Overview
Choosing endpoints for medical device trials is not merely a procedural step; it is essential, as these endpoints directly influence the trial's design, execution, and regulatory approval outcomes. Well-defined and clinically relevant endpoints significantly enhance the likelihood of successful trials. They align with regulatory expectations and address real-world patient needs, thereby facilitating smoother approval processes. This alignment is crucial in today's Medtech landscape, where the demands for transparency and efficacy are at an all-time high.
Introduction
In the intricate world of clinical trials, selecting endpoints is a cornerstone for evaluating the efficacy of medical devices. These predefined outcomes, which range from survival rates to quality of life improvements, dictate the trajectory of a study and play a pivotal role in regulatory approvals. Given the high stakes involved, understanding the nuances of endpoint selection is essential for researchers navigating the complexities of trial design and execution. As the landscape of medical device trials evolves, the importance of aligning endpoints with clinical relevance and regulatory expectations cannot be overstated. This article delves into the critical aspects of endpoint selection, offering insights into best practices that enhance trial success and ultimately improve patient outcomes.
Understanding Endpoints: The Foundation of Clinical Trials
Endpoints are predefined outcomes that serve as critical benchmarks for measuring the effectiveness of medical interventions in clinical studies. In the realm of medical device evaluations, these goals encompass a variety of metrics, including survival rates, symptom alleviation, and enhancements in quality of life. A comprehensive understanding of these endpoints is essential, as their selection for medical device trials not only guides the research process from study design to regulatory approval but also guarantees that the results yield significant and actionable insights.
Key Types of Endpoints
- Primary Endpoints: These represent the main outcomes that the study is specifically designed to assess. For instance, in a study evaluating a new cardiac device, the primary objective might focus on the rate of significant adverse cardiac incidents, directly reflecting the device's effectiveness.
- Secondary Objectives: These measures provide supplementary information regarding the treatment's effects, such as quality of life indicators or other relevant health outcomes. They contribute to a broader understanding of the intervention's impact.
- Exploratory Objectives: Often employed to gather additional information that may inform future investigations, exploratory objectives are not the main focus of the current experiment but can offer valuable insights for subsequent research.
The importance of well-defined endpoints cannot be overstated; research indicates that a significant percentage of medical studies fail due to issues related to endpoint selection in medical device trials. Specifically, when evaluating 100 different non-pre-specified subgroups, it is expected that approximately five will yield a p-value of less than 0.05 merely by chance, underscoring the necessity for meticulous outcome selection and definition.
Recent advancements in study design have highlighted the advantages of employing a Global Statistical Test (GST), particularly in studies with multiple objectives. The GST presents numerous benefits, including enhanced power, flexibility, and error control, while ensuring that the overall treatment effect remains clinically relevant. This approach is especially beneficial in scenarios characterized by multidimensional effects, as it leverages relationships among outcomes to clarify results.
As Mei-Jie Zhang, PhD, noted, "We hope this article will be of practical use to practitioners encountering the challenge of accurately interpreting research data," emphasizing the critical nature of clear outcome definitions.
As the landscape of medical device evaluations evolves, staying informed about the latest advancements and expert perspectives on outcome definitions is vital. Engaging with resources such as VIARES Academy can provide valuable updates on best practices in definition standards, job postings, and research courses, further supporting the ongoing education of professionals in the field. By prioritizing clear and well-defined objectives, the selection of endpoints for medical device trials can substantially enhance the likelihood of successful outcomes in medical device studies.
At bioaccess®, we leverage over 20 years of expertise in managing clinical evaluations, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, to ensure that outcome definitions are meticulously crafted, aligning with our commitment to regulatory excellence and innovation in Medtech research across Latin America.
The Importance of Endpoint Selection in Medical Device Trials
Choosing endpoints for medical device trials is critical, as appropriate targets significantly influence the trial's design, execution, and outcomes. The selection of access points can determine whether a device is deemed effective and safe by regulatory bodies. For instance, employing measures that lack medical significance or measurability can lead to ambiguous outcomes, ultimately prolonging the approval process.
Regulatory bodies, including the FDA, mandate clear proof of a device's effectiveness and safety. Selecting endpoints for medical device trials that are well-defined and align with regulatory expectations can streamline the approval process. Conversely, poorly chosen targets may lead to increased scrutiny or outright rejection of study outcomes.
Statistics indicate that the choice of endpoints for medical device trials can significantly impact the rate of regulatory approval. Higher approval rates are observed when the endpoints demonstrate clear medical relevance. For example, the approval rate for single-agent regimens was 89% for individuals with a maximum overall response rate (ORR) statistically exceeding 30%, and it reached 100% for those with a maximum ORR statistically exceeding 45%.
Experts emphasize the importance of selecting relevant outcomes in research studies. Findings suggest that high overall response rates (ORR) are suitable objectives for single-arm studies aimed at demonstrating the breakthrough activity of single-agent anticancer therapies. This perspective underscores the necessity of aligning endpoints for medical device trials with clinical goals to enhance study results.
Moreover, the change in FEV 1.0 from baseline is a measurement valued by clinicians in studies examining the optimal treatment of pulmonary exacerbations of cystic fibrosis, further illustrating the significance of relevant endpoints.
