Introduction
Clinical endpoints play a critical role in the evaluation of clinical trials, providing essential insights into the effectiveness of treatments or interventions. These endpoints are divided into primary and secondary categories. Primary endpoints are the main outcomes that a trial aims to assess, often forming the cornerstone for regulatory approval and guiding clinical decisions.
For instance, in trials involving terminally ill patients, primary endpoints might center on survival rates or significant improvements in quality of life.
Secondary endpoints, while still important, serve as supplementary measures that offer additional information on the treatment's effects, including symptom relief, side effects, or biomarkers. The meticulous selection and clear definition of these endpoints are crucial, as they directly influence the trial’s transparency and the reproducibility of its results. However, transparency in clinical trials is often inconsistent, with many studies not fully sharing protocols or raw data, thereby hindering comprehensive analysis and validation by the broader scientific community.
Due to their pivotal role in shaping patient care and outcomes, clinical trials must adhere to high standards. Overcoming traditional challenges in literature review processes and ensuring compliance with guidelines, such as PRISMA, can enhance the efficiency and reliability of these trials. The evolving regulatory landscape, with increasing scrutiny on data use and privacy, further underscores the necessity for meticulous endpoint selection and clear reporting to meet both ethical and legal standards.
Understanding Clinical Endpoints
Clinical objectives are essential metrics for assessing the results of research studies, providing crucial insights into the effectiveness of therapies or interventions. These connection points are categorized into primary and secondary types. Key objectives signify the main results that the study seeks to evaluate, frequently serving as the foundation for regulatory endorsement and influencing clinical choices. For instance, in studies involving patients with terminal illnesses, main objectives may concentrate on survival rates or notable enhancements in quality of life.
Secondary objectives, while still important, are supplementary measures that provide additional information on the treatment's effects. They can include various aspects such as symptom relief, side effects, or biomarkers. The rigorous selection and clear definition of these endpoints are crucial, as they directly impact the study's transparency and the reproducibility of its results. 'Regrettably, openness in medical studies remains uneven, with many studies not sharing complete protocols or raw data, which hinders thorough analysis and validation by the wider scientific community.'.
