Overview
Clinical research hubs in Latin America are gaining prominence due to their cost efficiency, diverse patient populations, and regulatory advancements. These factors render them attractive sites for global pharmaceutical firms. Notably, these hubs enhance participant recruitment and retention rates and benefit from robust government support and collaborations. Consequently, the region is positioning itself as a competitive player in the global clinical research landscape.
Introduction
As the landscape of clinical research evolves, Latin America emerges as a pivotal player, offering a wealth of opportunities for pharmaceutical companies and research institutions alike. With its diverse patient demographics, cost-effective trial execution, and improving regulatory frameworks, the region is increasingly recognized for its potential to transform clinical research initiatives.
However, despite these advantages, awareness of clinical trials remains surprisingly low among patients, presenting both a challenge and an opportunity for growth. This article delves into the key advantages, challenges, and future directions of clinical research in Latin America, highlighting the role of Contract Research Organizations and the strategic partnerships that are shaping this dynamic field.
As the region continues to enhance its capabilities, it stands poised to make significant contributions to global clinical research efforts.
Overview of Clinical Research Hubs in Latin America
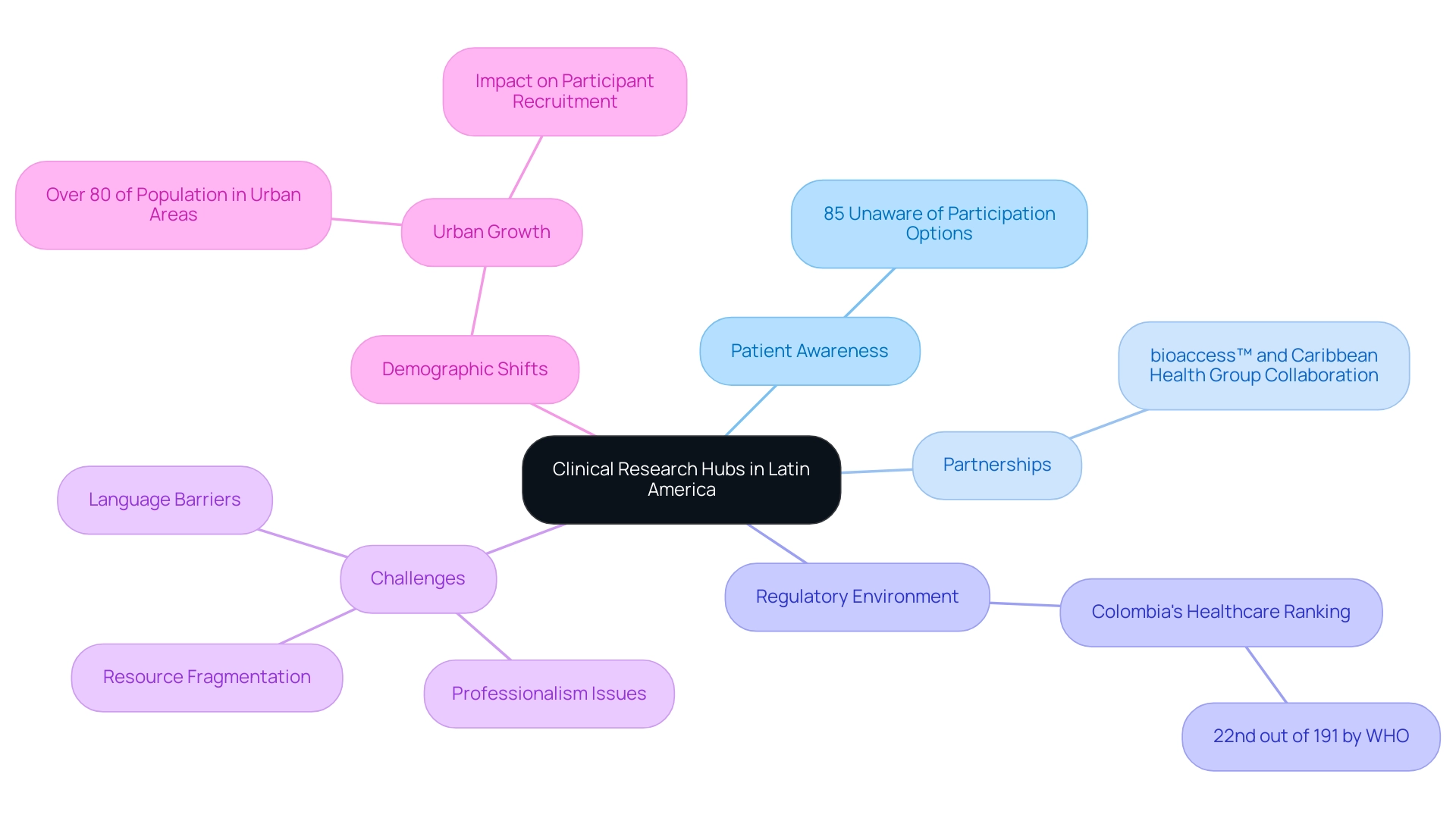
The emergence of clinical research hubs in Latin America has rendered these locations essential for conducting studies, driven by regulatory advancements, affordability, and diverse patient populations. With Colombia's healthcare system ranked 22nd out of 191 countries by the World Health Organization, the region is gaining recognition for its significant potential. However, awareness of medical studies remains alarmingly low, with 85% of patients unaware of their participation options, presenting a substantial opportunity for increased enrollment.
