Overview
The primary challenges in designing clinical trials in Argentina encompass:
- Navigating complex regulatory frameworks
- Enhancing patient recruitment
- Integrating cultural sensitivity into study designs
Addressing these challenges is essential for effective clinical research. By understanding regulatory processes and leveraging technology for trial management, researchers can significantly enhance trial efficiency. Furthermore, fostering collaboration with local stakeholders not only improves participant engagement but also leads to more successful research outcomes. This approach underscores the importance of a well-rounded strategy in overcoming obstacles within the Medtech landscape.
Introduction
Navigating the complexities of clinical trials in Argentina necessitates a multifaceted approach, integrating regulatory understanding, cultural sensitivity, and strategic collaboration. As the landscape evolves, researchers must confront a regulatory framework governed by the National Administration of Drugs, Food and Medical Technology (ANMAT), which dictates the approval processes and ethical considerations crucial for trial initiation.
Furthermore, enhancing patient recruitment through targeted community engagement and leveraging technology for efficient trial management has become essential. By incorporating local knowledge and fostering partnerships with healthcare providers and community organizations, researchers can streamline operations and build trust, ultimately leading to more successful clinical outcomes.
This article explores the key strategies that can empower researchers to excel in Argentina's dynamic clinical trial environment.
Navigate Argentina's Regulatory Framework
Argentina's regulatory structure for research studies is primarily overseen by the National Administration of Drugs, Food and Medical Technology (ANMAT). It is imperative for researchers to possess a comprehensive understanding of the specific regulations, particularly the often lengthy and intricate approval process. Key considerations encompass the requisite documentation, ethical approvals, and associated timelines. Recent reforms have notably streamlined these processes, leading to reduced approval times.
As Zulma Ortiz articulates in 'Clinical Trial Regulation in Argentina: Overview and Analysis of Regulatory Framework, Use of Existing Tools, and Researchers’ Perspectives to Identify Potential Barriers,' grasping the challenges inherent in designing trials for Argentina is essential for researchers. By 2025, the average timeline for medical research approvals has improved, with specific statistics indicating a decrease in approval durations, thereby facilitating a quicker commencement of studies.
Staying informed about these regulatory shifts is crucial for ensuring compliance and minimizing delays in initiating research. Engaging with compliance experts, including bioaccess's extensive network of over 4,000 professionals, can significantly enhance the understanding and navigation of the regulatory landscape.
Bioaccess provides comprehensive clinical trial management services, encompassing:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Ultimately, these services facilitate smoother trial operations.
Enhance Patient Recruitment Strategies
To enhance patient enrollment in Argentina, it is essential to address the challenges inherent in designing trials by implementing targeted strategies that resonate with the community. Digital platforms have emerged as powerful tools for outreach, enabling researchers to connect effectively with potential participants. Involving community leaders can significantly bolster hiring efforts, as they play a crucial role in building trust and promoting participation. Providing clear, accessible information about the study's benefits is paramount for clarifying the process and alleviating concerns.
Utilizing local healthcare providers not only fosters trust but also facilitates referrals, greatly enhancing participant involvement. Understanding the demographics and prevalent health issues of the target population allows for the customization of outreach messages, ensuring they are relevant and impactful. By 2025, effective patient engagement strategies in Argentina will increasingly rely on community-centered approaches that address the challenges in trial design, supported by data that underscores the efficiency of such methods in clinical studies. A notable example is the Flow-FX first-in-human trial in Colombia, where collaboration with regional partners and bioaccess™ led to a substantial improvement in participant enrollment efforts, achieving over a 50% reduction in enrollment duration and 95% retention rates, thereby fostering trust within the community. Furthermore, as highlighted by Dushyanth Surakanti, 'My experience with bioaccess® during the initial human study in Colombia demonstrated the effectiveness of local CROs in navigating the regulatory framework,' illustrating the importance of local involvement in hiring strategies. To further bolster recruitment efforts, it is advisable to implement regular feedback loops with participants to continuously refine the recruitment process.