'Clinical studies must adhere to high standards due to their significant role in shaping patient care and outcomes.'. As highlighted by assessment experts, overcoming conventional obstacles in literature review procedures and ensuring adherence to standards like PRISMA can improve the effectiveness and dependability of these studies. The evolution of regulatory environments, especially with the growing examination of data utilization and privacy, further emphasizes the necessity for careful selection of connections and clear reporting to satisfy both ethical and legal standards.
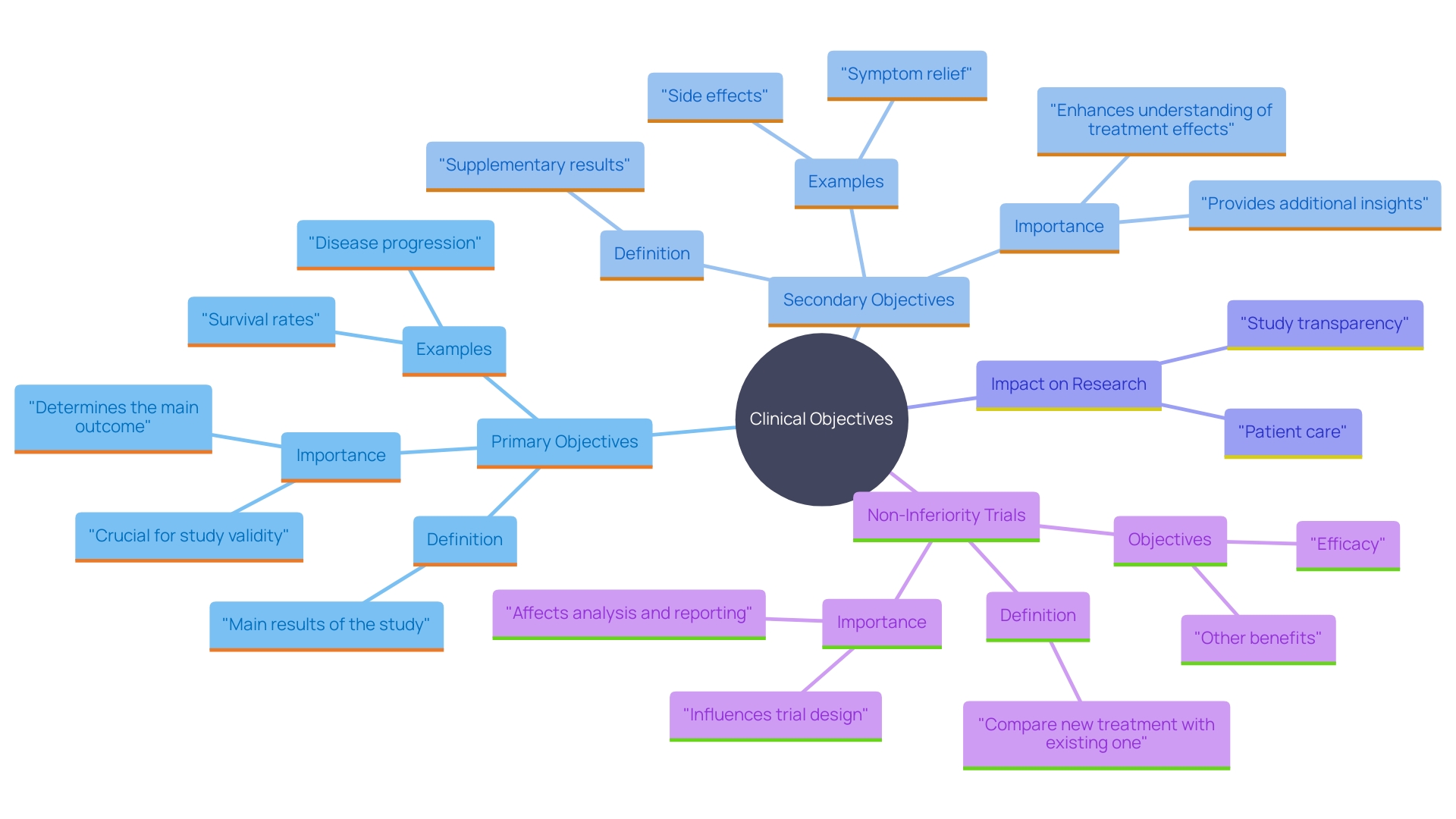
Primary Endpoints: Definition and Importance
'Main objectives are pre-defined results that act as the central emphasis in clinical studies, directly affecting the research's sample size and statistical strength.'. Their significance lies in their capacity to offer essential evidence regarding the efficacy of a treatment. For instance, in cancer medication studies, the main goal frequently pertains to overall survival rates. This endpoint provides concrete data on the treatment's effectiveness in extending patient lifespan compared to existing therapies. As emphasized by the recent updates to ICH E6(R3), ensuring the quality of study data is essential for making informed decisions. 'This is especially important in studies such as those involving new cancer medications, where trustworthy information on survival results can result in major progress in treatment alternatives, as highlighted in various development initiatives.'.
Challenges in Selecting Primary Endpoints
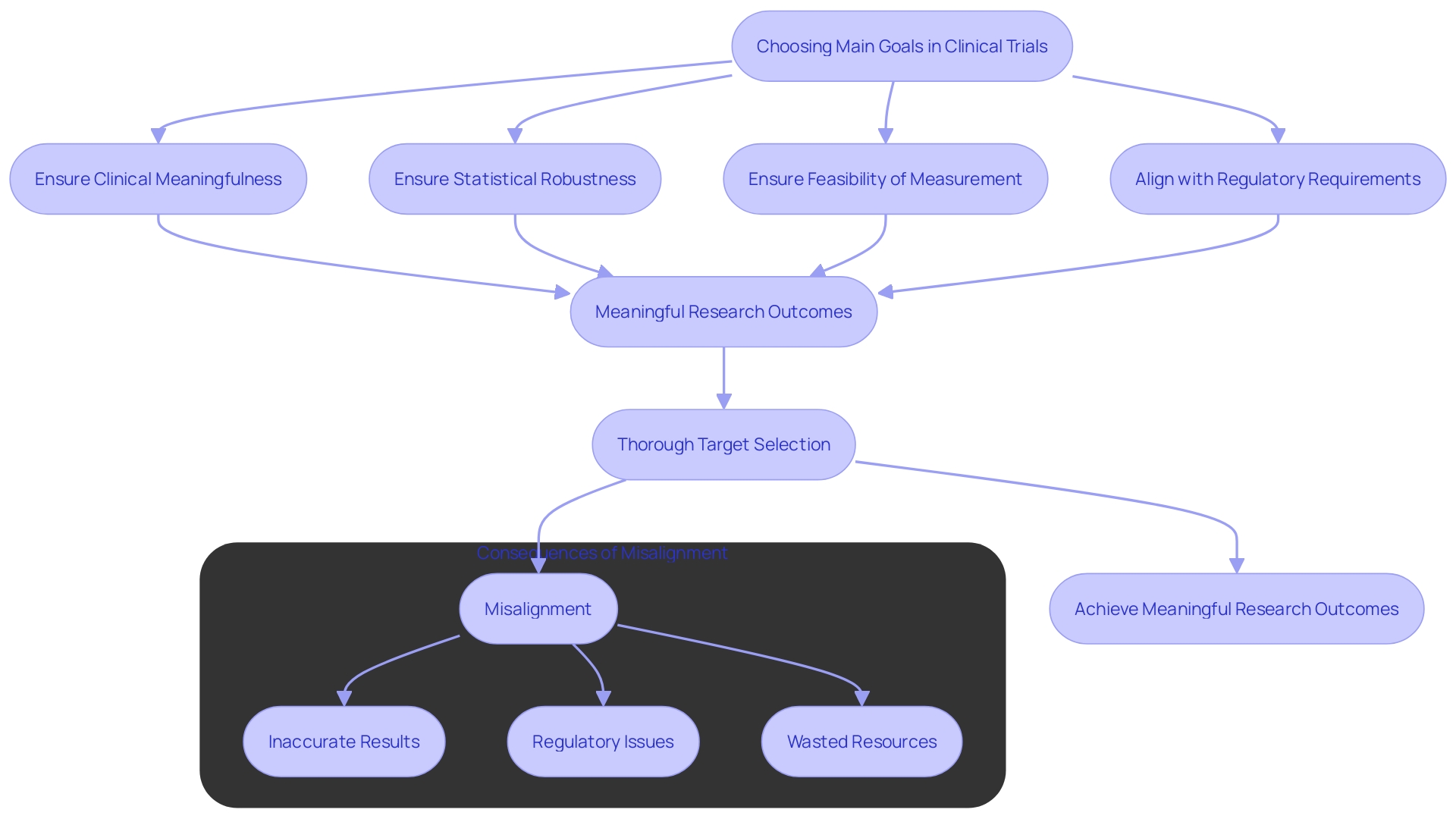
Choosing suitable main goals in clinical trials presents considerable difficulties. Researchers must ensure that these targets are clinically meaningful, statistically robust, and feasible to measure. Misalignment between the selected target and the study's objectives can lead to inconclusive results. Moreover, regulatory bodies often have stringent requirements, necessitating extensive justification for the chosen measures. This complexity is evident in the ongoing discussions around leveraging AI and data innovation in healthcare. For example, a recent meta-analysis of paclitaxel-coated devices for peripheral artery disease (PAD) involved a comprehensive review by a three-physician steering committee to meet FDA standards. This emphasizes the significance of thorough selection of targets and alignment with regulatory expectations to guarantee meaningful and impactful research outcomes.