A pertinent case study involves a study for a new orthopedic implant. If the primary objective is defined as a decrease in pain levels, yet the data collected fails to robustly support this assertion, the study may struggle to demonstrate the device's effectiveness. Such deficiencies can create substantial regulatory hurdles, highlighting the critical nature of endpoint selection in achieving successful study outcomes.
The use of multi-component measures, which aggregate various pre-specified outcomes into a single score using multi-attribute instruments, can offer a more comprehensive evaluation of treatment effects. This approach differs from composite measures, as individual elements may lack significance when examined in isolation, necessitating careful consideration of the relative importance of each component.
In summary, choosing endpoints for medical device trials is not merely a procedural step; it is a strategic decision that can profoundly influence the trajectory of medical device studies and their subsequent regulatory approval. By leveraging extensive research study management services, such as those provided by bioaccess®, organizations can enhance their study designs, ensuring that objectives are both relevant and aligned with regulatory standards, ultimately contributing to economic development and advancements in healthcare.
Types of Endpoints: Objective vs. Subjective Measures
Outcomes in research studies are essential for assessing the effectiveness of medical devices and can be classified into two main categories: objective and subjective measures.
Objective Outcomes
Objective outcomes are quantifiable and measurable results that provide clear data independent of personal opinions or interpretations. Common examples include:
- Survival Rates: This metric reflects the percentage of patients alive after a defined period, serving as a fundamental indicator of treatment efficacy.
- Biomarker Levels: These are measurable biological indicators, such as blood pressure or cholesterol levels, which can provide concrete evidence of a device's impact on physiological parameters.
The significance of objective outcomes is underscored by current research, which indicates that approximately 70% of clinical trials in 2025 utilize objective measures to ensure robust data collection and analysis. This trend highlights the growing emphasis on quantifiable outcomes in the assessment of medical technologies. Furthermore, the Duration of Response (DoR) measures the time from randomization to disease progression or death in patients with complete or partial response, making it a useful metric for assessing treatments that provide durable responses.
Subjective Measures
In contrast, subjective measures are based on personal perceptions and experiences, often relying on patient-reported outcomes. Examples include:
- Quality of Life Assessments: These surveys evaluate a patient's overall well-being and satisfaction with their health status, providing valuable insights into the impact of a medical device on daily life.
- Pain Scales: These measures capture patient-reported pain intensity, offering a subjective perspective on treatment effectiveness.
While subjective measures can enrich the understanding of patient experiences, they are inherently variable and can be influenced by numerous factors, including psychological and social contexts. Consequently, they are frequently utilized alongside objective metrics to offer a more comprehensive perspective on treatment results.
Choosing endpoints for medical device trials involves a selection between objective and subjective measures, which is primarily determined by the particular objectives of the study. Objective measures produce clear, quantifiable data that can aid regulatory approval and decision-making in healthcare. On the other hand, subjective measures can highlight patient experiences and satisfaction, which are essential for comprehending the real-world effects of medical devices.
In recent case analyses, such as those investigating dose-finding assessments in early experimental phases, the combination of both outcome types has shown to be advantageous. These investigations highlight the moral aspects of reducing patient risk while efficiently establishing ideal dosages, demonstrating the significance of meticulous outcome selection in research design. The methodologies examined in these studies, including dose-escalation and the continual reassessment method (CRM), emphasize the intricacy of determining optimal doses while guaranteeing patient safety.
Ultimately, choosing endpoints for medical device trials involves a strategic combination of objective and subjective measures that can enhance the robustness of research, ensuring compliance with regulatory standards while also addressing patient needs and experiences. As Robert Peter Gale observed, the meticulous choice of outcomes is essential for the success of medical studies, emphasizing the necessity for a balanced strategy in outcome design.
Criteria for Choosing Effective Endpoints
Selecting appropriate endpoints for clinical trials is a pivotal step that requires careful consideration of several essential criteria:
-
Relevance: Endpoints must be closely aligned with the clinical questions being investigated. Choosing endpoints for medical device trials should involve capturing outcomes that are significant to both patients and healthcare providers, ensuring that the study addresses real-world needs.
-
Measurability: It is crucial that targets are quantifiable and can be consistently measured across all study participants. This consistency is vital for ensuring the reliability and validity of the data collected throughout the trial.
-
Sensitivity: Effective measures should possess the sensitivity necessary to detect meaningful changes over time, particularly in response to the intervention being evaluated. This sensitivity is key when considering choosing endpoints for medical device trials to demonstrate the efficacy of the medical device under investigation.
-
Feasibility: Researchers must evaluate whether the outcomes can be realistically measured within the study's constraints, including time limitations, available resources, and participant accessibility. This practical consideration helps ensure that the trial can be conducted smoothly and efficiently.
-
Regulatory Alignment: It is essential for targets to align with regulatory expectations to streamline the approval process. Familiarity with guidelines from regulatory bodies, such as the FDA and INVIMA, can significantly aid in choosing endpoints for medical device trials that meet necessary standards.