Incorporate Cultural Sensitivity in Trial Design
Incorporating cultural sensitivity into study design is crucial for effectively engaging the Argentine population, particularly given the challenges associated with conducting trials in Argentina. This necessitates a profound understanding of regional customs, beliefs, and health practices, as highlighted by Steve Garchow's emphasis on the critical role of community knowledge in navigating the complexities of the Latin American market. Involving community stakeholders in the design process ensures that materials resonate with the population, thereby enhancing accessibility and relevance. For instance, providing consent forms and educational materials in local languages significantly improves participant comprehension and comfort. Furthermore, identifying and addressing potential cultural barriers fosters trust and encourages a greater willingness to participate in studies. As Kate Williamson articulates, "Patient satisfaction is intricately linked to cultural competence in healthcare, as individuals are more likely to be content with their healthcare experiences when their cultural backgrounds are acknowledged and respected." Additionally, addressing financial barriers to patient participation, as emphasized in the ASCO policy statement, can further bolster engagement. By prioritizing cultural significance, clinical studies can achieve higher enrollment rates and improved participant satisfaction, ultimately leading to more favorable outcomes. To begin integrating cultural sensitivity into trial design, consider the following strategies:
- Conduct focus groups with local communities to gather insights on their specific needs and preferences.
- Provide consent forms and educational materials in local languages.
- Recognize and address potential cultural barriers to foster trust.
- Address financial barriers to patient participation.
These strategies align with the proactive market access approach outlined by Garchow, ensuring that the challenges in designing trials for Argentina are effectively addressed by developing research studies that consider the unique context of the Argentine population.
Leverage Technology for Efficient Trial Management
Utilizing technology in medical studies is essential for enhancing efficiency and ensuring data integrity. The implementation of a Clinical Trial Management System (CTMS) significantly streamlines vital processes, including participant tracking, data collection, and adherence to regulatory requirements. For instance, adopting electronic data capture (EDC) systems enables real-time data entry and monitoring, minimizing errors and enhancing data reliability. Furthermore, virtual platforms for remote monitoring and telehealth consultations have proven effective in increasing participant engagement and retention, particularly amid ongoing health concerns.
In Argentina, the advantages of CTMS are particularly evident in overcoming challenges associated with designing trials, as they provide modular solutions tailored to the unique requirements of research. This ultimately results in a more efficient management process. Notably, pharmaceutical companies that implement end-to-end data management solutions increase their likelihood of regulatory approval by 23%. Moreover, bioaccess® has demonstrated its commitment to innovation and regulatory excellence by overseeing a variety of studies, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Clinical Follow-Up studies, all crucial for Medtech companies navigating the complexities of research in Latin America.
The recent collaboration between GlobalCare Clinical Trials and bioaccess™ exemplifies these advancements, achieving over a 50% reduction in recruitment duration and a 95% retention rate for study participants in Colombia. As the landscape of medical studies evolves, leveraging these technologies will be vital for Medtech firms aiming to navigate the intricacies of the regulatory environment and achieve favorable outcomes.
