Introduction
Navigating the intricate landscape of medical device approval, the FDA De Novo pathway stands out as a pivotal regulatory mechanism designed for low- to moderate-risk devices lacking prior classification. This pathway is instrumental in overcoming the challenges manufacturers face when introducing innovative devices that do not conform to the conventional 510(k) process. By providing a structured avenue for demonstrating safety and efficacy, the De Novo pathway ensures that groundbreaking medical technologies can reach the market efficiently while maintaining rigorous safety standards.
The process mandates extensive pre-market research, development, and clinical evidence to substantiate the device's safety and effectiveness. Notable examples, such as the heart monitor in the Apple Watch and medical technologies from Medtronic, illustrate how the De Novo pathway facilitates market entry for cutting-edge devices, enabling real-world use to inform further regulatory adjustments. Administrative and substantive reviews are essential components of this pathway, ensuring that only safe and effective devices receive approval.
In essence, the FDA De Novo pathway is crucial in promoting the introduction of innovative medical devices, fostering a robust regulatory environment that adapts to emerging technologies without compromising on safety.
What is the FDA De Novo Pathway?
The FDA's new route functions as an essential regulatory system for low- to moderate-risk medical instruments that do not have a previous classification. 'This route is crucial for overcoming the challenges producers encounter in introducing novel products to the marketplace, especially when these items do not conform to the current 510(k) procedure.'. By enabling producers to showcase the safety and effectiveness of their products, the De Novo route aids in obtaining marketing approval.
Pre-market research, development, and clinical evidence are integral to this process. Manufacturers must compile and evaluate extensive scientific and clinical data related to the post-market safety, efficacy, and performance of the products. The FDA's established methods for evaluating technologies help streamline market entry, as seen with the heart monitor embedded in the Apple Watch. 'Originally approved for identifying abnormal heart rhythms in 2018, this product illustrates how innovative technologies can integrate into established oversight routes, facilitating faster market access and enabling subsequent real-world application to guide further oversight modifications.'.
The Medtronic example emphasizes the importance of this route. As a prominent worldwide healthcare technology organization, Medtronic's mission to address significant health issues through groundbreaking instruments highlights the necessity of having a strong oversight structure like the De Novo system. The organization's varied assortment of technologies, including cardiac instruments and surgical tools, benefits from clear regulatory standards that ensure safety and effectiveness.
Administrative and substantive reviews are critical steps in the De Novo process. Issues such as submission errors or insufficient evidence can delay approvals. However, overcoming these hurdles is vital for ensuring that only safe and effective products reach the market. 'The FDA's commitment to adapting its oversight policies based on practical experience ensures that emerging technologies are accommodated without compromising safety.'.
In summary, the FDA De Novo procedure plays a crucial role in enabling the market entry of innovative medical products, ensuring they meet rigorous safety and effectiveness standards while allowing for the practical evolution of regulatory policies.
