Introduction
The Dominican Republic's healthcare system, characterized by a blend of public and private sectors, presents a unique landscape for clinical research and medical device development. The public sector, predominantly funded by the government, serves a substantial portion of the population despite facing persistent resource constraints and infrastructural challenges. Conversely, the private sector is known for offering more advanced services, catering to those who can afford them.
This dual system significantly influences the scope and execution of clinical research in the country.
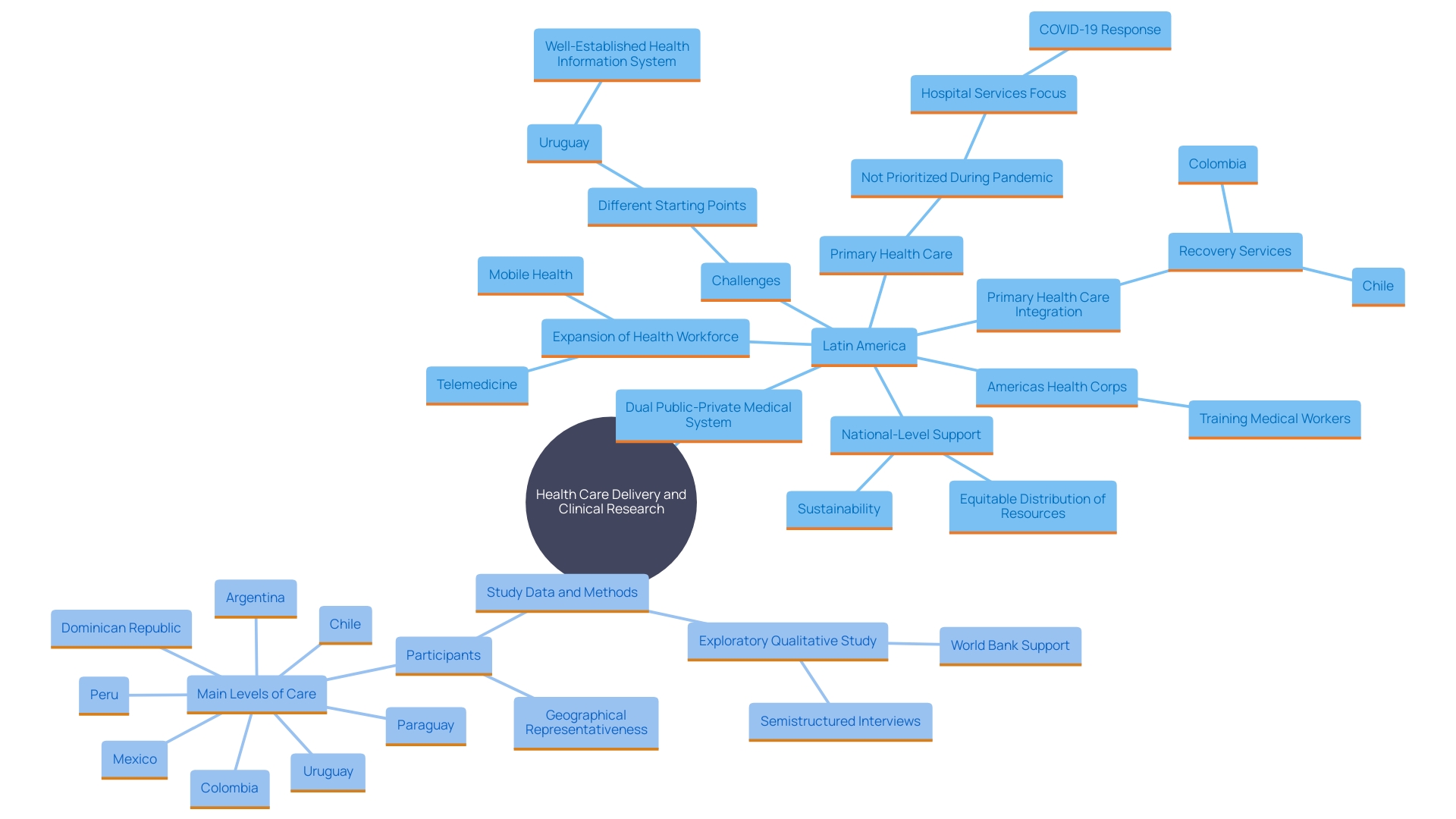
A comprehensive assessment of healthcare systems across Latin America, including the Dominican Republic, highlights prevalent challenges such as limited resources, access barriers, and the need for enhanced service quality. The Dominican Republic's inclusion in studies surveying national healthcare facilities underscores the critical role of primary health care (PHC) in managing public health and individual care efficiently and equitably.