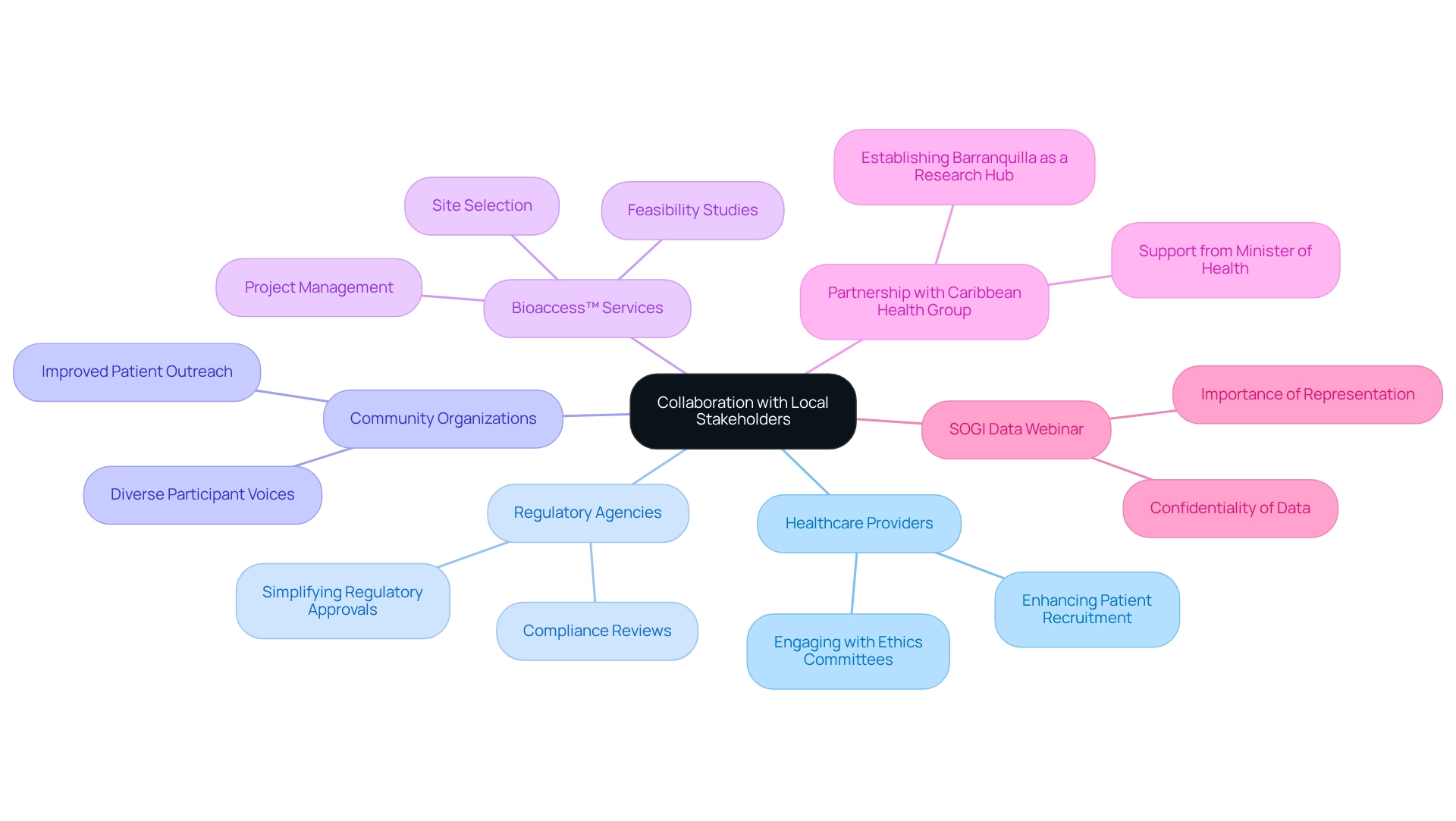
Foster Collaboration with Local Stakeholders
Collaborating with regional stakeholders, including healthcare providers, regulatory agencies, and community organizations, is crucial for addressing the challenges in designing trials for Argentina. Such collaborations simplify regulatory approvals, enhance patient recruitment, and provide vital insights into community health, thereby tackling these challenges effectively. Engaging with community ethics committees and patient advocacy organizations is essential, as it ensures that studies prioritize participant welfare, fostering trust and improving the overall quality of research. Notably, community involvement projects have showcased how civil society organizations significantly contribute to health service provision, directly influencing research outcomes.
