Introduction
Clinical trials represent a cornerstone of medical research, providing the framework for developing new treatments and therapies. Navigating the complex landscape of clinical trials requires a thorough understanding of key terminology, the structured phases of the trials, and the ethical and regulatory considerations that underpin these studies. Familiarizing oneself with terms such as 'informed consent,' 'placebo,' and 'randomization' is essential for grasping the intricacies of clinical trial processes.
Each phase of a clinical trial, from Phase I to Phase IV, plays a distinct and critical role in assessing safety, efficacy, and long-term effects of treatments. Moreover, ethical and regulatory frameworks ensure the protection of participants and the integrity of the research. This article provides a detailed overview of these fundamental aspects, offering a glossary of essential terms to aid in comprehending the multifaceted world of clinical trials.
Key Terminology in Clinical Trials
Gaining a comprehensive understanding of clinical trials involves becoming familiar with specific terminology essential to the field. One of the primary terms is 'informed consent,' a critical process where individuals are provided with all necessary information regarding the study, including its purpose, potential risks and benefits, length, and procedures. This information is presented clearly and concisely to assist individuals in making an informed decision about their involvement. The draft guidance encourages the use of various methods to communicate this information, such as written documents, oral explanations, and visual aids like videos, ensuring accessibility for all participants, including those with language barriers or other impairments.
'Another key term is 'placebo,' referring to a substance with no therapeutic effect used as a control in experiments to compare the efficacy of the actual treatment.'. 'Endpoints' refer to the main results assessed to evaluate a treatment's efficacy, which can encompass health outcomes, quality of life indicators, or other important measures.
'Randomization' is a procedure used to allocate individuals to various categories in an experiment in a way that reduces bias, ensuring the dependability and accuracy of the findings. This method helps in achieving comparable groups and balancing known and unknown factors that could affect the outcomes.
Comprehending these terms is essential for anyone engaged in clinical studies, as they establish the basis of the stringent and ethical practices anticipated in clinical research. The informed consent process, in particular, plays a crucial role in protecting the rights of individuals and ensuring their voluntary involvement based on a clear understanding of the study.
Clinical Trial Phases
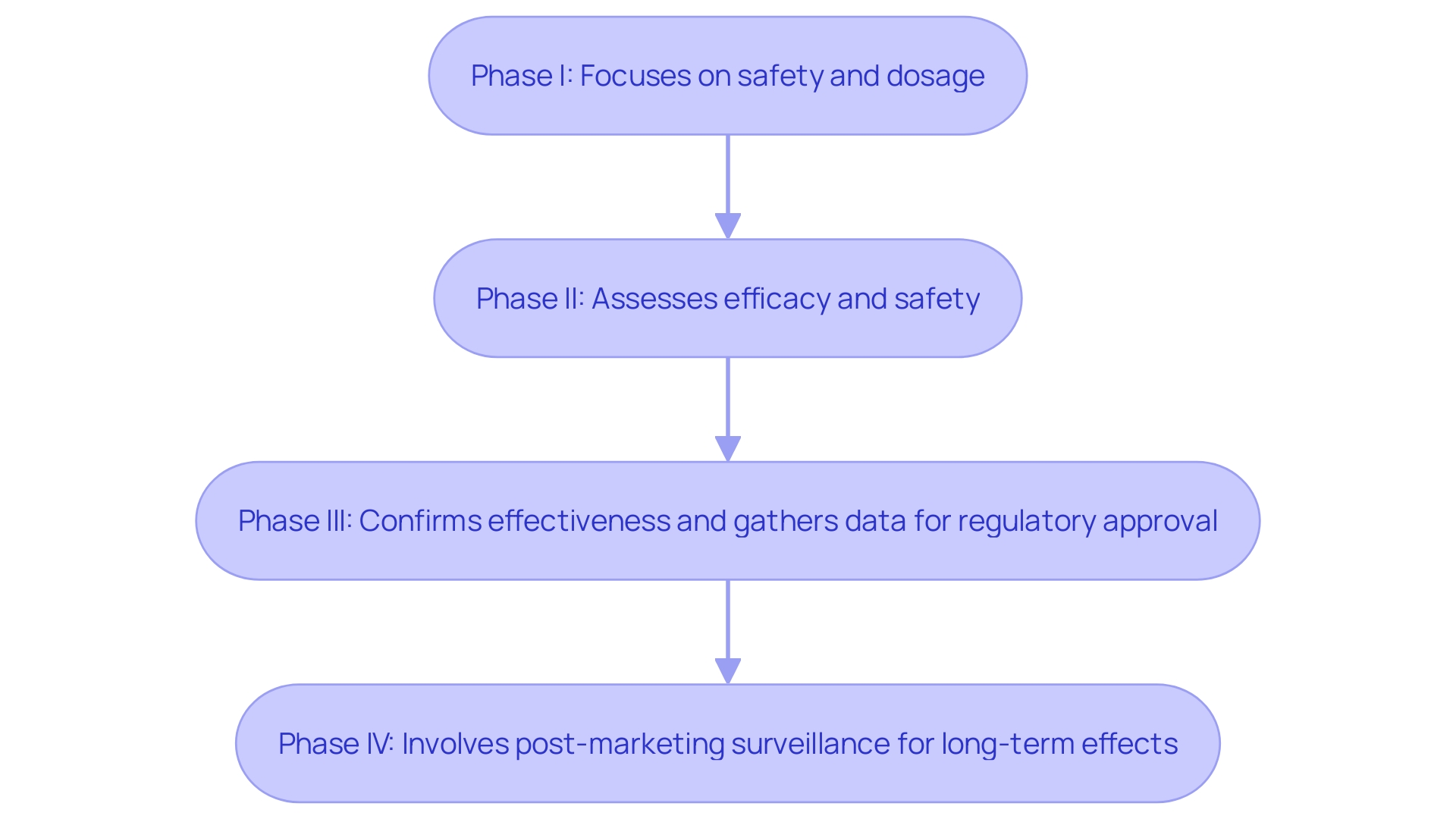
Clinical studies are meticulously organized into distinct phases, each serving a pivotal role in the drug development process. Phase I studies mainly concentrate on evaluating the safety and ideal dosage of a new treatment in a limited group of individuals. This initial phase is crucial for determining the safe dosage range and identifying any potential side effects. Phase II studies build on this by evaluating the treatment's efficacy while continuing to monitor its safety. These studies offer initial information on whether the drug is effective in individuals with a specific condition or illness and include a larger cohort of participants in comparison to Phase I.