Notably, partnerships such as that between bioaccess™ and Caribbean Health Group are strategically positioning Barranquilla as a leading clinical research hub in Latin America for clinical trials, with the endorsement of Colombia's Minister of Health, Juan Pablo Uribe. This highlights the critical role of governmental support in these initiatives. Countries such as Brazil, Argentina, and Mexico are also at the forefront of this transformation, establishing themselves as clinical research hubs in Latin America that attract global pharmaceutical firms and academic institutions. These hubs not only provide access to new patient populations but also streamline study processes, contributing to lower dropout rates compared to the U.S. and EU.
Nevertheless, U.S. Medtech companies encounter challenges such as professionalism, language barriers, and resource fragmentation, which can hinder effective collaboration. Mariana Bei, Sr. Director of Clinical Operations at Parexel, underscores the necessity of regional presence, stating, 'Having a local presence and leadership that is based in the LATAM region strengthens the relationships that Parexel builds with local talent, regulatory authorities based in the area, local associations, and the global network of sites overall.' As urban growth continues—over 80% of the population now resides in urban areas—clinical research hubs in Latin America are uniquely positioned to leverage this demographic shift, enhancing participant recruitment and achieving over a 50% reduction in recruitment time and 95% retention rates. This positions LATAM as an optimal ally for adapting to customer needs in trial initiatives.
Key Advantages of Conducting Clinical Research in Latin America
Latin America presents a compelling landscape for conducting clinical research, driven by several key advantages:
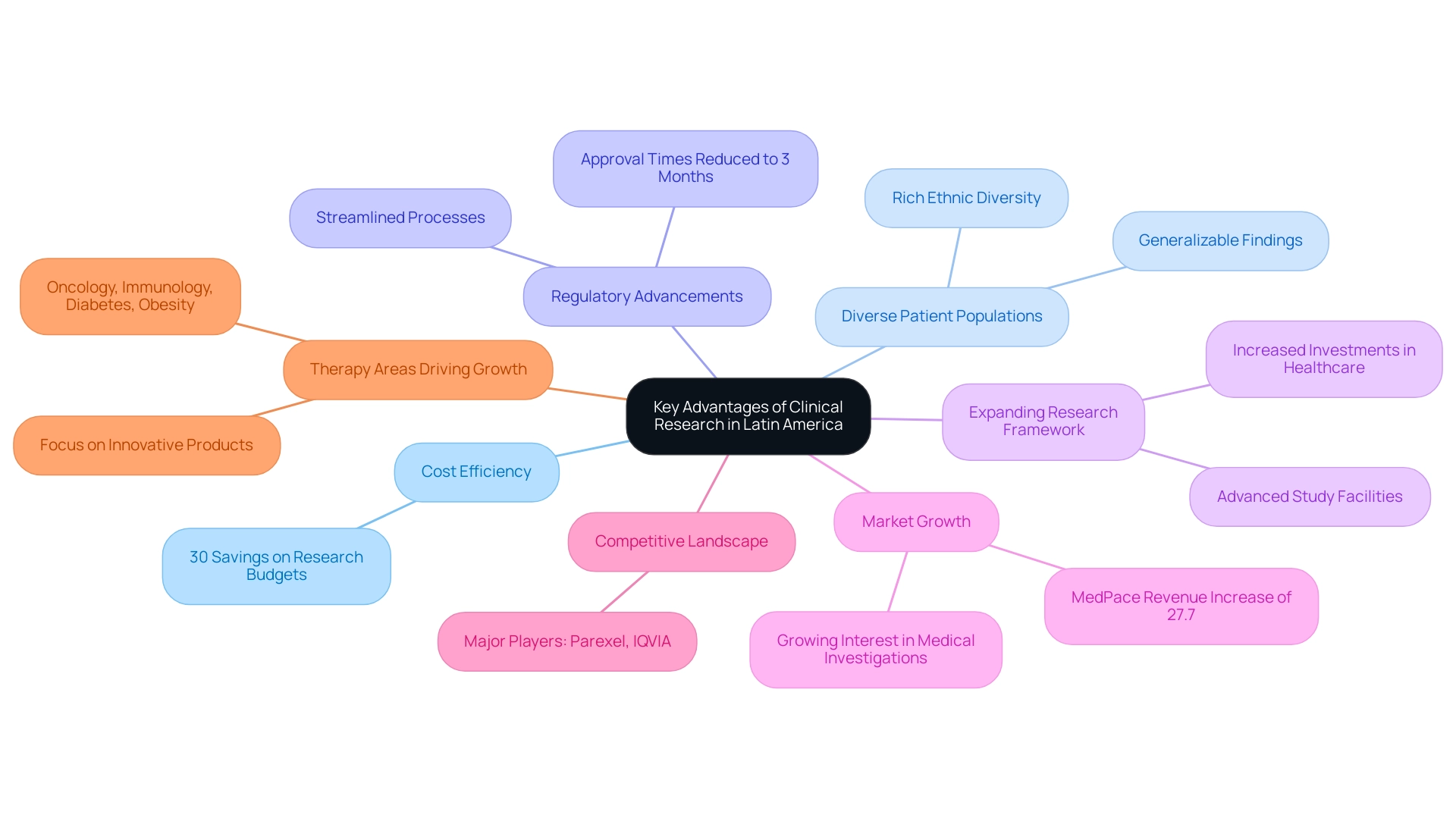
- Cost Efficiency: The operational expenses in this region are considerably lower than those in the United States and Europe, allowing pharmaceutical firms to realize substantial savings on research budgets. Research indicates that conducting medical studies in Latin America can reduce expenses by as much as 30%, making it an appealing choice for cost-conscious organizations.
- Diverse Patient Populations: The rich ethnic diversity of Latin America enhances the pool of participants available for research studies. This diversity not only enriches the data collected but also ensures that findings are more generalizable across various demographics. The region has witnessed an increase in participation from diverse ethnic groups, which is crucial for understanding how treatments impact different populations.
