using active comparison groups tend to yield results that are not only more substantial but also more dependable in comparison to those depending on passive comparisons. This conclusion arises from a thorough examination of numerous studies, which emphasized how active interventions effectively reduce bias and offer a clearer evaluation of treatment effectiveness. For example, a recent research published in the Journal of Political Economy examined 509 double-blind randomized experiments (RCTs) concentrating on antidepressants and antipsychotics. It underscored that RCTs are considered the gold standard for evaluating drug effectiveness, primarily because they reduce potential biases inherent in other study designs.
The results of this analysis correspond with the wider agreement that active measures improve the credibility of clinical studies. In particular, a notable example from this study involved the antidepressant Effexor, where Wyeth Pharmaceuticals conducted a 15-year comparison against Prozac. In 12 out of 14 experiments solely financed by Wyeth, Effexor showed greater efficacy than Prozac, highlighting the significance of well-organized active comparisons in assessing drug effectiveness. The information distinctly demonstrates the benefits of including active participant sets in clinical study frameworks, thereby strengthening their crucial function in enhancing medical understanding and elevating patient results.
Implications for Trial Design and Interpretation
The choice of oversight cohorts is a crucial element of clinical trial design, carrying notable consequences for both the study's integrity and the relevance of its results. Active treatment teams, in particular, can enhance the credibility of results and support more informed clinical decision-making. By comparing a new approach against a well-established one, researchers can better gauge the effectiveness and safety of the experimental intervention.
Employing active comparison groups allows for a more detailed understanding of therapy effects, as they offer a standard against which new methods can be assessed. This is particularly vital in experiments where the effectiveness of a new method might be uncertain. For example, platform studies that adjust control interventions based on emerging findings can enhance resource distribution while preserving strong statistical strength. Such designs enable the evaluation of various approaches efficiently, enhancing the potential for discovering superior interventions.
Furthermore, present trends suggest an increasing dependence on Bayesian approaches, which provide adaptability in modifying study designs as new information arises. Bayesian methods enable more regular evaluations of treatment effectiveness, aiding in the early cessation of unproductive therapies without facing repercussions for prolonging studies in ambiguous situations. This adaptability is essential for maintaining the relevance and applicability of clinical research in a rapidly evolving medical landscape.
As the FDA continues to enhance its recommendations on the use of historical information in clinical studies, the significance of choosing suitable comparison groups becomes even more apparent. These considerations are not merely academic; they directly influence regulatory outcomes and patient care practices. By aligning trial designs with best practices in control selection, researchers can ensure their findings are both valid and applicable, ultimately enhancing patient outcomes.
Conclusion
The exploration of active and passive control groups in clinical trials reveals their critical influence on the validity and reliability of research outcomes. Active control groups, which compare new treatments to established therapies, provide a robust framework for evaluating efficacy and safety. This comparative analysis not only mitigates biases commonly associated with passive controls but also addresses ethical considerations by ensuring that participants receive effective treatments.
The importance of well-structured active control trials is further underscored by recent meta-analyses, which demonstrate that studies utilizing active controls yield more reliable results. By offering a clearer assessment of treatment effectiveness, active controls enhance the credibility of findings and support informed clinical decision-making. The integration of advanced methodologies, such as machine learning and Bayesian approaches, signifies a shift towards more adaptive trial designs that can respond to emerging data, ultimately improving the efficiency of clinical research.
As regulatory bodies like the FDA continue to refine their guidelines, the emphasis on appropriate control group selection will remain paramount. The implications of these decisions extend beyond research, directly affecting patient care and treatment methodologies. By prioritizing the use of active control groups, researchers can contribute to a more nuanced understanding of treatment efficacy, advancing medical knowledge and improving patient outcomes in diverse therapeutic areas.