Phase III studies are more extensive, involving larger populations to confirm the treatment's effectiveness, monitor side effects, and compare it to standard or equivalent treatments. This phase is instrumental in gathering the comprehensive data required for regulatory approval. The heightened quantity of evaluations in these phases has grown from an average of 17 (2013-2016) to 21 (2017-2020), imposing a greater burden on patients, which can adversely impact enrollment rates and participation during the research.
Finally, Phase IV trials commence once a treatment has received approval. These post-marketing investigations collect additional information about the drug's long-term effects, new applications, and previously unrecognized adverse reactions. As highlighted, “Once a drug is approved in Phase 4, a post-marketing review takes place to ensure that the new medicine remains safe for public use.” This phase often includes pediatric studies or special safety studies to ensure ongoing compliance with FDA regulations and to build trust in the treatment's safety and efficacy for the broader population.
Ethical and Regulatory Considerations
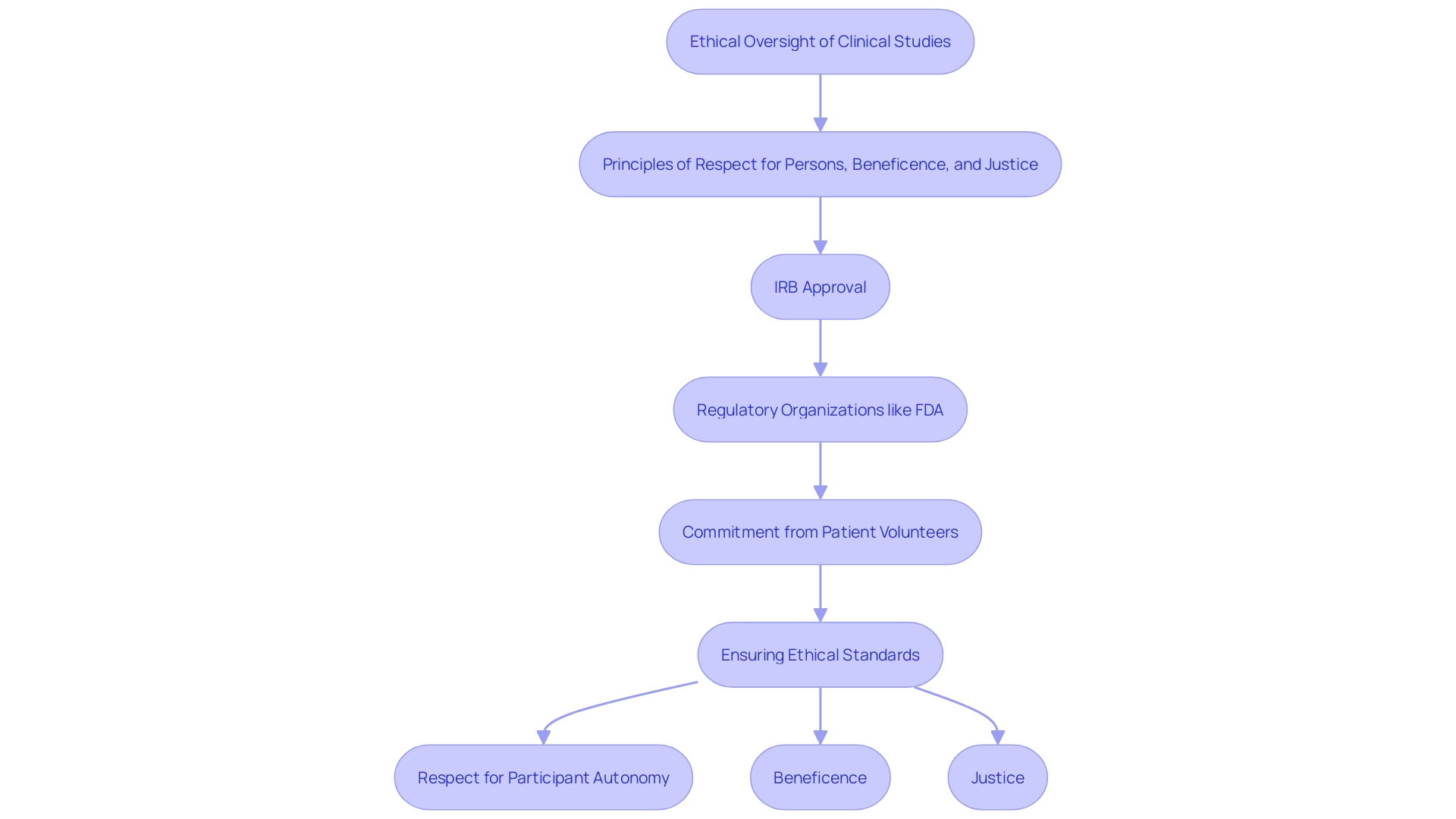
Ethical considerations are paramount in clinical studies, guided by the principles of respect for persons, beneficence, and justice. Regulatory organizations, like the FDA in the United States, establish strict standards to guarantee the integrity and safety of studies. This includes the requirement for Institutional Review Board (IRB) approval, which evaluates the ethical aspects of the study before it begins, ensuring that participant rights and welfare are protected.