- Regulatory Advancements: Numerous Latin American countries have made significant strides in streamlining their regulatory processes. These improvements have led to quicker approvals and a more direct pathway for initiating medical studies, enabling organizations to accelerate their timelines. A recent report noted that regulatory efficiency in the region is on the rise, with some countries reducing approval times to as little as three months.
- Expanding Research Framework: With increasing investments in healthcare and research facilities, Latin America is enhancing its capabilities to conduct high-quality medical studies. The establishment of advanced study facilities and collaborations with local organizations, such as bioaccess®, has bolstered the region's reputation in medical research.
- Market Growth: The research studies sector in Latin America is gaining momentum, as evidenced by MedPace's annual revenue increase of 27.7% in 2022, reaching $394.1 million. This growth underscores the rising interest and investment in the region's medical investigation capabilities.
- Competitive Landscape: Major players in the medical studies market, including Parexel and IQVIA, are recognizing the potential of Latin America, further underscoring its attractiveness for conducting medical investigations.
- Therapy Areas Driving Growth: According to the case study titled "Forecast for Therapy Areas," oncology, immunology, diabetes, and obesity drugs are projected to be the leading contributors to growth in the next five years. This focus on innovative products aligns seamlessly with the region's capabilities and resources.
These factors collectively create a favorable environment for advancing medical investigation initiatives within clinical research hubs in Latin America. As emphasized by Precedence Research,
Trials serve as the primary method used by scientists to examine if a new therapy, a medical device, or a novel drug is safe and effective in individuals.
Furthermore, collaborations with specialists such as bioaccess®, focusing on comprehensive study management services—including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF)—enhance the efficiency of investigative efforts in the region. Thus, clinical research hubs in Latin America not only serve as a cost-effective option but also as a strategic site for various high-quality medical studies.