-
Independence of Censoring Mechanisms: It is important to consider the independence of censoring mechanisms and events to avoid underestimating event rates, which is crucial for a comprehensive understanding of outcome selection.
In 2025, the significance of relevance at the conclusion continues to be emphasized by clinical research leaders, who advocate for involving diverse end-user groups in choosing endpoints for medical device trials. This method not only improves the significance of study results but also guarantees that the objectives align with the needs and preferences of patients and healthcare professionals alike. For instance, a recent case study titled "Challenges in Individualized Endpoint Selection" illustrates the complexities involved and emphasizes the value of stakeholder perspectives in refining endpoint criteria.
By prioritizing these best practices, researchers can enhance the quality and relevance of their studies, especially when it comes to choosing endpoints for medical device trials, ultimately advancing the development of medical devices that significantly improve patient outcomes.
As Stephanie Pugh, PhD at NRG Oncology Statistics and Data Management Center, states, "It is an important responsibility of both the principal investigator and the statistician that the outcomes of trials are published, regardless of what those outcomes are." This emphasizes the significance of transparency in medical research. With more than 20 years of experience in the Medtech sector, bioaccess® recognizes the potential advantages that medical devices can offer to individuals' lives, emphasizing the importance of the selected measures concerning real-world effects.
Our extensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting, ensure that your research initiatives are designed and executed with the highest level of expertise.
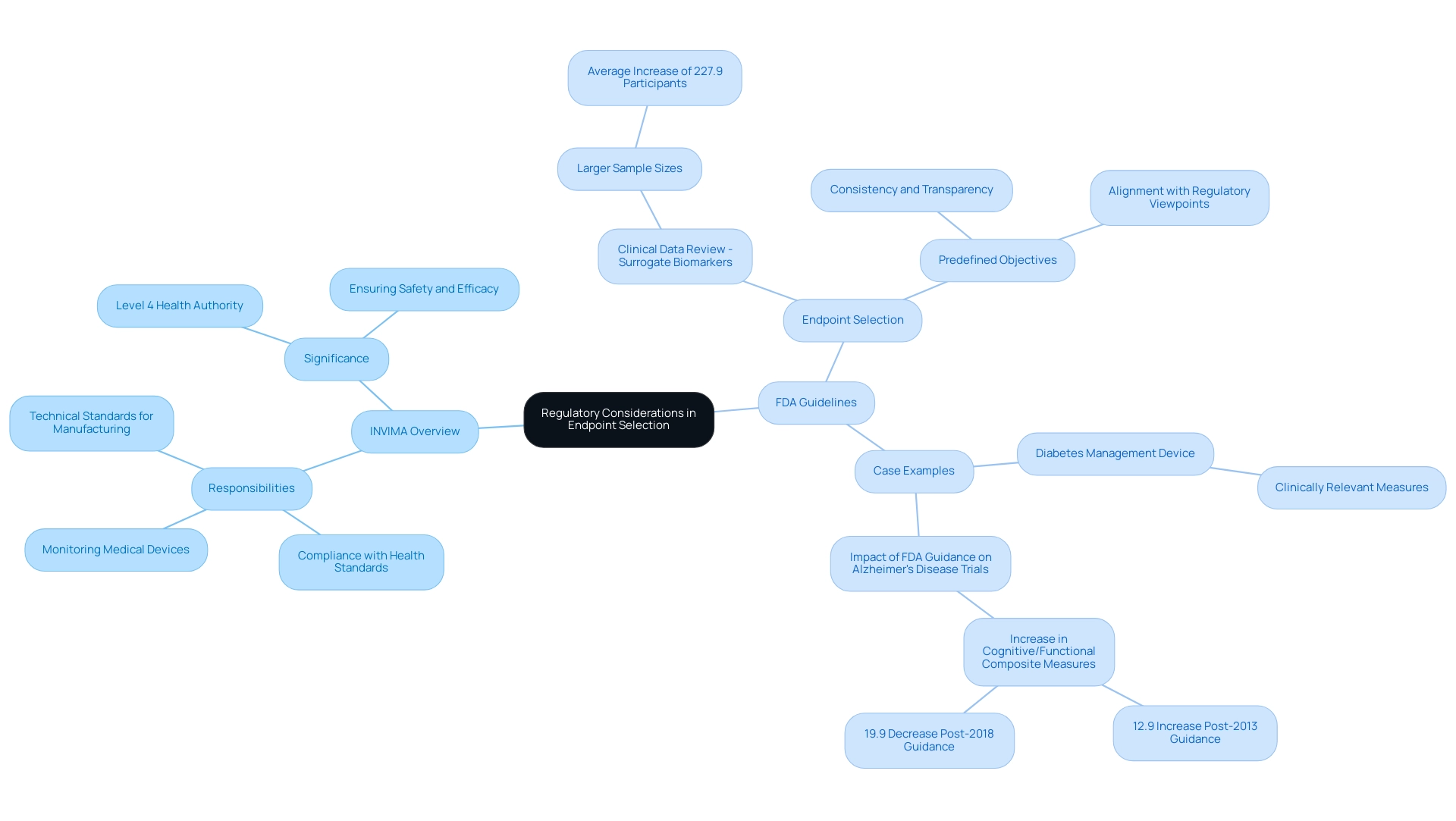
Regulatory Considerations in Endpoint Selection
Regulatory authorities play a vital role in selecting endpoints for medical device trials, making it essential for researchers to comprehend their guidelines for successful outcome selection.