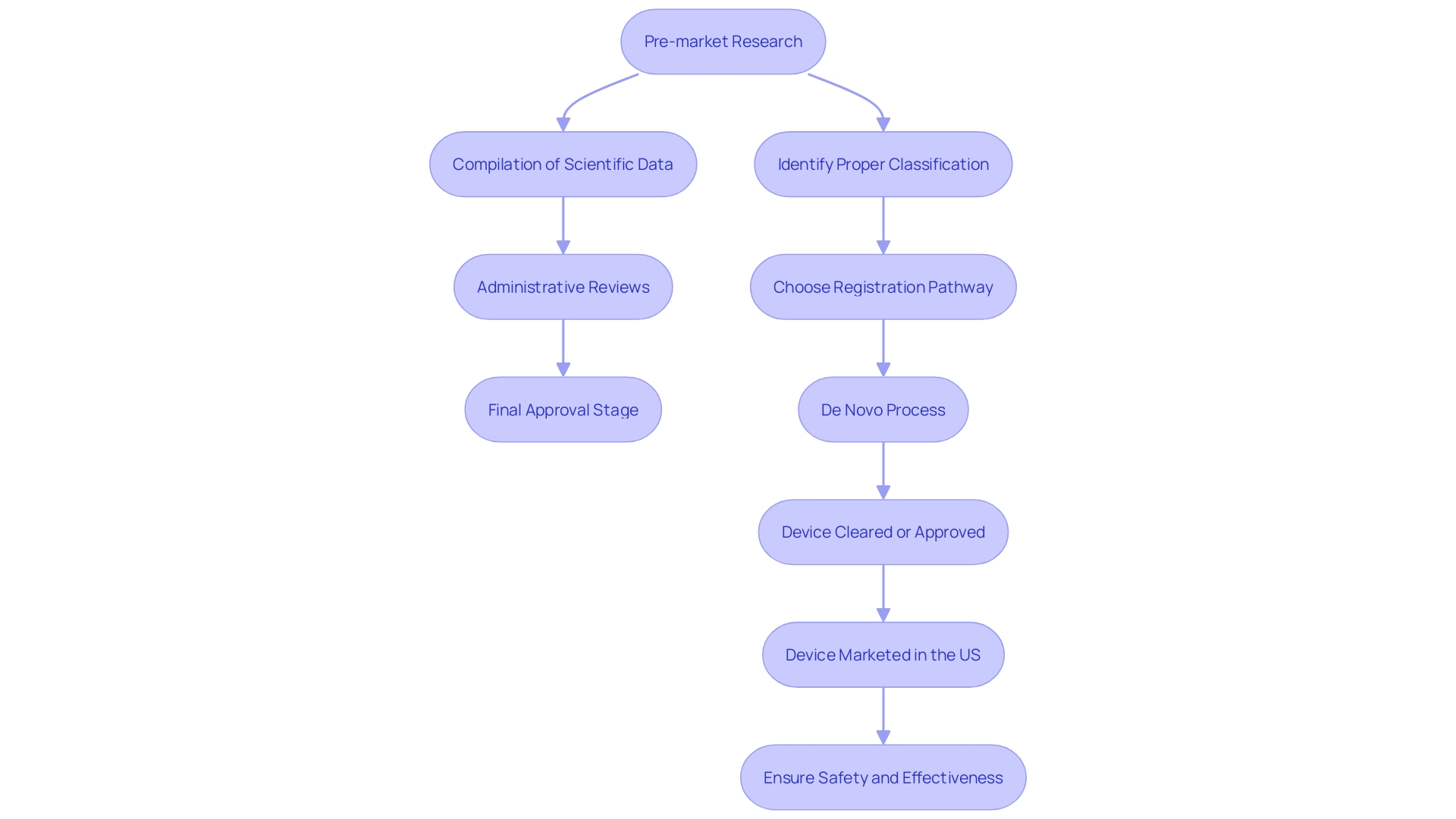
Determining Eligibility for De Novo Classification
Eligibility for the new classification is determined by the product's intended use, risk level, and absence of a legally marketed predicate product. Devices must demonstrate substantial evidence of safety and effectiveness for their intended use. For instance, on May 20, 2021, the FDA issued final guidance on Implanted Brain-Computer Interface (BCI) Devices for patients with paralysis or amputation, underscoring the importance of non-clinical testing and clinical considerations. If an object is found to lack a predicate and poses low to moderate risk, it can proceed through the De Novo process. This pathway is particularly relevant for innovative devices, such as neurological devices, which are often classified into one of three categories based on their risk: Class I (lowest risk), Class II (moderate risk), and Class III (high risk). The FDA uses these classifications to ensure appropriate regulatory controls and mitigate health risks.