The Role of Contract Research Organizations in Latin America
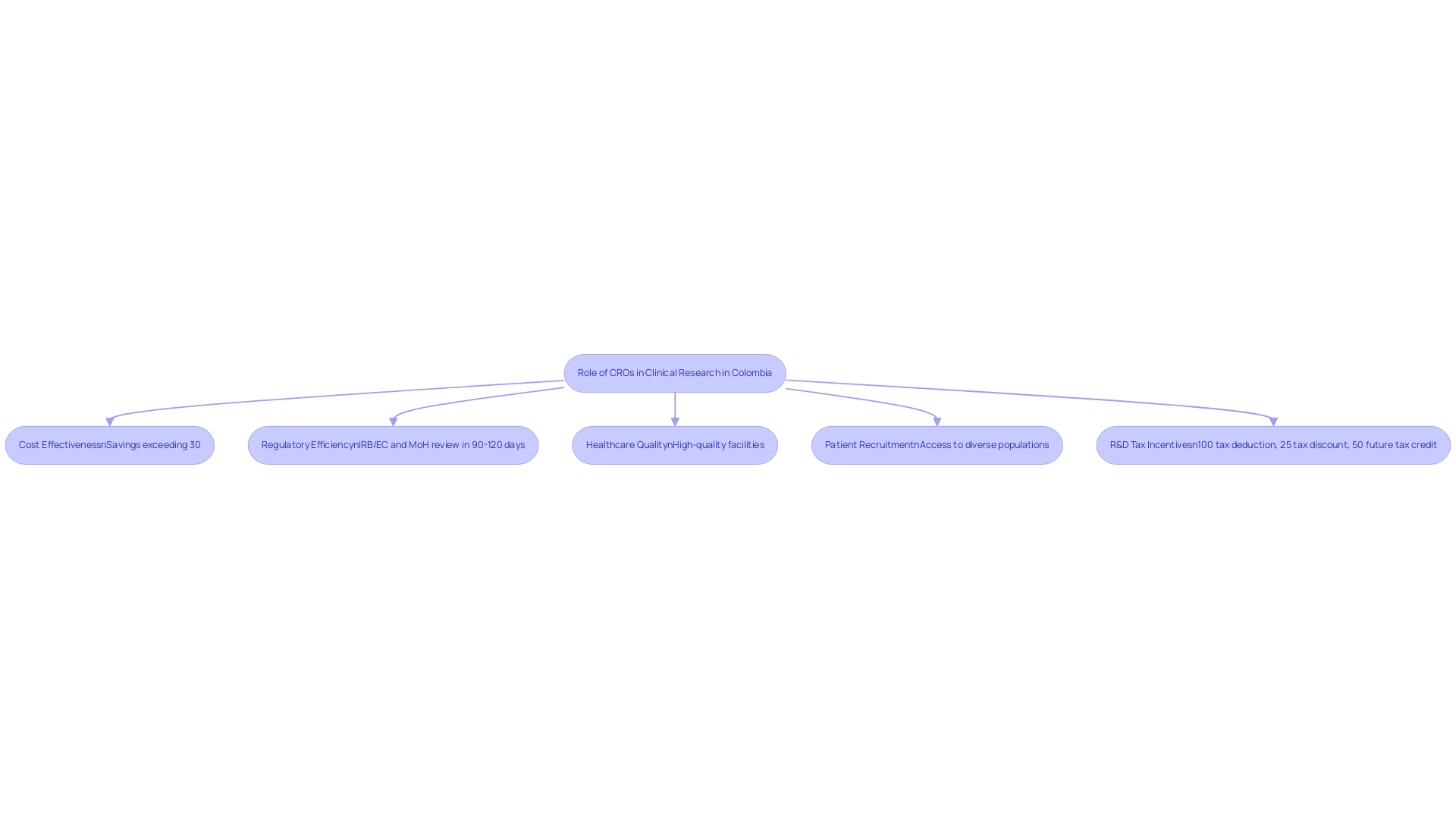
Contract Research Organizations (CROs) play a pivotal role in the clinical research landscape of Latin America, particularly in Colombia, which stands out for its competitive advantages in first-in-human studies. The country offers cost effectiveness, with savings exceeding 30% compared to similar trials in North and Western Europe. Regulatory processes are efficient, with the total review by IRB/EC and MoH (INVIMA) completed in a mere 90-120 days, facilitating prompt project initiation.
