Introduction
In the world of clinical trials, choosing the right consulting company is crucial for success. This article dives into key factors that should be considered when selecting a consulting company for your clinical trial. It emphasizes the importance of industry expertise, a proven track record, and a comprehensive range of services.
Cost-effectiveness is also addressed, promoting the search for a firm that offers an excellent return on investment. Drawing from real-life examples and expert insights, this article provides valuable information to guide readers in their decision-making process. Whether you're a pharmaceutical company, biotech firm, or academic institution, this article is a must-read to ensure you make an informed and strategic choice.
Key Factors to Consider When Choosing a Consulting Company
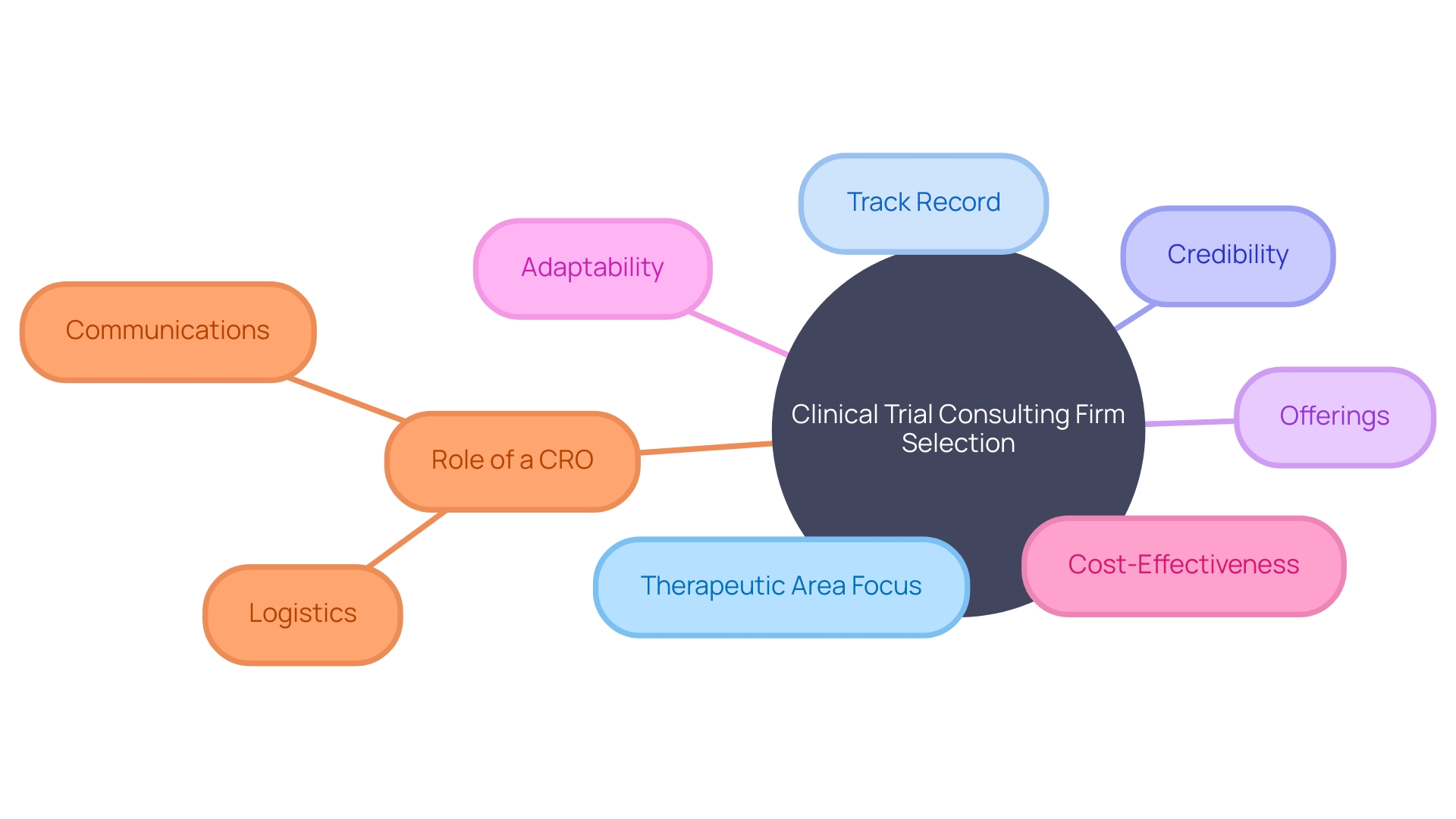
Selecting the right clinical trial consulting company is a multifaceted process that necessitates a thoughtful approach. Ideally, the company you choose should boast direct experience in your focused therapeutic area.
A proven track record is essential, implying deep regulatory insights and an ability to navigate various challenges along the development pathway. Credibility in this space is crucial.
