Introduction
Navigating the complex landscape of regulatory compliance for medical devices is crucial for manufacturers aiming to enter the European Union market. CE Marking serves as a vital certification mark, indicating that a medical device meets the stringent health, safety, and environmental protection legislation set forth by the EU. This mark is not merely a formality but a significant assurance of a device's safety and efficacy, aligned with the rigorous standards established by the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR).
As the medtech industry evolves, encompassing not only physical devices but also software such as Software as a Medical Device (SaMD), the scope of CE Marking has broadened to ensure comprehensive regulatory oversight.
The importance of CE Marking extends beyond compliance; it fosters trust among healthcare professionals and patients by guaranteeing that medical devices have undergone thorough evaluation and testing. Key regulations such as the MDR and IVDR provide a transparent framework, emphasizing the necessity for high safety and performance standards. This article delves into the essential aspects of CE Marking, including its significance, the critical regulations governing it, and a step-by-step guide to obtaining this certification.
It also highlights recent regulatory updates aimed at enhancing transparency and efficiency, underscoring the EU's commitment to robust medical device oversight.
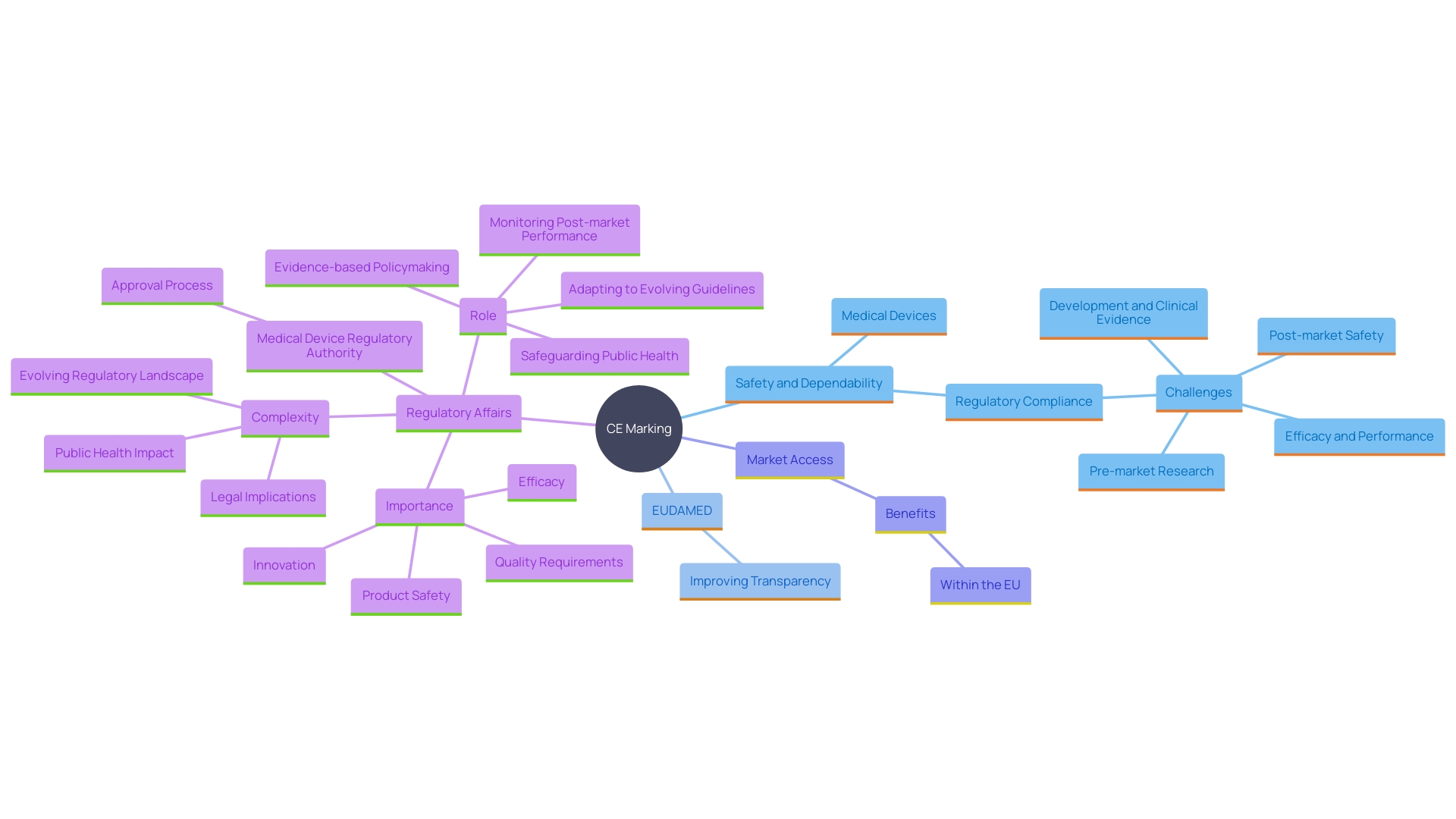
What is CE Marking for Medical Devices?