The function of patient volunteer work in research studies is becoming more intricate. 'As stated by the Tufts Center for the Study of Drug Development, the typical individual in a Phase 3 study is anticipated to participate in 18 to 20 scheduled visits and undergo 34 to 38 procedures, indicating a considerable commitment of their time, energy, and resources.'. This commitment highlights the significance of recognizing contributors' efforts by taking into account their wish for information regarding the outcomes of the research they assist. The Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard published a Guidance, Toolkit, and Principles in 2017, revised in 2022, highlighting the necessity for openness in research studies.
Research has indicated that many individuals anticipate obtaining their personal results, motivated by a natural curiosity regarding their health and the findings of the study to which they contributed. 'Ethical oversight systems must balance the competing interests of patient desires, ethical principles, and the practical considerations of conducting a trial.'. The FDA has made important moves to align clinical research regulations with the U.S. Department of Health and Human Services (HHS) Common Rule, encouraging effective clinical research while safeguarding the rights of those involved.
IRBs play a critical role in this process. All human research is reviewed by an IRB, which evaluates proposals detailing the purpose of the investigation, procedures, risks, benefits, and consent forms. Since 2021, about half of the IRBs that review medical studies are for-profit and serve private companies. This system aids in stopping unethical research behaviors, guaranteeing that studies are carried out morally and individuals are completely informed.
In summary, the moral structure for medical studies is intended to safeguard individuals while promoting healthcare progress. By following rigorous protocols and ensuring openness, research organizations can uphold the trust and collaboration of individuals involved, ultimately resulting in more effective and dependable research results.
Glossary of Essential Terms
A comprehensive glossary of essential terms in clinical research serves as an invaluable reference. For example, an 'adverse event' describes any undesirable experience linked to the treatment, which is critical for safety assessments. ''Blinding' is a methodological approach to prevent bias by ensuring that participants and/or investigators are unaware of group assignments. The term 'sponsor' denotes the individual or entity that finances the study, emphasizing the financial and managerial foundation of the research. 'The 'Clinical Trial Protocol' is a crucial document outlining the project's objectives, design, methodology, and statistical considerations, ensuring structured and regulated progress. These terms, along with a multitude of other data elements, are integral to ClinicalTrials. Gov study records, evolving over 25 years to enhance trial reporting and compliance with regulatory guidelines.
Conclusion
The exploration of clinical trials reveals their foundational role in advancing medical research and treatment development. A thorough understanding of key terminology such as "informed consent," "placebo," and "randomization" is essential for navigating the complexities of these studies. These terms encapsulate the ethical and methodological frameworks that uphold the integrity of clinical trials, ensuring participant safety and informed decision-making throughout the research process.
Furthermore, the structured phases of clinical trials—Phase I through Phase IV—highlight the meticulous approach required to assess new treatments. Each phase serves a distinct purpose, from initial safety assessments to post-marketing evaluations, thereby providing a comprehensive understanding of a drug's efficacy and long-term effects. The data gathered during these phases not only informs regulatory approvals but also contributes to ongoing patient safety and treatment optimization.
In addition to the scientific and procedural aspects, ethical and regulatory considerations play a pivotal role in clinical trials. Adherence to ethical principles and regulatory guidelines safeguards participant rights and fosters trust in the research process. The evolving expectations of participants, particularly regarding transparency and access to their results, underscore the importance of ethical oversight in maintaining the integrity of clinical trials.
In summary, an informed understanding of clinical trials—including their terminology, structured phases, and ethical frameworks—is crucial for anyone engaged in or impacted by medical research. By prioritizing transparency, participant welfare, and rigorous methodology, the clinical trial landscape can continue to evolve, ultimately leading to safer and more effective treatments for patients worldwide.