Case Study: A Successful De Novo Approval Journey
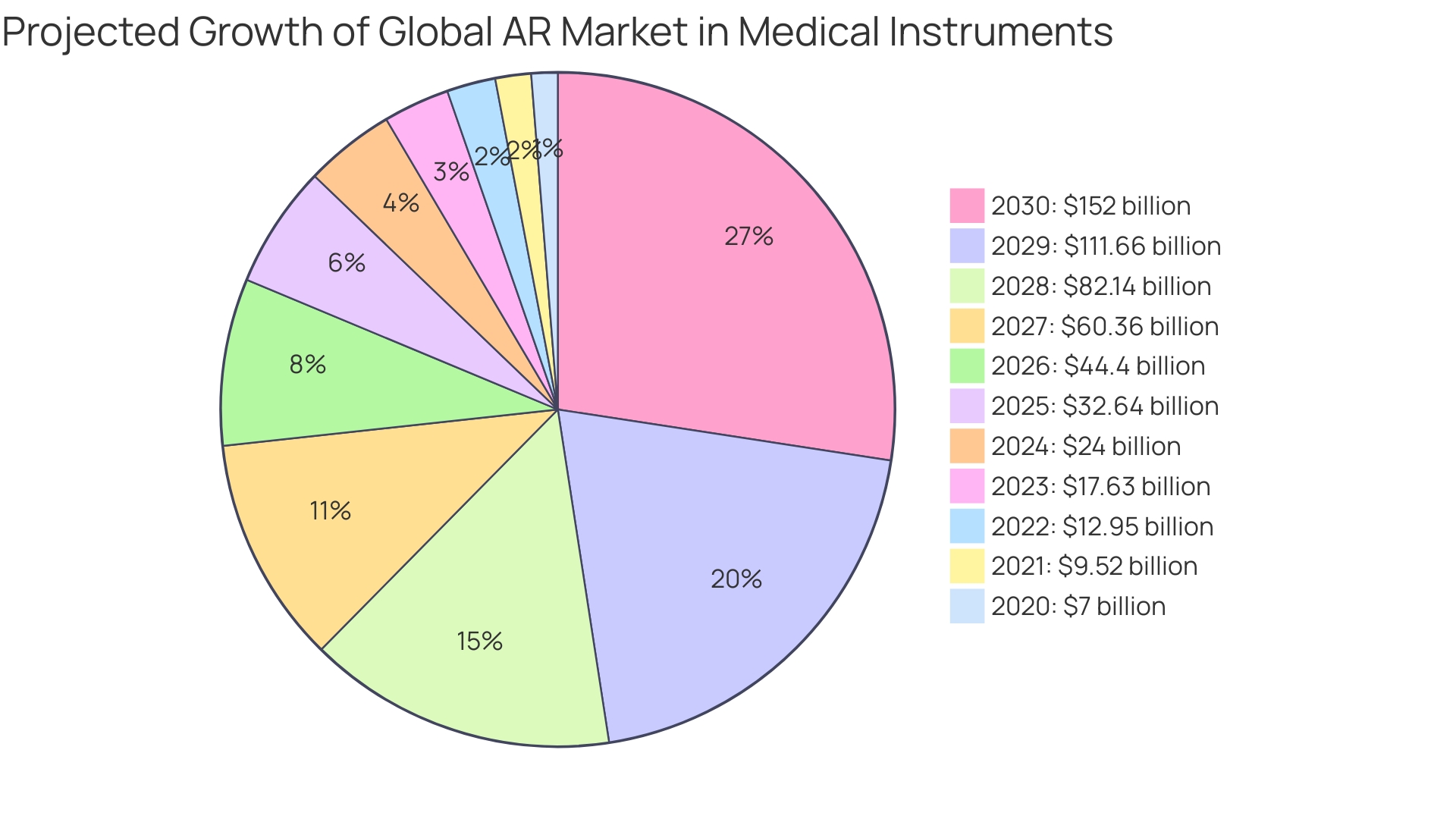
'A remarkable instance of a successful new submission is the case of the Atheer Augmented Reality System.'. Designed to assist surgeons by providing real-time overlay information during procedures, the system highlights the potential of innovative technology in the medical field. The manufacturer submitted a De Novo classification request, including comprehensive clinical data and evidence of safety and effectiveness. Following an extensive evaluation, the FDA granted approval, enabling the product to enter the market. This approval is part of a broader trend, with the global market for augmented reality (AR) in medical instruments projected to grow from $7 billion in 2020 to $152 billion by 2030, reflecting a compound annual growth rate (CAGR) of 36%.