Moreover, Colombia's healthcare system, ranked 22nd by the World Health Organization, features high-quality facilities, with hospitals acknowledged among the finest in Latin America. These hospitals must undergo a rigorous ICH/GCP certification process before conducting trials with pharmaceutical drugs, ensuring adherence to high standards of quality and compliance. Patient recruitment is also advantageous, supported by a population exceeding 50 million and universal healthcare coverage for approximately 95% of residents, thereby enhancing access to diverse patient populations.
The CRO bioaccess® serves as a prime example of how these organizations bolster operational efficiency and compliance with local regulations. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, underscores his experience during the bioaccess® first human study in Colombia as a testament to the region's capabilities. Additionally, the R&D tax incentives available in Colombia position it as one of the premier clinical research hubs in Latin America, where investments in science and technology projects can receive:
- a 100% tax deduction
- a 25% tax discount
- a 50% future tax credit
- approximately $10 million in government grants
This renders it an attractive locale for health-related initiatives.
As the demand for strategic alliances escalates, collaborations such as that between LEO Pharma and ICON illustrate how pharmaceutical companies leverage local expertise to enhance the quality and speed of their studies. Julio G. Martinez-Clark, CEO of bioaccess, highlights Colombia's commitment to evolving into a knowledge economy through its ambitious science, technology, and innovation plan from 2022 to 2031. Furthermore, investing in digital technologies presents an opportunity for CROs to enhance collaboration and optimize processes, thereby strengthening their role in advancing medical studies in Latin regions.
Challenges and Barriers to Clinical Research in Latin America
Carrying out medical investigations in clinical research hubs in Latin America presents a distinct array of challenges, which bioaccess® is committed to addressing to enhance the testing environment for medical device trials. Key barriers include:
-
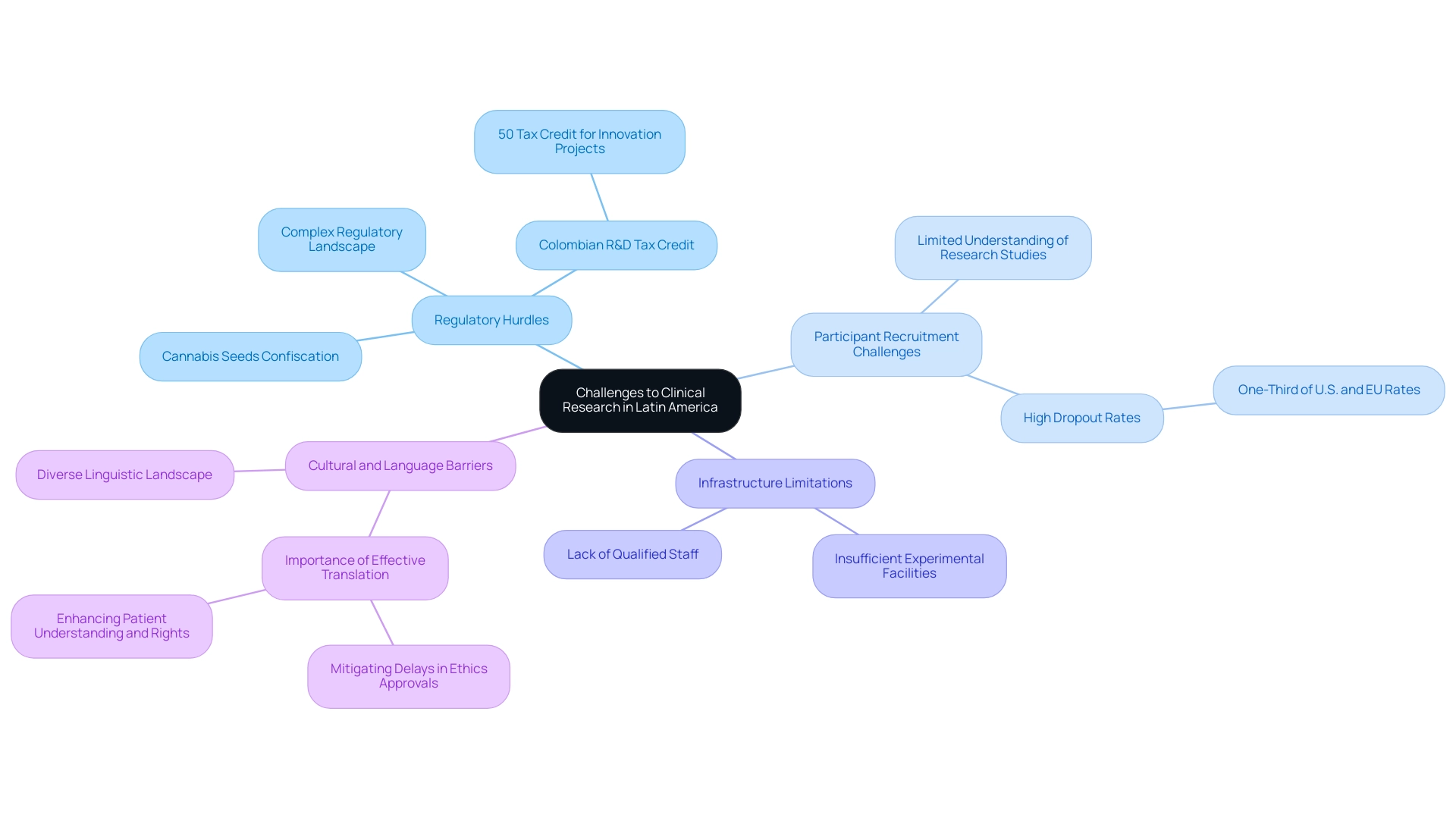
Regulatory Hurdles: While significant strides have been made in recent years, the regulatory landscape remains complex, with variations that can differ markedly across countries.