"CE Marking is a certification symbol that indicates a medical product's adherence to the fundamental health, security, and environmental protection laws of the European Union (EU).". This mark guarantees that the apparatus complies with the rigorous criteria established by the EU for protection and effectiveness, allowing it to be sold within the EU member nations. 'The adoption of CE Marking is crucial, as it aligns with the comprehensive rules framework established by the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR).'. These regulations offer comprehensive guidance on the documentation needed, including Clinical Evaluation Reports (CER), which are crucial for demonstrating the safety and performance of the product.
Furthermore, the EU has made significant strides in increasing transparency and efficiency in the oversight process. For instance, the European Database on Medical Equipment (EUDAMED) is being developed to provide a comprehensive overview of all healthcare products available on the European market, thereby enhancing the visibility of compliance status and facilitating market surveillance. The Commission's proposal to expedite parts of EUDAMED by late 2025 underscores the commitment to robust regulatory oversight.
Alongside hardware, the description of a healthcare instrument now encompasses software, such as Software as a Healthcare Instrument (SaMD), mirroring the changing landscape of the medtech sector. This wider definition guarantees that all components of healthcare instruments, whether tangible or virtual, are held to the same stringent criteria, thus protecting public health.
The OECD's Due Diligence Guidance for Responsible Supply Chains, although primarily focused on conflict minerals, highlights the importance of understanding market-specific regulations and maintaining compliance on a global scale. This approach is particularly relevant in the context of CE Marking, where adherence to EU standards can serve as a benchmark for regulatory compliance in other regions.
Why is CE Marking Important for Medical Devices?
CE Marking is crucial as it indicates that health instruments have undergone rigorous assessment and testing, guaranteeing their safety and dependability for patients and users. This mark also enables the free movement of medical devices within the EU market, offering manufacturers access to a broader consumer base. In an evolving oversight environment, CE Marking fosters confidence among healthcare professionals and patients by ensuring compliance with rigorous standards. According to industry experts, achieving market approval and maintaining regulatory compliance have become top priorities, as tougher legislation and increasing volumes of literature pose significant challenges. 'The European Commission's initiative to accelerate the introduction of EUDAMED components aims to improve transparency and simplify the accessibility of healthcare products, further strengthening the significance of CE Marking in maintaining security and effectiveness in health services.'.