'The success of the Atheer Augmented Reality System highlights the significance of the FDA's De Novo route in enabling the introduction of groundbreaking technologies.'. This route enables producers to showcase the safety and effectiveness of their products, which is essential considering the changing global oversight environment. The authorization was partially based on results from the US Investigational Device Exemption (IDE) clinical study conducted by the company, showcasing the rigorous process involved.
Moreover, the approval of such innovative devices aligns with broader social goals aimed at improving human health outcomes. By traversing the new process, companies can ensure their products meet strict compliance standards, ultimately improving the quality of patient care. As the market for AR in medical applications continues to expand, the role of oversight structures in supporting technological advancements becomes increasingly significant.
Challenges and Considerations in the De Novo Journey
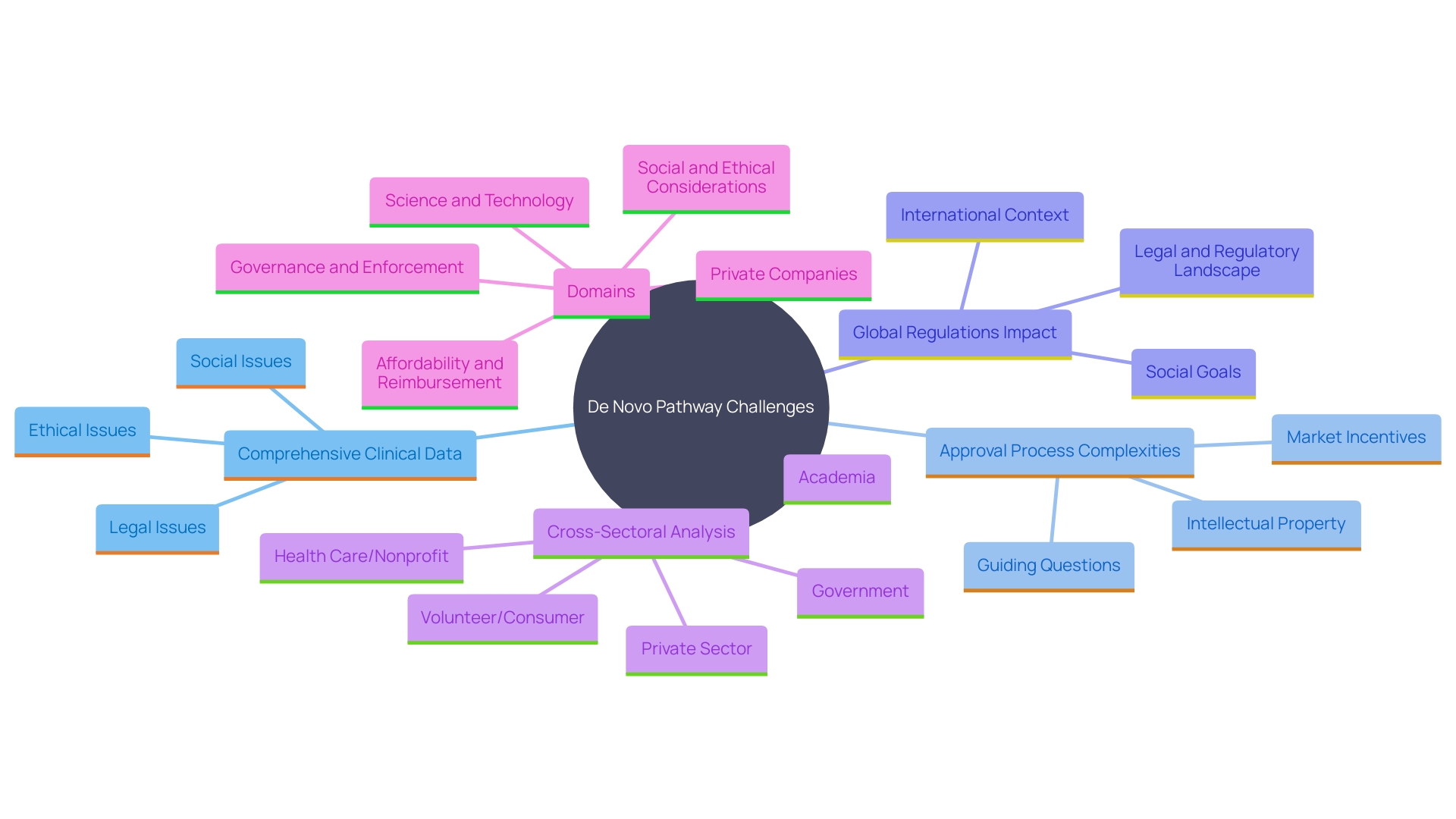
Despite the advantages of the De Novo pathway, manufacturers face substantial challenges. One of the primary hurdles is the necessity for comprehensive clinical data, requiring extensive pre-market research and development. 'The governance landscape is complex and continually evolving, often presenting legal and ethical issues that need careful navigation.'.
Moreover, the approval process can be protracted, with potential delays arising from the rigorous scrutiny by the FDA. This scrutiny frequently involves iterative submissions and responses to feedback, demanding considerable time and resources. According to a study involving medical equipment leaders, gaining market approval and ensuring compliance with regulatory bodies are top priorities, yet these tasks are increasingly difficult as legislation becomes more stringent.
Manufacturers must also contend with a global industry landscape, which introduces additional complexities such as varying international regulations and market incentives. Real-world examples underscore these challenges; for instance, the FDA has been noted for its detailed attention to ensuring the safety, effectiveness, and security of medical devices, which can extend the approval timeline significantly.