For example, the Colombian government has recently implemented an R&D tax credit enabling small and midsize companies to claim a 50% tax credit on their investments in innovation projects, indicating a positive shift. However, deeper regulatory understanding is required for firms to fully capitalize on such incentives. Furthermore, recent news concerning cannabis seeds confiscated in Latin regions emphasizes ongoing regulatory difficulties that may affect research processes.
-
Participant Recruitment Challenges: Ensuring enrollment can be especially difficult, particularly in areas where understanding of research studies is limited. This is underscored by dropout rates in Latin America being one-third of those observed in the U.S. and EU. These dropout rates indicate substantial obstacles in patient retention and engagement, which must be tackled to enhance recruitment strategies and ensure successful research outcomes.
-
Infrastructure Limitations: In specific regions, insufficient experimental facilities and a lack of qualified staff can significantly impede the advancement of trials, further complicating the investigative landscape.
-
Cultural and Language Barriers: The diverse linguistic and cultural landscape presents additional challenges. As demonstrated in the case study on 'Language Barriers and Translation in Clinical Research,' effective translation of regulatory and patient-related materials is crucial.
Effectively addressing these linguistic and cultural differences can reduce delays in ethics committee approvals and improve patient understanding and rights. Acknowledging these challenges, bioaccess® stands as a prominent Contract Research Organization supporting medical device trials in the clinical research hubs in Latin America. As Julio G. Martinez-Clark, CEO of Bioaccess, aptly stated,
Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy.
This emphasizes the ongoing efforts to improve the framework across the region and highlights the significance of tackling these challenges for future success. Furthermore, bioaccess® is committed to ensuring data protection and compliance, as outlined in our FAQs, reinforcing our dedication to safeguarding client information. We also invite our clients to share their experiences with us, as customer testimonials highlight our successful navigation of these challenges and the tailored pricing strategies we implement to meet diverse market needs.