A recent study revealed that 38 partners identified various impacts within their projects, such as improved patient outreach and enhanced data collection methods, underscoring the multifaceted benefits of local collaboration. Moreover, bioaccess™ has demonstrated its commitment to improving research management through extensive services, including:
- feasibility studies
- site selection
- compliance reviews
- setup
- import permits
- project management
- reporting
Their partnership with Caribbean Health Group aims to establish Barranquilla as a prominent hub for medical research in Latin America, supported by Colombia's Minister of Health. This partnership exemplifies how community collaboration can drive global health enhancement and innovation in Medtech.
Furthermore, a recent webinar on Sexual Orientation and Gender Identity (SOGI) data highlighted the significance of representation in research, illustrating how community partnerships can enhance study outcomes by ensuring diverse participant voices are included. By leveraging these partnerships, clinical trials can achieve more successful outcomes, ultimately advancing medical technology in the region. To maximize the benefits of collaboration, stakeholders should actively engage with local communities and continuously assess the impact of their initiatives.
Conclusion
Navigating the complexities of clinical trials in Argentina necessitates a strategic approach that encompasses:
- An understanding of the regulatory landscape
- Enhancing patient recruitment
- Incorporating cultural sensitivity
- Leveraging technology
- Fostering collaboration with local stakeholders
A comprehensive grasp of the ANMAT regulations enables researchers to streamline trial initiation and ensure compliance, while targeted recruitment strategies that engage community leaders and utilize digital platforms can significantly enhance participant engagement.
Cultural sensitivity is essential; by acknowledging local customs and addressing potential barriers, trial designs can resonate more deeply with participants, leading to improved enrollment and satisfaction. Furthermore, the integration of advanced technology, such as Clinical Trial Management Systems and electronic data capture, bolsters operational efficiency and data integrity—both critical for successful trial outcomes.
Collaboration with local stakeholders enriches the research process, providing insights into community health needs and fostering trust. By engaging with healthcare providers, ethics committees, and advocacy groups, researchers can ensure that clinical trials are not only compliant but also ethically sound and community-focused.
Ultimately, the success of clinical trials in Argentina hinges on a multifaceted strategy that embraces regulatory knowledge, cultural awareness, technological innovation, and local partnerships. By implementing these strategies, researchers can effectively navigate the dynamic clinical trial environment, ultimately leading to advancements in medical technology and improved health outcomes for the Argentine population.
Frequently Asked Questions
Who oversees the regulatory structure for research studies in Argentina?
The regulatory structure for research studies in Argentina is primarily overseen by the National Administration of Drugs, Food and Medical Technology (ANMAT).
What is important for researchers to understand regarding the approval process in Argentina?
Researchers must have a comprehensive understanding of the specific regulations, particularly the lengthy and intricate approval process, which includes requisite documentation, ethical approvals, and associated timelines.
How have recent reforms impacted the approval times for medical research in Argentina?
Recent reforms have streamlined the approval processes, leading to reduced approval times and facilitating a quicker commencement of studies.
What is the significance of staying informed about regulatory shifts in Argentina?
Staying informed about regulatory shifts is crucial for ensuring compliance and minimizing delays in initiating research.
What services does Bioaccess provide for clinical trial management?
Bioaccess provides comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, and project management.
How can patient enrollment be enhanced in Argentina?
Patient enrollment can be enhanced by addressing challenges in trial design through targeted strategies, utilizing digital platforms for outreach, involving community leaders, and providing clear information about study benefits.
What role do local healthcare providers play in participant involvement?
Local healthcare providers foster trust and facilitate referrals, greatly enhancing participant involvement in clinical trials.
What is an example of effective patient engagement strategies in clinical studies?
A notable example is the Flow-FX first-in-human trial in Colombia, which achieved over a 50% reduction in enrollment duration and 95% retention rates through collaboration with regional partners and local involvement.
Why is local involvement important in recruitment efforts for clinical trials?
Local involvement is essential as it helps navigate the regulatory framework and builds trust within the community, leading to improved recruitment outcomes.
What strategy can be implemented to refine the recruitment process continually?
Implementing regular feedback loops with participants can help continuously refine the recruitment process.