The region's commitment to training 500,000 health workers by 2027, under the Americas Health Corps initiative, emphasizes the necessity for a robust healthcare workforce. This initiative aims to address the uneven distribution of health workers and improve care quality, especially in underserved rural and peri-urban areas.
Given the mixed healthcare system, the Dominican Republic offers distinct opportunities and challenges for clinical research and medtech development. As healthcare needs evolve, focusing on infrastructure improvements, resource allocation, and workforce training will be vital in advancing medical knowledge and patient outcomes in the region.
Overview of the Dominican Republic's Healthcare System
The nation's medical system is a mix of public and private sectors, each contributing significantly to service provision. The public sector, funded by the government, serves a significant portion of the population but often grapples with resource constraints and infrastructural challenges. In contrast, the private sector offers more advanced services, appealing to those who can afford it. This dual system significantly impacts clinical research and medical device development.
A systematic evaluation of medical systems throughout Latin America, including the nation of Dominica, highlights shared difficulties. These include limited resources, access barriers, and the need for improved quality of services. Significantly, the Dominican Republic was involved in a study that examined national samples of medical facilities to comprehend these challenges more effectively. This comprehensive method, involving interviews with various medical system levels, underscores the importance of primary health care (PHC) in managing public wellness and individual care efficiently and equitably.
'The commitment to training 500,000 medical workers in the Latin American and Caribbean region by 2027, as supported by the Americas Health Corps initiative, further emphasizes the need for a robust medical workforce.'. This initiative aims to address the uneven distribution of health workers and enhance the quality of care, particularly in underserved rural and peri-urban settings.
The combined medical system in the country presents unique opportunities and challenges for clinical research and medtech development. As health requirements change, the emphasis on enhancing infrastructure, resource distribution, and workforce education will be essential in progressing health knowledge and patient results in the area.