Such assurance might come from robust client testimonials and in-depth case studies, which reflect the company's reliability. Offerings of a consulting firm are pivotal, with the anticipation that they deliver an extensive portfolio covering everything from protocol design to regulatory strategy, and advanced data management to sophisticated statistical analysis.
This breadth of services ensures a cohesive strategy and execution plan for trails at any scale. The adaptability of a consulting company is another paramount factor; they must be able to customize their approach and align services with the evolving requirements of your project.
While quality should never be compromised, cost-effectiveness remains an important consideration. Prospective clients should seek a balance, aiming for a consulting firm that delivers an excellent return on investment.
In clarifying these factors, let's reference CMIC Group, which has been pioneering in the Contract Research Organization (CRO) business for over 30 years. As Japan's first and largest CRO, they offer an array of services for the pharmaceutical lifecycle and have become a testament to the innovation in this industry. Moreover, the complexity of clinical trials is highlighted by cases such as a patient from rural Pennsylvania needing to participate in a trial in Turkey. This scenario underscores the role of a CRO not just in technical aspects, but potentially also in facilitating logistics and communications. According to a statement by an industry expert, time and careful consideration in the planning stages significantly affect the quality and success rate of clinical trials. This expertise is what clinical trial companies bring to the table, optimizing decisions that affect the entire lifecycle of drug development.
Understanding the Role of Independent Consultants in Clinical Research
Navigating the intricate world of clinical trials, independent consultants serve as vital contributors by providing specialized knowledge and impartial advice to organizations like pharmaceutical companies, biotech firms, and academic institutions. In their role, they facilitate the development and optimization of clinical trial protocols, aid in the selection of suitable sites, impart essential training to investigators, and heed to stringent regulatory compliance.
One prime illustration of their impact on the industry is the CMIC Group, which has revolutionized the Contract Research Organization (CRO) space in Japan over the past three decades, reflecting the comprehensive nature of services these consultants provide. Drawing from 1,200 data points, it has been observed that nearly 80% of the decisions influencing a study's outcome are pre-determined years in advance.
Acknowledging this, independent consultants focus on meticulously refining these pivotal decisions, thus ensuring an optimized approach tailored to each phase of the company's timeline. This dedication translates to enhanced trial efficiency and mitigated risk, fortifying each study's integrity. The Chronic Pain Network (CPN) exemplifies the collaborative synergy between patient partners and researchers, establishing that such partnerships are the cornerstone of inclusive and impactful clinical research. With insights gleaned from various sectors and the central ethos of patient-centered development, independent consultants are poised to not only propel individual studies but also contribute significantly to the global evolution of clinical trials and their methodologies.
Professional Development for Independent Consultants
As independent consultants in clinical research, continuous professional development is not just beneficial, it's imperative for staying competitively informed and for furnishing clients with the most valuable insights and top-tier services. Familiarity with crucial regulatory frameworks, such as Good Clinical Practice (GCP) and International Conference on Harmonization (ICH) guidelines, is paramount for ensuring that all clinical trials adhere to the latest regulatory expectations.
Mastery over these ever-evolving guidelines is akin to being fluent in a vital language of clinical research—a language that shapes the very landscape of health policy and patient care. Aligning with progressive technology an essential skill, with emphasis on the integration of electronic data capture, remote monitoring systems, wearable health devices, and the execution of virtual trials.
These innovations not only advance the rigor of clinical trials but also broaden their reach—potentially transporting trials to patients across borders, as with a patient in rural Pennsylvania participating in a trial in Turkey. Project management prowess stands as a cornerstone of successful clinical trial coordination, encompassing the orchestration of project timelines, risk mitigation, budget adherence, and team leadership.
It is a multifaceted skill set that hands the reins of a clinical trial to the consultant, allowing them to steer study outcomes toward excellence. In addition, honing communication skills is indispensable, paving the way for effective collaboration with clients, stakeholders, and research teams.
It empowers consultants to facilitate discussions, mediate conflicts, and produce results that echo the collective intent of all parties involved. Professional development avenues for independent consultants are manifold, ranging from attending industry conferences and engaging in webinars, to pursuing specialized courses. Whether it's extracting pivotal insights on maternal-child health or scrutinizing ethical considerations in participant compensation, professional growth fuels the engine of innovation and impacts practices on a global scale. Rigid self-assessment to pinpoint one's own educational needs is sometimes inspired by something as intuitive as dialogues with peers or as structured as systematic literature reviews. The result? A perpetually sharpened edge in a field where knowledge equates to influence and progress.
Ethical Guidelines for Clinical Research Consulting
Navigating the ethical landscape in clinical trial consultancy is analogous to applying data science principles: knowledge of and adherence to established rules are crucial. Consultants are tasked with safeguarding the dignity and rights of human participants, preserving the integrity of data, and upholding the research's esteem.