INVIMA Overview
In Colombia, the INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) serves as the national regulatory authority overseeing the marketing and manufacturing of health products, including medical devices. Established in 1992 under the Ministry of Health and Social Protection, INVIMA is responsible for ensuring compliance with health standards and implementing best practices. Its Directorate for Medical Devices and other Technologies specifically monitors medical devices, suggesting technical standards for their manufacturing, marketing, and quality assurance, as well as overseeing the import and export of these products.
Acknowledged as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA's role is essential in ensuring the safety, efficacy, and quality of medical devices in Colombia, including those created by firms like bioaccess®.
FDA Guidelines
The FDA outlines specific recommendations for choosing endpoints for medical device trials, emphasizing the necessity for outcomes to be clinically meaningful and directly relevant to patient results. Familiarity with these guidelines is vital for researchers, especially when selecting endpoints for medical device trials, to ensure compliance and enhance the credibility of their studies. For instance, studies including Clinical Data Review - Surrogate Biomarkers (CDR-SB) have demonstrated notably larger sample sizes, averaging an increase of 227.9 participants compared to those lacking such objectives, highlighting the influence of strategic goal selection on study design.
Additionally, the Surrogate Endpoint Table is revised twice a year by CBER and CDER, guaranteeing that researchers have access to the most recent regulatory insights.
Importance of Predefined Objectives
Predefined objectives must be clearly expressed before the study begins and carefully recorded in the research protocol. This approach not only promotes consistency and transparency throughout the process but also aligns with current regulatory viewpoints that highlight the significance of choosing endpoints for medical device trials to achieve successful results. The FDA's guidelines on outcome selection are revised twice a year, ensuring that researchers have access to the most recent regulatory insights.
Case Example
A recent study for a new diabetes management device illustrates the significance of aligning outcome selection with regulatory expectations. The FDA required that the main goal be a clinically relevant measure of glycemic control, reinforcing the necessity for definitions that resonate with both regulatory standards and patient needs. Furthermore, a study examining the effect of FDA recommendations on Alzheimer's disease studies indicated that the yearly application of cognitive/functional composite measures rose by 12.9% after the 2013 guidance, demonstrating how compliance with FDA regulations can notably affect study success rates and the overall progress of medical devices.
This work was supported by the National Institutes of Health (Award R01AG062277), adding further authority to the discussion of regulatory influences on clinical studies.
Challenges in Selecting Endpoints for Medical Device Trials
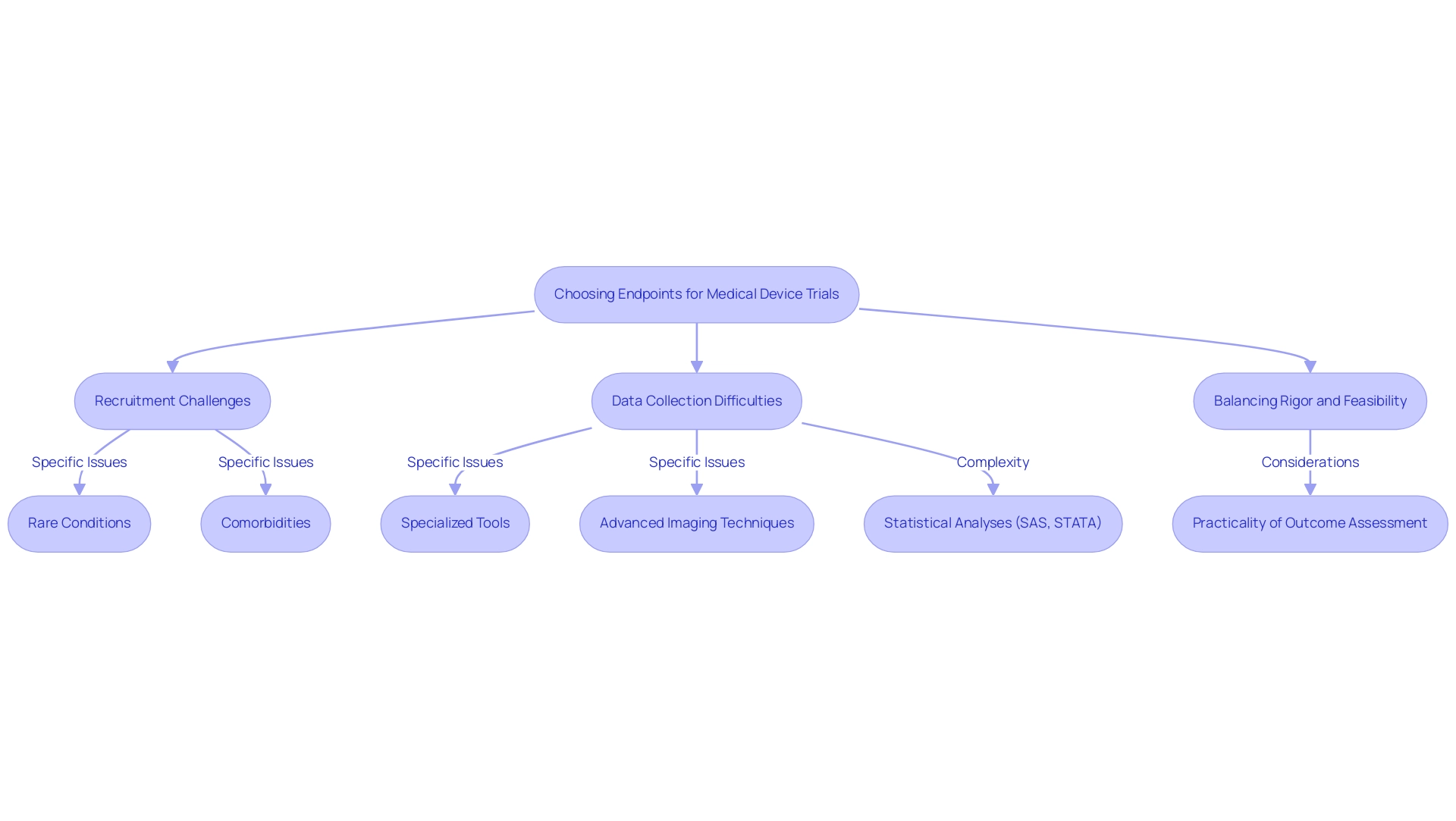