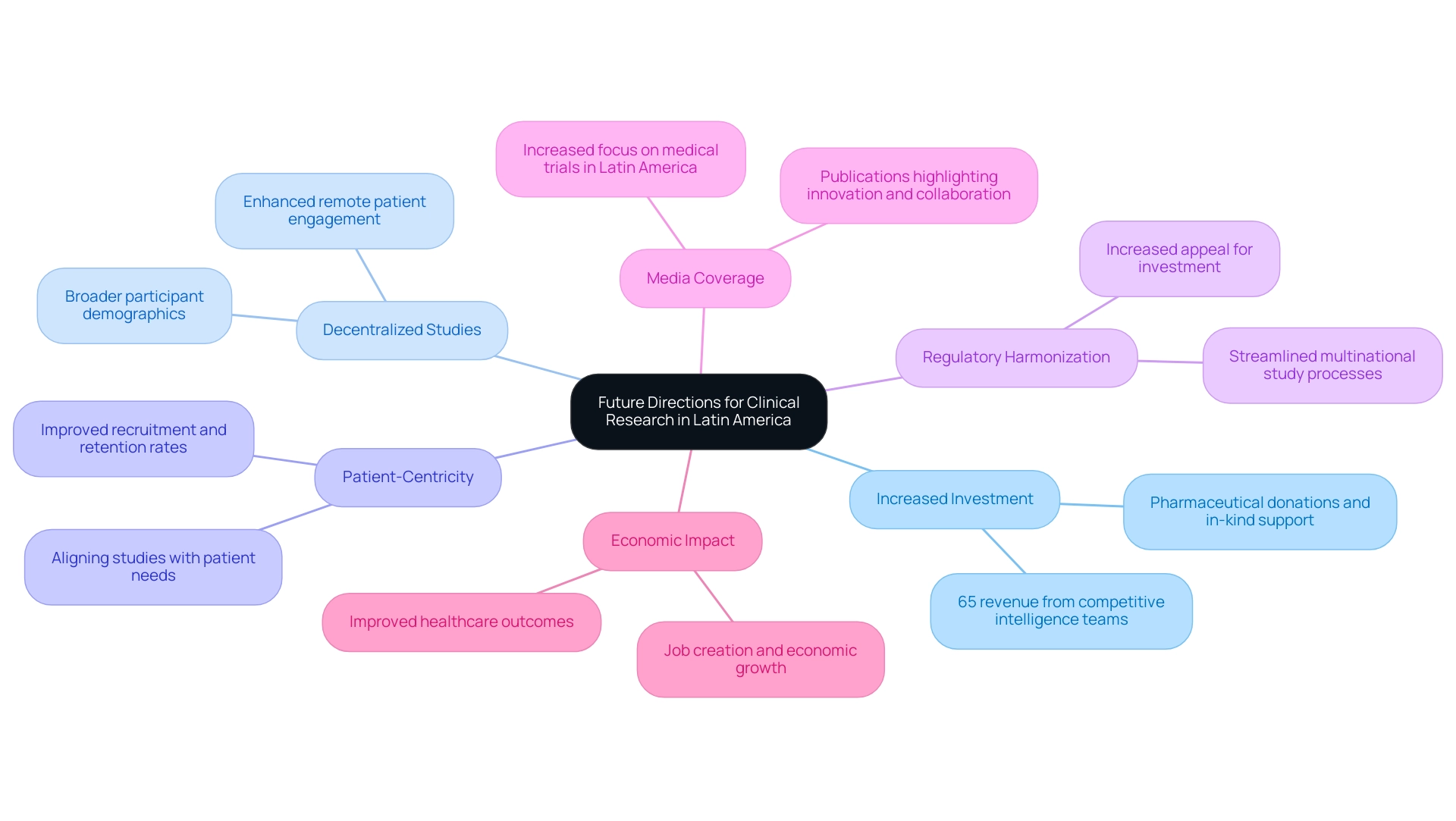
Future Directions for Clinical Research in Latin America
The future of clinical studies in Latin America is poised for significant advancement, driven by several key trends:
- Increased Investment: The region is progressively recognized for its robust development capabilities, leading to a projected rise in investment from pharmaceutical companies. These companies contribute not only through direct investments but also through donations and in-kind support, thereby enhancing the overall research landscape. Approximately 65% of revenue in the industry now arises from competitive and market intelligence teams, underscoring the critical importance of strategic investment in this landscape.
- Decentralized Studies: The adoption of decentralized clinical studies is gaining traction, utilizing technology to enhance remote patient engagement. This trend not only improves accessibility but also broadens participant demographics, thus enriching data quality.
- Focus on Patient-Centricity: A notable shift is occurring towards designing studies that prioritize patient needs and preferences. By aligning study structures with patient expectations, companies can enhance recruitment and retention rates, leading to more successful outcomes.
- Regulatory Harmonization: Ongoing efforts to align regulations across Latin American nations promise to streamline the processes involved in conducting multinational studies. This initiative is crucial in making clinical research hubs in Latin America more appealing for investment in studies.
- Media Coverage: The press has increasingly concentrated on medical trials in Latin regions, particularly in Colombia, emphasizing the area's rising significance in the worldwide study landscape. Publications like Clinical Leader have reported extensively on these developments, showcasing the potential for innovation and collaboration.