Understanding these multifaceted challenges is crucial for success in the De Novo process, as it enables manufacturers to better prepare for the stringent requirements and potential hurdles they may encounter.
Conclusion
The FDA De Novo pathway serves as a vital mechanism for the approval of low- to moderate-risk medical devices that lack a prior classification. By allowing manufacturers to demonstrate safety and efficacy, this pathway addresses the challenges faced in bringing innovative technologies to market. The rigorous requirements for pre-market research and clinical evidence ensure that only safe and effective devices are authorized, which ultimately benefits public health.
Determining eligibility for the De Novo classification hinges on the device's intended use, risk level, and the absence of a legally marketed predicate device. This structured approach enables the FDA to adapt its regulatory policies according to emerging technologies while maintaining high safety standards. Notable examples, such as the heart monitor in the Apple Watch and the Atheer Augmented Reality System, illustrate the pathway's effectiveness in facilitating the introduction of groundbreaking devices that have the potential to enhance patient care.
Despite the advantages, manufacturers must navigate several challenges within the De Novo process, including the need for comprehensive clinical data and the complexities of an evolving regulatory landscape. Potential delays in approvals due to rigorous scrutiny and iterative feedback can test the resources and patience of manufacturers. Understanding these challenges is essential for successfully navigating the De Novo pathway and ensuring that innovative medical devices can reach the market efficiently and safely.
In conclusion, the FDA De Novo pathway is indispensable in fostering innovation within the medical device industry. By maintaining a robust regulatory framework that adapts to new technologies, it ensures that advancements can be brought to market without compromising safety, ultimately supporting the goal of improving health outcomes for patients.