Central to these responsibilities is the ethical imperative of informed consent. It mandates the provision of lucid, comprehensive information, enabling participants to make well-founded decisions about their involvement.
Alongside this is the unbreachable duty to protect personal data, upholding stringent measures against unauthorized exposure. Furthermore, the reality of potential conflicts of interest cannot be ignored.
It is incumbent upon consultants to disclose any such conflicts to maintain the ethos of transparency and integrity, which are pivotal to sustaining trust amongst all parties involved. Finally, the onus is on consultants to meticulously align research activities with pertinent regulatory mandates, ensuring compliance with protocols set forth by ethics committees and other regulatory bodies.
Adherence to such ethical guidelines is imperative not just for participant welfare but also for securing the scientific method's credibility and contributing to medical advancements. The American Statistical Association's Ethical Guidelines, revised as recently as 2022, highlight the importance of aligning practice standards to societal and professional shifts over time, striving for clarity and applicability. Similarly, the UN Fundamental Principles of Official Statistics underscore the need for statistical data to be compiled based on professional ethics and scientific principles. These statistics serve as an anchor, providing a framework for consultants to benchmark their ethical compliance. Embracing the notion that 'principles matter,' as underscored by Gillikin (2017), consultants recognize that ethical standards not only define their profession but also help in mediating personal and professional conduct, underscoring uniformity across the field.
Case Studies: Successful Clinical Trials with Consulting Companies
Clinical trial companies, epitomized by pioneers like CMIC Group in Japan, are champions in navigating the complexities of medical research. Their consultancy in study design is invaluable, particularly when facing intricate challenges like those outlined in their extensive service provision, including the seamless integration of development, manufacturing, and even marketing. For instance, ensuring that a study's endpoints are well-defined and that its control groups are meticulously selected is fundamental for deriving conclusive results.
Moreover, the project management prowess of these organizations cannot be understated. They skillfully steward trials to adhere to strict timelines, efficiently allocate necessary resources, and strategically mitigate risks, thus safeguarding the integrity of the research. Regulatory compliance is another critical domain where these companies excel.
Their expertise encompasses obtaining essential approvals, rigorous documentation, and the diligent reporting of any adverse events, thereby maintaining the highest standards of regulatory adherence. A noteworthy example is the effort taken to optimize decision-making processes, as highlighted by the 1,200 data point study conducted by a transaction advisory, where it was revealed that more thorough planning could drastically enhance the outcomes of clinical trials. This meticulous approach is also reflected in data management and analysis, where accuracy, completeness, and timeliness are of paramount importance.
It is through these rigorous practices that clinical trial companies like CMIC equip pharmaceutical entities with the means not only to develop drugs but also to navigate the Japanese market effectively. Furthermore, poignant quotes from industry professionals reveal a desire for a more comprehensive framework in real-world data (RWD) quality, underscoring the necessity for relevance and reliability in data to inform scientific inferences. In essence, clinical trial companies are fundamental in bridging the gap between innovative medical discoveries and their successful market release, thus playing a pivotal role in the advancement of healthcare.