- Economic Impact: Medtech studies are contributing significantly to local economies through job creation, economic growth, and improved healthcare outcomes. By fostering international collaboration, these studies not only enhance local healthcare infrastructure but also attract further investments.
These developments indicate a transformative change towards a more efficient and robust medical study environment in Latin America, establishing it as one of the prominent clinical research hubs within the global investigation arena. bioaccess® exemplifies this commitment with its comprehensive clinical trial management services, focusing on Early-Feasibility Studies, First-In-Human Trials, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies. The case of Horizon Databook demonstrates this potential; generating 30% of its revenue from investment firms and 65% from competitive intelligence and market intelligence teams, while also supporting academic institutions at subsidized rates, reflects a commitment to fostering innovation and excellence in scholarship. Furthermore, as North America is projected to lead the regional market in terms of revenue by 2030, and Asia Pacific is expected to be the fastest-growing market, Latin America must capitalize on these trends to solidify its role in the international landscape. The anticipated investment trends in clinical research for 2024 will further enhance this position.
Conclusion
Latin America is emerging as a vital hub for clinical research, characterized by a unique blend of advantages that position the region favorably in the global landscape. With cost efficiency, diverse patient populations, and improving regulatory frameworks, the region provides a compelling environment for conducting clinical trials. The significant decrease in trial costs, coupled with the rich ethnic diversity of participants, enhances the quality and generalizability of research findings. Furthermore, the swift regulatory processes and growing infrastructure bolster Latin America's appeal to pharmaceutical companies and research institutions.
However, the journey is not without its challenges. Low awareness of clinical trials among patients, coupled with regulatory complexities and infrastructure limitations, presents hurdles that must be addressed. By navigating these challenges through strategic partnerships and leveraging local expertise, organizations like bioaccess® are setting the stage for continued growth and innovation in the region.
Looking ahead, the future of clinical research in Latin America is bright, fueled by increased investment, the rise of decentralized trials, and a greater focus on patient-centric approaches. As regulatory harmonization efforts continue and media coverage amplifies the region's potential, Latin America is poised to solidify its role as a key player in the global clinical research arena. Embracing these trends will not only enhance the capabilities of the region but also contribute significantly to advancing healthcare outcomes worldwide.
Frequently Asked Questions
Why are clinical research hubs in Latin America becoming essential for studies?
Clinical research hubs in Latin America are essential due to regulatory advancements, affordability, and diverse patient populations, making them attractive locations for conducting studies.
What is Colombia's healthcare ranking and why is it significant?
Colombia's healthcare system is ranked 22nd out of 191 countries by the World Health Organization, highlighting the country's potential in the clinical research landscape.
What challenges do patients face regarding participation in medical studies in Latin America?
Awareness of medical studies is low, with 85% of patients unaware of their participation options, creating an opportunity for increased enrollment.
What partnerships are helping to establish Barranquilla as a clinical research hub?
The partnership between bioaccess™ and Caribbean Health Group is positioning Barranquilla as a leading clinical research hub in Latin America, supported by Colombia's Minister of Health.
Which other countries are emerging as clinical research hubs in Latin America?
Brazil, Argentina, and Mexico are also establishing themselves as clinical research hubs, attracting global pharmaceutical firms and academic institutions.
What advantages do clinical research hubs in Latin America offer compared to the U.S. and EU?
Latin American hubs provide access to new patient populations and streamline study processes, resulting in lower dropout rates compared to the U.S. and EU.
What challenges do U.S. Medtech companies face when collaborating in Latin America?
U.S. Medtech companies encounter challenges such as professionalism, language barriers, and resource fragmentation that can hinder effective collaboration.
How does urban growth affect clinical research in Latin America?
With over 80% of the population residing in urban areas, clinical research hubs can leverage this demographic shift to enhance participant recruitment and achieve faster recruitment times.
What are the key advantages of conducting clinical research in Latin America?
Key advantages include cost efficiency, diverse patient populations, regulatory advancements, an expanding research framework, market growth, a competitive landscape, and focus on therapy areas such as oncology and immunology.
How much can conducting medical studies in Latin America reduce expenses?
Conducting medical studies in Latin America can reduce expenses by as much as 30% compared to the United States and Europe.
What role do collaborations with organizations like bioaccess® play in clinical research?
Collaborations with organizations like bioaccess® enhance the efficiency of clinical investigations by providing comprehensive study management services, including Early-Feasibility Studies and Post-Market Follow-Up Studies.