Choosing endpoints for medical device trials presents a myriad of challenges that can significantly affect the trial's success and validity. Key issues include recruitment challenges, data collection difficulties, and the need to balance rigor with feasibility.
Recruitment issues arise when identifying suitable participants who meet specific endpoint criteria, particularly in trials focused on rare conditions or niche populations. For instance, studies requiring participants with specific comorbidities may struggle to recruit enough subjects, leading to delays and potential compromises in study integrity. Notably, studies utilizing substitute measures have indicated a greater chance of producing favorable outcomes, raising concerns about possible biases in study results.
Data collection difficulties often necessitate specialized tools or methodologies, complicating the trial process and escalating costs. For example, systems relying on advanced imaging techniques or biomarker analyses may require additional training for staff and investment in technology, straining resources and timelines. Statistical analyses for these trials are typically conducted using software such as SAS and STATA, adding another layer of complexity to data management.
Researchers face the critical task of balancing scientifically rigorous endpoints with those that are practical to measure within the study's constraints. This balance is essential to ensure that the targets are not only relevant but also achievable. A well-structured study must consider the practicality of outcome assessment alongside scientific goals, necessitating ongoing conversations among stakeholders.
Recent insights indicate that recruitment challenges are exacerbated by stringent endpoint criteria, particularly in 2025. A study named 'Funding Source and Outcomes of Experiments' revealed that studies financed by commercial organizations were more prone to indicate favorable results when substitute measures were utilized. This underscores the necessity for clarity and thoroughness in selection, especially considering that the capacity to identify substantial variations in study characteristics based on funding sources was determined to be 79% for surrogate measures and 41% for disease-specific mortality compared to all-cause mortality.
Experts emphasize that choosing endpoints for medical device trials is crucial, noting that the choice of endpoints can significantly influence trial outcomes and regulatory approval. As Dr. Bettina Martin aptly stated, "You owe this not only to yourself and the notified bodies but, above all, to your patients." This highlights the ethical responsibility researchers have in selecting endpoints that are not only scientifically sound but also aligned with patient needs.
By addressing these challenges thoughtfully, researchers can enhance the design and execution of medical device studies, ultimately leading to more reliable outcomes and advancements in patient care. Moreover, partnerships such as that of bioaccess® and Caribbean Health Group, revealed on March 29, 2019, are crucial in establishing Barranquilla as a prominent location for medical research in Latin America, backed by the Colombian Minister of Health. With bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, the environment for studies in the region is poised to enhance significantly, achieving over 50% reduction in recruitment time and 95% retention rates.