Advantages of Conducting Medical Device Clinical Trials in the Dominican Republic
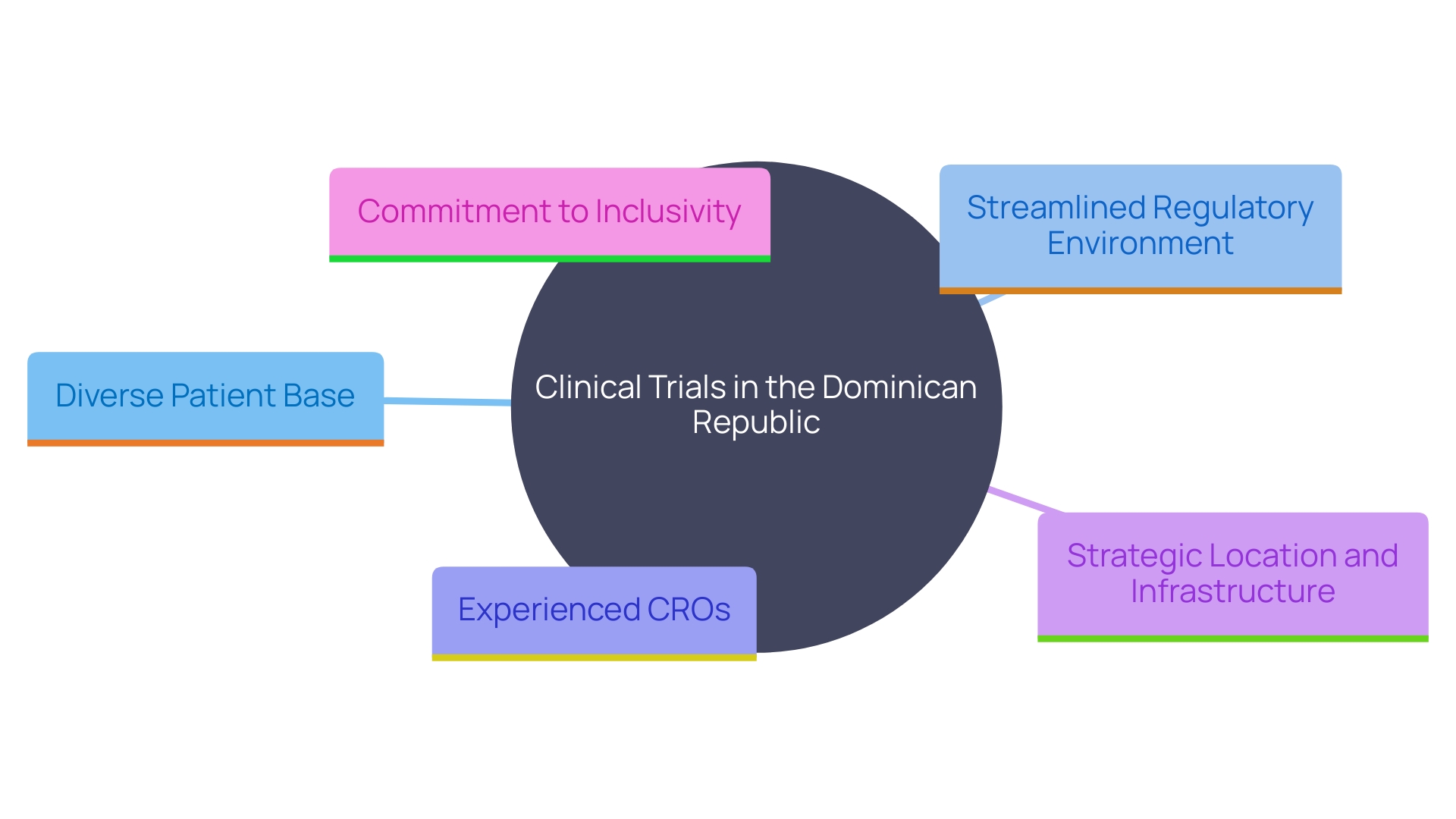
The Dominican Republic offers a multitude of advantages for conducting clinical trials for medical devices. With a growing population experiencing rising medical needs, the country provides a diverse and substantial patient base essential for robust clinical studies. The regulatory environment has become more streamlined, simplifying the process for companies to initiate and conduct trials effectively. This regulatory efficiency is complemented by the presence of experienced Clinical Research Organizations (CROs) and a collaborative atmosphere among healthcare professionals, further enhancing the feasibility and success of clinical trials.
Additionally, the country's strategic location and developed infrastructure make it an attractive destination for global trade and clinical research activities. 'As observed by the Biomedical Research Unit of Profamilia in Santo Domingo, which is pioneering studies on innovative contraceptive implants, the nation is equipped with skilled personnel and essential healthcare resources.'. This environment not only supports diverse medical research but also ensures that clinical trials are conducted with high standards of quality and efficiency.
Furthermore, the nation's commitment to inclusivity and diversity in clinical trial design ensures that research findings are representative and applicable to real-world scenarios. The nation's strategic investments in healthcare infrastructure and human resources highlight its potential as a leading center for clinical trials in Latin America.
Regulatory Framework and Ethical Oversight for Clinical Trials
'The regulatory framework for clinical trials in that country is governed by the National Health System and the Ministry of Public Health, ensuring adherence to international standards and ethical guidelines.'. This framework is critical for maintaining the integrity of research and protecting participant rights. Ethical oversight is provided by institutional review boards (IRBs), which rigorously evaluate study protocols to safeguard participants' welfare. Such comprehensive oversight is essential for researchers and companies aiming to conduct clinical trials in the Caribbean nation, as it aligns with global expectations for ethical research practices. The importance of understanding and navigating this framework cannot be overstated for those looking to contribute to the advancement of medical knowledge within the region.