Best Practices for Selecting a Consulting Company
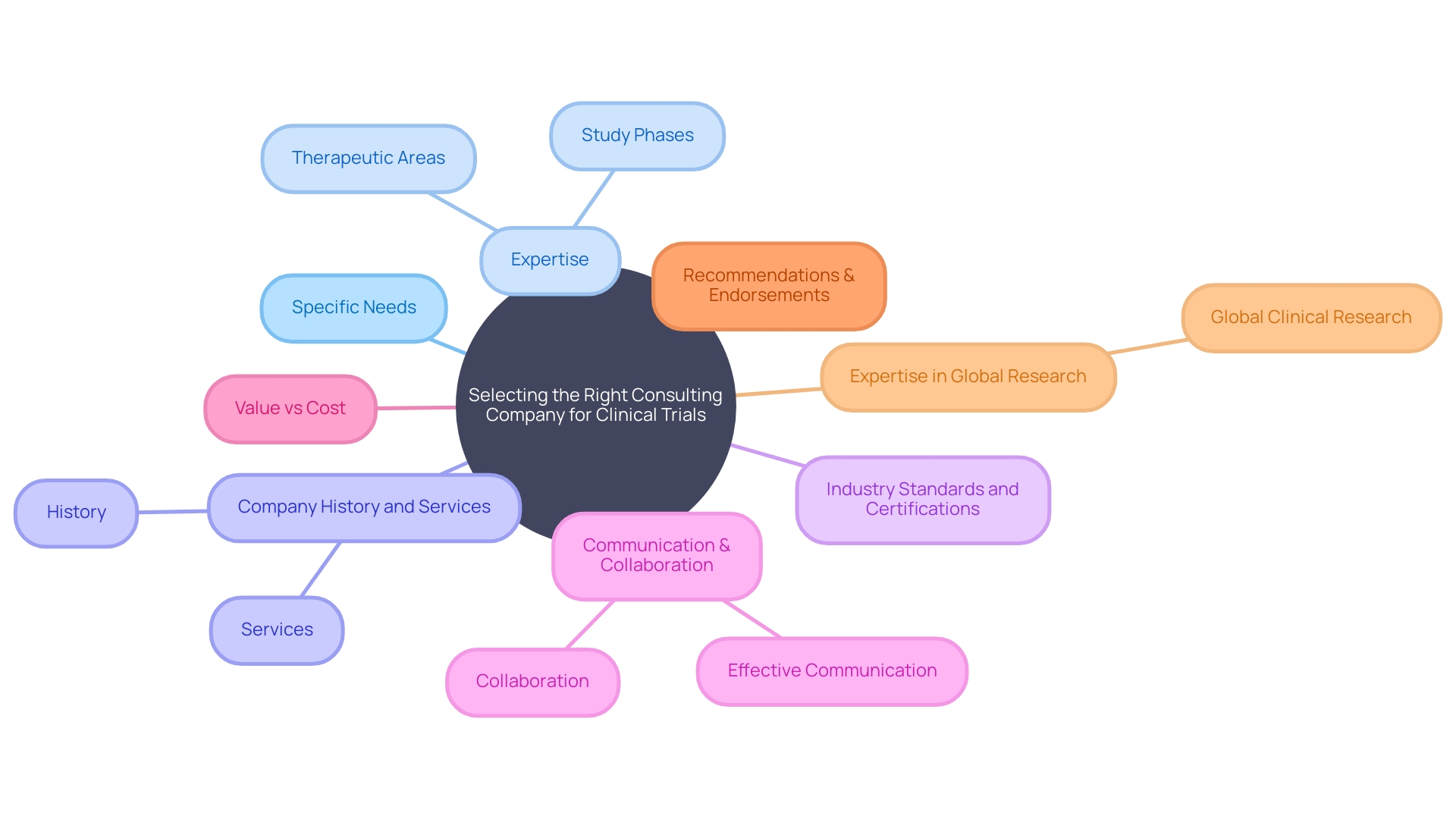
In the multifaceted world of clinical trials, the choice of a consulting company can be as important as the trial itself. It demands a discerning eye and an understanding that the company you choose will be an extension of your own team. Starting with a definition of your specific needs is imperative.
This encompasses the therapeutic area, the phase of the study, and the particularities of the services required. Seeking out a consulting firm that boasts substantial expertise in these areas can be the cornerstone of your project's success. An exhaustive evaluation is not only recommended but necessary to make a well-informed selection.
Look beyond surface-level attributes and delve into the company's history and breadth of services. This, paired with an examination of client testimonials and meaningful discussions via proposals or interviews, will shed light on their potential fit with your objectives. A company's adherence to industry standards can often be measured through their credentials and certifications.
These are the badges of their commitment to quality and continual professional development. Understanding this is critical in a sphere as complex as clinical research. Furthermore, effective communication and the ability to work collaboratively should be at the forefront of your considerations.
A company that is both transparent in its dealings and proactive in its communication ensures a synergistic relationship with your team. While budget constraints are a reality, they should not be the sole deciding factor. Weigh the value provided against the cost; sometimes, investing a little more upfront can pay dividends in the long run.
Hearing from colleagues who have tread the same ground can provide real-world insights that are invaluable. Endorsements within the industry and from the consulting company's clientele offer a candid look at what you might expect. An illustrative case highlighting the imperative nature of expert guidance is evident when a Pennsylvania patient with an ultra-rare disease is afforded a chance to join a clinical trial overseas.
The complexities of international travel, language barriers, and medical documentation underscore the need for a consulting company that can navigate the intricacies of global clinical research. In conclusion, the selection of a consulting company is not a decision to be taken lightly. By adhering to these best practices and seeking a partner that understands the nuances of your project, you can significantly improve the prospects of your clinical trials success.
Conclusion
In conclusion, choosing the right clinical trial consulting company requires careful consideration of several key factors. Look for a company with industry expertise in your specific therapeutic area, as well as a proven track record demonstrated through client testimonials and case studies. Ensure the consulting firm offers a comprehensive range of services, covering everything from protocol design to regulatory strategy and data management.
Adaptability is crucial, allowing them to tailor their approach to meet your evolving project requirements. While cost-effectiveness is important, prioritize a company that offers an excellent return on investment without compromising quality. Remember that investing upfront can yield long-term benefits.
Overall, the selection of a consulting company is a critical decision for the success of your clinical trial. Consider their expertise, track record, range of services, adaptability, and cost-effectiveness. Find a partner who understands your project's nuances, and you'll significantly enhance the prospects of your trial's success.