Key Regulations for CE Marking
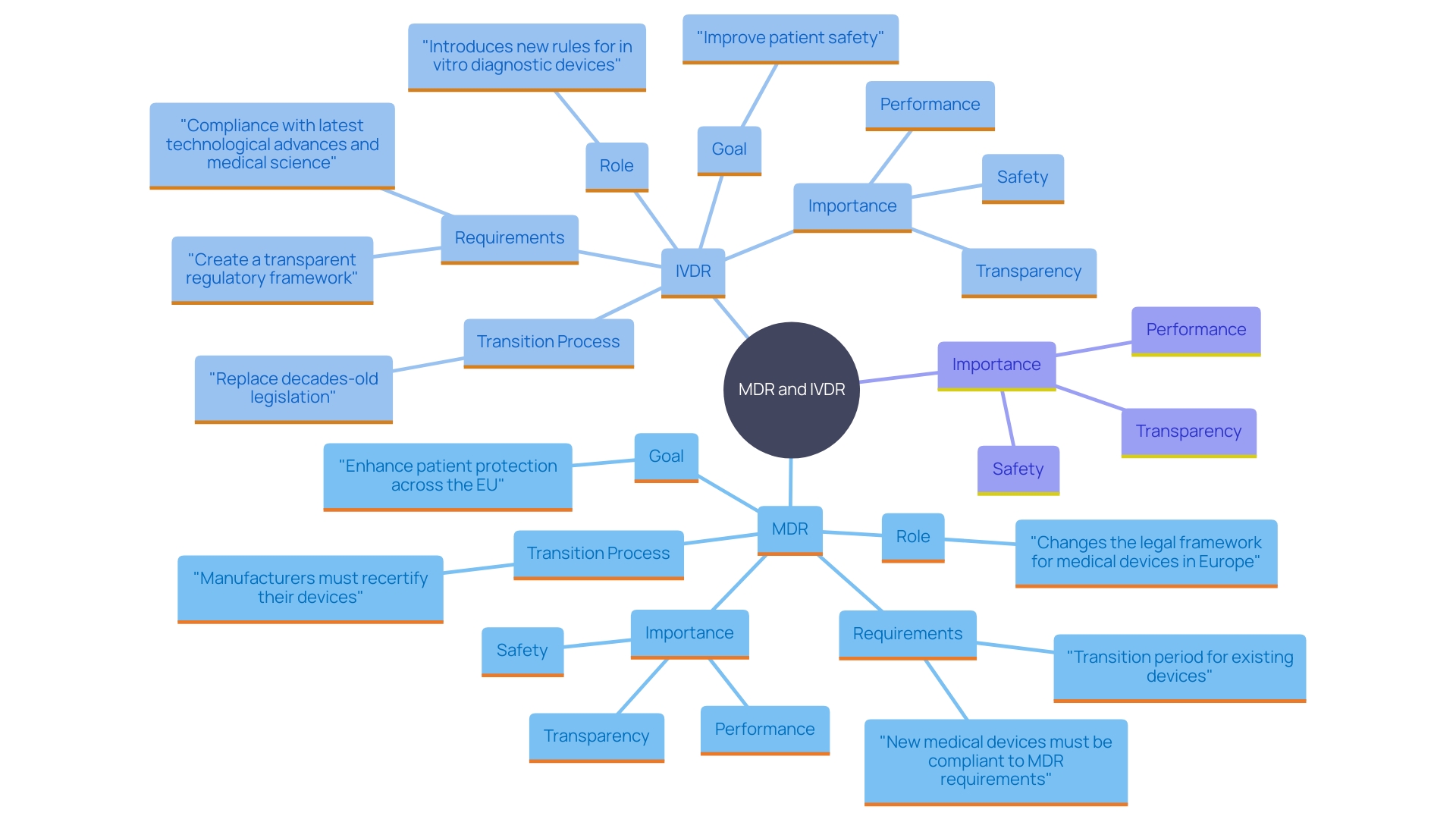
The fundamental rules for CE marking of healthcare instruments in the EU encompass the Medical Instrument Regulation (MDR) (EU) 2017/745. 'This regulation, which overrides the Medical Equipment Directive (MDD), establishes stringent requirements for safety, performance, and transparency of healthcare products.'. Producers of new healthcare products must ensure adherence to MDR before entering the European market, while existing items have a transition period for recertification.
Additionally, the In Vitro Diagnostic Medical Device Regulation (IVDR) (EU) 2017/746 governs in vitro diagnostic devices. Enacted to replace outdated legislation, the IVDR introduces comprehensive rules aligned with the latest technological advancements and health science. It seeks to establish a clear oversight structure, improving patient protection. In vitro diagnostics, which include tests like blood analysis and disease detection, are critical tools both in traditional healthcare settings and remote environments such as at-home testing.
Both MDR and IVDR stress the importance of a clear and strict regulatory environment, guaranteeing that healthcare products meet high standards of safety and effectiveness, ultimately benefiting patients throughout the EU.
Step-by-Step Guide to Obtaining CE Marking
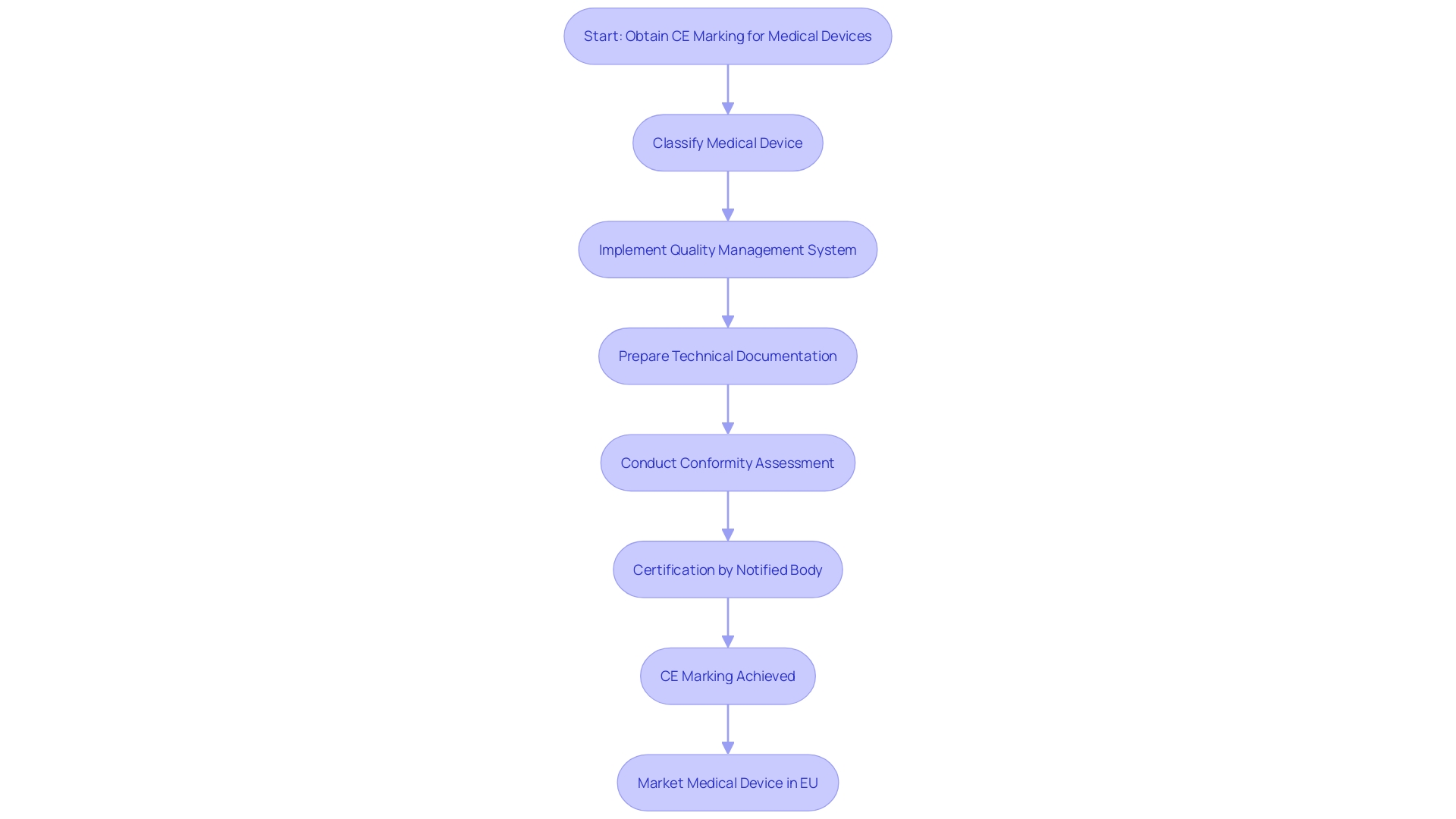
To achieve CE Marking for a medical product, manufacturers must navigate several critical steps. Initially, the instrument must be classified in accordance with the classification rules outlined in the Medical Device Regulation (MDR). Proper classification is essential as it determines the subsequent regulatory pathway and requirements. After categorization, an extensive Quality Management System (QMS) must be implemented to guarantee the consistent quality and security of the product throughout its lifecycle.
Preparing detailed technical documentation that demonstrates compliance with applicable standards is another crucial step. This documentation must include a well-written Clinical Evaluation Report (CER), which evaluates the safety and performance of the instrument based on clinical data. Such reports are vital for meeting compliance requirements and are often compiled with the assistance of CER Consultants.
Once the technical documentation is complete, a conformity assessment must be conducted. This process may involve a Notified Body, particularly for higher-risk products. The assessment ensures that all regulatory requirements are met and verifies the product's compliance with the MDR.