Challenges and Opportunities in Clinical Research in the Dominican Republic
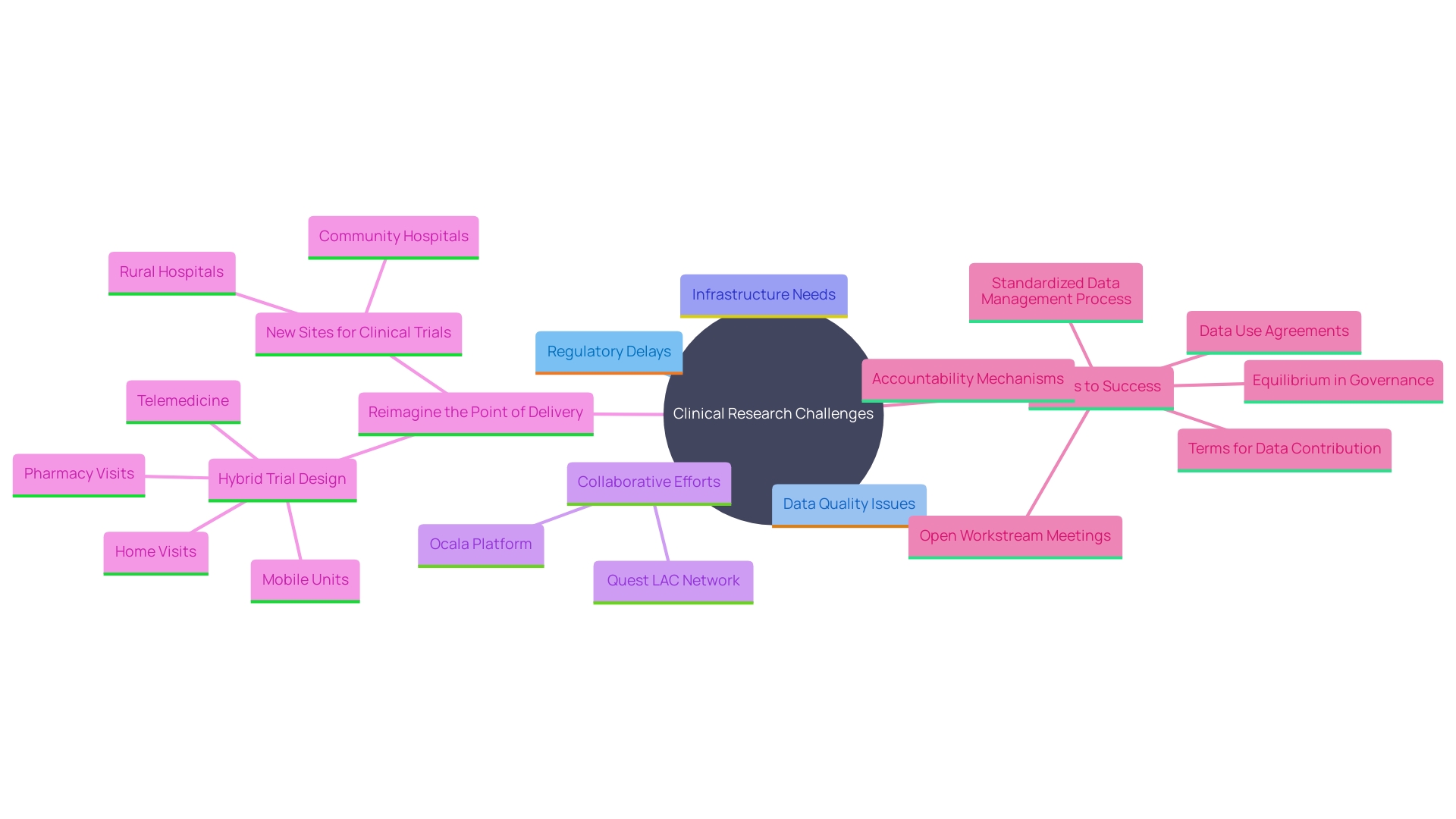
'The potential for clinical research in that nation is significant, yet several challenges continue to impede progress.'. Regulatory delays and variability in data quality are prominent issues that need addressing. Additionally, the need for improved infrastructure is critical for the advancement of research. Tackling these obstacles requires a collaborative effort between local institutions and international stakeholders. For instance, the Ocala platform by the Academy of Sciences of Latin America enhances connectivity among researchers and facilitates the exchange of scientific events and ideas across the region. This kind of partnership can lead to innovative solutions and significantly enhance the research environment. Moreover, the Quality Evidence for The Transformation of Health Systems for Latin America and the Caribbean (Quest LAC) network exemplifies how regional collaboration can build human capacity in health systems research and develop high-quality medical systems. Investing in such networks and platforms can address the existing challenges and foster a thriving research ecosystem in that nation.