Case Study: Application of Primary Endpoints in Clinical Trials
A compelling example can be seen in the clinical study for Canagliflozin, a drug aimed at managing diabetes. 'The main objective of this study was the alteration in HbA1c levels from baseline to a specified follow-up period.'. This endpoint was meticulously chosen due to its critical role in evaluating glycemic control, a pivotal aspect of diabetes management. 'The trial's results revealed significant reductions in HbA1c levels, which played a crucial role in the drug receiving regulatory approval.'. This success has paved the way for further investigations into the long-term outcomes associated with Canagliflozin.

Conclusion
The exploration of clinical endpoints reveals their essential role in assessing the efficacy of treatments within clinical trials. Primary endpoints serve as the cornerstone of these studies, providing vital information that influences regulatory approval and clinical decision-making. The focus on survival rates or quality of life improvements in trials, particularly for terminally ill patients, underscores the significance of these measures.
Meanwhile, secondary endpoints enrich the understanding of treatment effects, offering insights into symptom relief and side effects. However, the inconsistency in transparency regarding trial protocols and data sharing poses challenges for comprehensive analysis and validation.
Selecting appropriate primary endpoints is fraught with challenges, requiring researchers to ensure that these measures are both clinically meaningful and statistically robust. Misalignment between endpoints and study objectives can lead to inconclusive results, complicating the regulatory approval process. The emphasis on rigorous endpoint selection is further highlighted by recent advancements in regulatory standards, which necessitate thorough justification for chosen measures.
The case study of Canagliflozin exemplifies the successful application of well-defined primary endpoints, showcasing how they can lead to significant advancements in treatment options.
In conclusion, the meticulous selection and clear definition of clinical endpoints are vital to the integrity and success of clinical trials. Upholding high standards in endpoint selection not only facilitates regulatory compliance but also enhances the overall quality of patient care. As the regulatory landscape continues to evolve, the importance of transparent reporting and robust data usage cannot be overstated, ensuring that clinical trials remain a reliable foundation for advancing medical knowledge and treatment efficacy.