Best Practices for Endpoint Selection in Clinical Trials
To optimize endpoint selection in clinical trials, researchers must adhere to the following best practices:
-
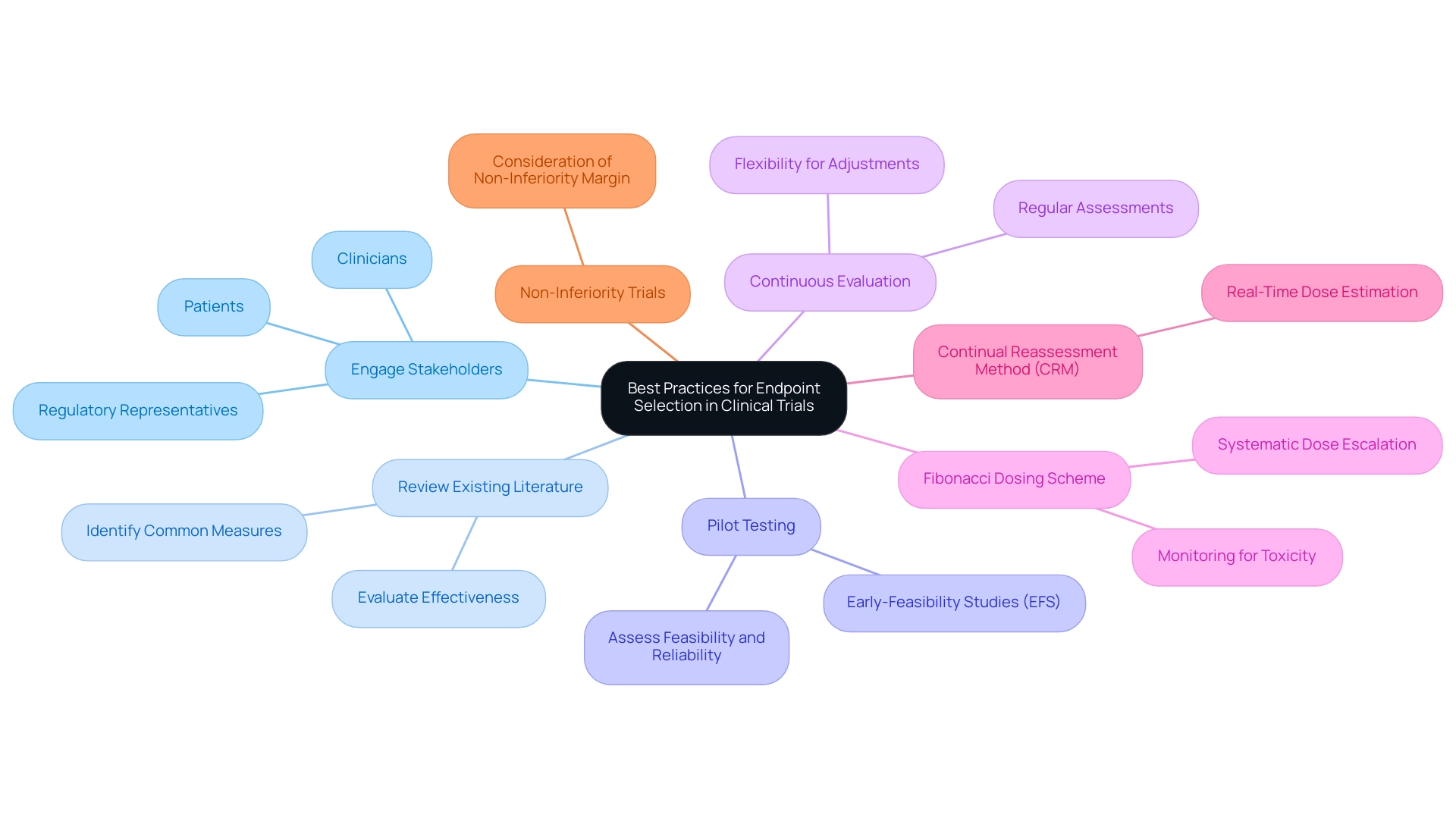
Engage Stakeholders: Actively involve key stakeholders—such as clinicians, patients, and regulatory representatives—in the endpoint selection process. This collaboration ensures that the chosen measures are not only relevant but also meaningful to all parties involved, enhancing the overall quality of the process. At bioaccess®, we emphasize the importance of stakeholder engagement, leveraging our 20+ years of expertise in managing clinical studies to foster collaboration that enhances study credibility.
-
Review Existing Literature: Conduct a comprehensive review of existing studies to identify commonly used measures and evaluate their effectiveness. This analysis can yield valuable insights into successful strategies used in similar experiments, guiding researchers toward informed decisions. Our team at bioaccess® is skilled at navigating existing literature to guide selection, ensuring alignment with best practices in the field.
-
Pilot Testing: Conduct preliminary assessments to evaluate the feasibility and reliability of the chosen endpoints before the full-scale launch. This preliminary testing can uncover potential issues early, allowing for adjustments that enhance the robustness of the main study. bioaccess® focuses on Early-Feasibility Studies (EFS) that serve this purpose, offering essential insights before larger-scale evaluations.
-
Continuous Evaluation: Regularly assess the relevance and effectiveness of the selected endpoints throughout the study. Researchers should remain flexible and ready to make necessary adjustments to ensure that the study consistently aligns with its objectives. Our comprehensive clinical study management services at bioaccess® include ongoing project management and reporting to facilitate this continuous evaluation.
In 2025, the emphasis on stakeholder engagement in endpoint selection processes is more critical than ever. Involving stakeholders not only promotes collaboration but also strengthens the credibility of the results. For instance, the Fibonacci dosing scheme exemplifies a structured approach to dose-ranging research, where doses are assigned based on a sequence derived from Fibonacci numbers. This method permits systematic escalation of doses while monitoring for toxicity, ensuring patient safety and optimizing study design.
Moreover, the continual reassessment method (CRM) is essential for real-time estimation of optimal doses during studies, allowing researchers to adapt to emerging data effectively. Current expert views emphasize the significance of stakeholder involvement in choosing endpoints for medical device trials, highlighting that effective communication and collaboration can greatly influence success. Additionally, non-inferiority studies require careful consideration of the non-inferiority margin, which varies widely and impacts the interpretation of results.