Upon successful completion of the conformity assessment, manufacturers can obtain certification, allowing the CE Mark to be attached to the product. This mark is a testament to the product's adherence to strict European Union standards, ensuring that it is safe and effective for use in the healthcare market.
Recent regulatory updates, including the proposal to expedite parts of the EUDAMED database, aim to enhance transparency and streamline the evaluation process. These modifications are expected to be compulsory by late 2025, further enhancing the efficiency and supervision of healthcare instruments in the EU market.
In summary, obtaining CE Marking involves a detailed and structured approach, requiring strict adherence to MDR guidelines, robust quality management, thorough documentation, and rigorous conformity assessment. These steps are imperative for ensuring that medical devices meet the high standards of safety and efficacy demanded by the European market.
Conclusion
Achieving CE Marking for medical devices is a critical process that signifies compliance with the European Union's stringent health, safety, and environmental regulations. This certification not only facilitates market access within EU member states but also builds trust among healthcare professionals and patients by ensuring that devices have undergone thorough evaluation and testing. The Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) provide a comprehensive framework that governs this process, underscoring the importance of high safety and performance standards.
The significance of CE Marking extends beyond mere regulatory compliance; it represents a commitment to patient safety and enhances transparency within the medical device market. The evolving landscape of the medtech industry, including the inclusion of software like Software as a Medical Device (SaMD), necessitates a broader understanding of regulatory requirements. As the EU continues to enhance its regulatory framework, including initiatives like the European Database on Medical Devices (EUDAMED), manufacturers must stay abreast of these changes to ensure compliance and maintain market relevance.
In summary, the journey to obtain CE Marking involves a structured approach, encompassing device classification, establishment of a Quality Management System, and rigorous conformity assessments. These steps are essential for demonstrating that medical devices not only meet regulatory requirements but also uphold the highest standards of safety and efficacy. As the regulatory landscape continues to evolve, manufacturers must prioritize compliance to foster confidence in their products and contribute positively to public health.