As Galileo Galilei wisely noted, "There are those who reason well, but they are greatly outnumbered by those who reason badly." By incorporating these best practices, researchers can improve the quality and significance of their studies, ultimately promoting the advancement of innovative medical devices with the support of bioaccess®. Moreover, bioaccess® provides a complete array of research, including Pilot Trials, Pivotal Trials, and Post-Market Follow-Up Research (PMCF), specifically designed to address the requirements of the Latin American market, ensuring expedited medical device examination services.
Key Takeaways: Enhancing Trial Success through Strategic Endpoint Selection
The process of selecting endpoints for medical device trials is critical, significantly influencing the overall success of the research. Essential considerations for optimizing endpoint selection include:
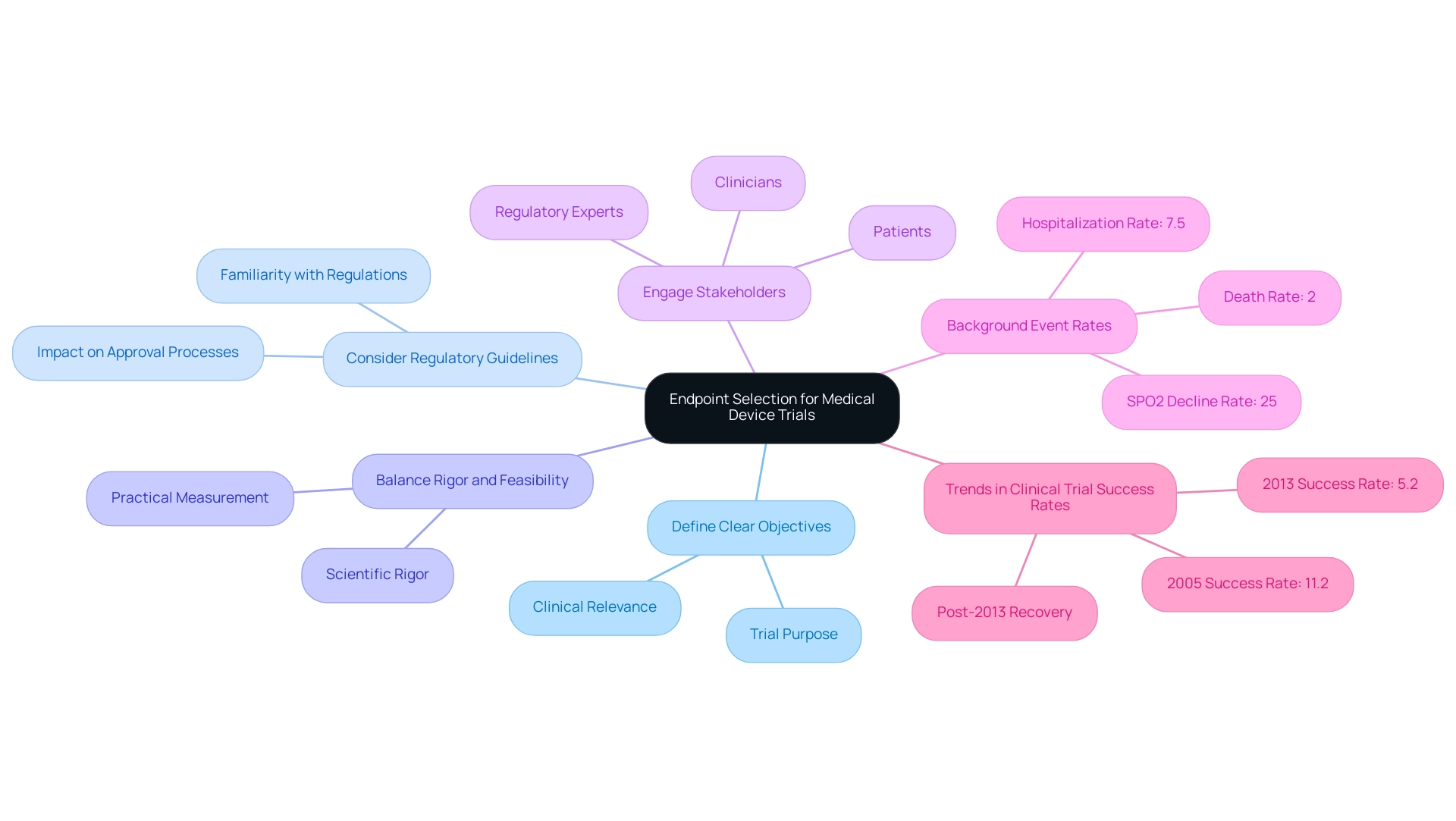
- Define Clear Objectives: Endpoints must be directly aligned with the trial's objectives and possess clinical relevance. This alignment clarifies the trial's purpose and enhances the interpretability of results.
- Consider Regulatory Guidelines: Familiarity with regulatory expectations is vital for facilitating smoother approval processes. Understanding these guidelines can significantly affect the approval of targets by regulatory bodies, particularly given bioaccess®'s expertise in Regulatory Affairs for medical devices and in vitro diagnostics in Latin America.
- Balance Rigor and Feasibility: It is essential to choose endpoints that are both scientifically rigorous and practical to measure. This balance ensures that objectives can be reliably evaluated without compromising the study's integrity, a principle that bioaccess® implements in its comprehensive clinical research management services, including endpoint selection for medical device trials such as Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Engaging stakeholders—clinicians, patients, and regulatory experts—in the endpoint selection process enhances the relevance and applicability of the chosen measures.
Recent analyses indicate that strategic selection of goals can lead to improved results in experiments. For instance, a study analyzing trends in research success rates revealed a significant decline in overall success rates from 11.2% in 2005 to 5.2% in 2013, followed by a recovery post-2013, coinciding with increased FDA approvals for new medications. This underscores the importance of adapting strategies to align with evolving regulatory landscapes and market needs.
As Mei-Jie Zhang, PhD, noted, "We hope this article will be of practical benefit to practitioners confronting the challenge of accurately interpreting research data."
Furthermore, understanding background event rates (CER) for various health outcomes—such as 2% for death, 7.5% for hospitalization, and 25% for SPO2 decline—provides a more comprehensive context for selecting endpoints in medical device trials, particularly regarding practical relevance. Additionally, the methodology for calculating the probability of success (POS) has improved, allowing for the imputation of missing data from experiments, although challenges with data processing persist.
By adhering to these guidelines, researchers can significantly enhance the quality and reliability of their clinical studies, particularly in endpoint selection for medical device trials. This ultimately leads to better patient outcomes and successful regulatory approvals. The impact of strategic endpoint selection is not merely theoretical; it has been evidenced through various case studies where optimized endpoints have yielded more favorable trial results and expedited pathways to market, contributing to job creation and economic growth in local communities.
Conclusion
Selecting appropriate endpoints in medical device trials transcends mere procedural formality; it represents a strategic decision that can profoundly influence the success of a study. This article has illuminated the multifaceted nature of endpoint selection, emphasizing the necessity for a comprehensive understanding of various endpoint types—primary, secondary, and exploratory—while aligning these endpoints with clinical relevance and regulatory expectations.
Moreover, the discussion underscores the critical role of regulatory bodies such as the FDA and INVIMA in shaping endpoint selection. These organizations ensure that researchers adhere to guidelines that facilitate smoother approval processes. By engaging stakeholders in the endpoint determination process and considering both objective and subjective measures, researchers can enhance the credibility and applicability of their trials.
The challenges encountered in endpoint selection, including recruitment issues and data collection difficulties, further illustrate the complexities involved. However, by adopting best practices—such as conducting pilot studies, continuously evaluating endpoint relevance, and leveraging existing literature—researchers can mitigate these challenges and improve trial outcomes.
Ultimately, strategic endpoint selection is essential not only for achieving regulatory approval but also for enhancing patient outcomes and advancing medical technology. As the landscape of clinical trials continues to evolve, prioritizing well-defined, clinically relevant endpoints will remain a cornerstone for success in the medical device sector. The insights gained from this article serve as a vital resource for researchers aiming to navigate the intricacies of endpoint selection effectively, ensuring that their trials are not only rigorous but also aligned with the needs of patients and the healthcare community.
Frequently Asked Questions
What are endpoints in clinical studies?
Endpoints are predefined outcomes that serve as critical benchmarks for measuring the effectiveness of medical interventions in clinical studies. They include various metrics such as survival rates, symptom alleviation, and enhancements in quality of life.
What are the main types of endpoints in medical device trials?
The main types of endpoints are: 1. Primary Endpoints: Main outcomes the study is designed to assess, such as the rate of significant adverse cardiac incidents. 2. Secondary Objectives: Supplementary measures that provide additional information about the treatment's effects, like quality of life indicators. 3. Exploratory Objectives: Gather additional information that may inform future research but are not the main focus of the current study.
Why is endpoint selection important in medical device evaluations?
Well-defined endpoints are crucial as their selection guides the research process from study design to regulatory approval. Poorly chosen endpoints can lead to ambiguous outcomes, increased scrutiny, or rejection by regulatory bodies.
How do endpoints affect regulatory approval rates?
Higher approval rates are observed when endpoints demonstrate clear medical relevance. For example, approval rates for single-agent regimens significantly increase when overall response rates exceed certain statistical thresholds.
What challenges can arise from poorly defined endpoints?
If the data collected fails to robustly support the primary objectives, such as a decrease in pain levels for an orthopedic implant, the study may struggle to demonstrate the device's effectiveness, creating regulatory hurdles.
What is the role of multi-component measures in endpoint selection?
Multi-component measures aggregate various pre-specified outcomes into a single score, offering a comprehensive evaluation of treatment effects. This approach is different from composite measures, which may lack significance when examined individually.
How can organizations enhance endpoint selection for medical device trials?
Organizations can leverage research study management services, like those provided by bioaccess®, to enhance study designs, ensuring that objectives are relevant and aligned with regulatory standards, which contributes to successful outcomes in medical device studies.