Market Trends and Future Prospects for Medical Devices in the Dominican Republic
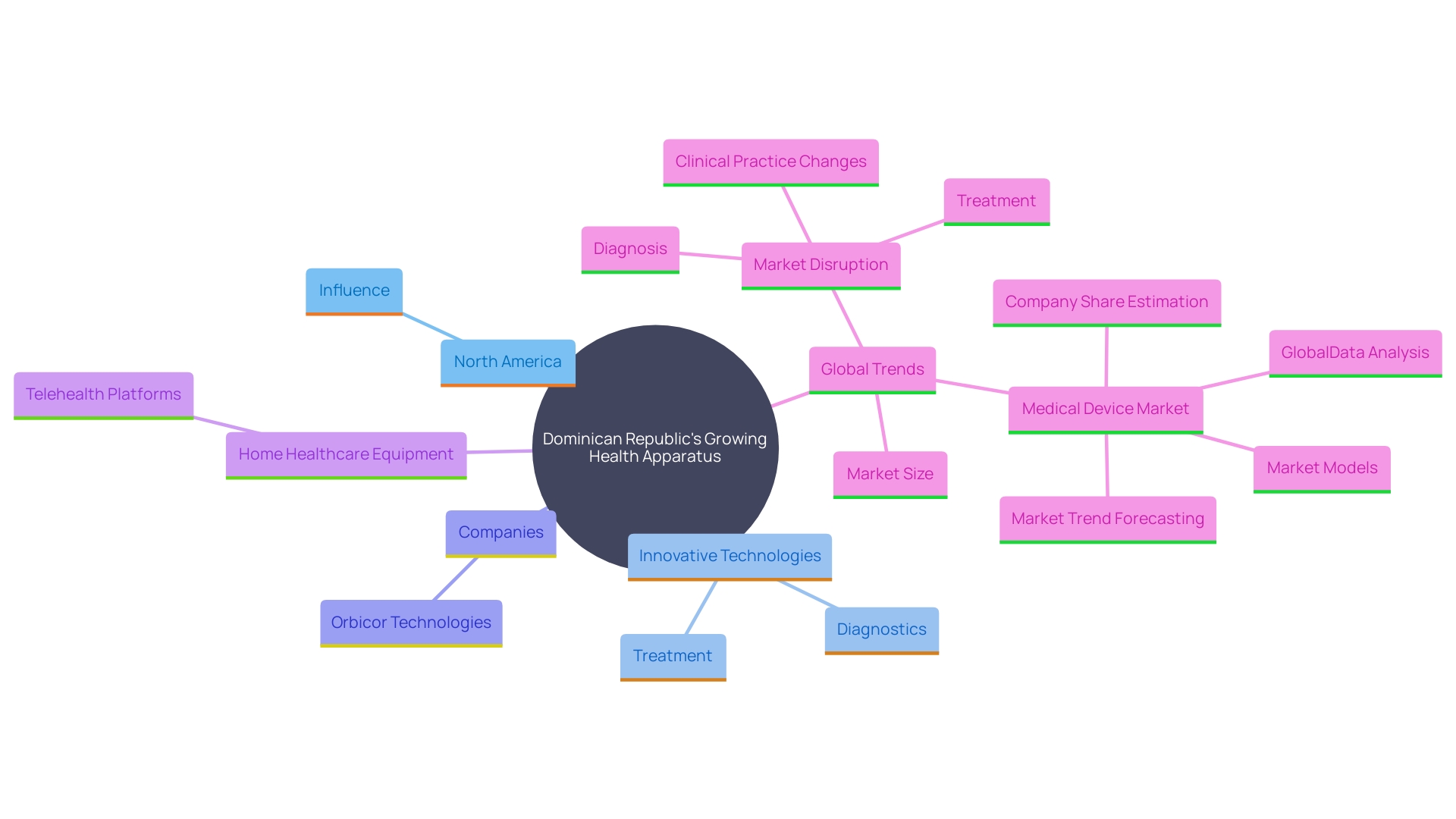
The Dominican Republic is experiencing a growing need for health apparatus, driven by a swiftly developing health sector and considerable investments in health infrastructure. This surge is part of a broader trend observed in the medical device market, notably dominated by North America, which held 39.9% of the total revenue share in 2022. The U.S., with its robust healthcare system and rigorous regulatory framework overseen by the FDA, has been a key player, emphasizing the importance of continued innovation and investment.
In the Dominican Republic, market trends reveal a growing acceptance of innovative technologies, especially in diagnostics and treatment options. This corresponds with international progress where heightened investments in research and development are fueling the creation of sophisticated healthcare devices. For instance, the FDA authorized 54 new wellness devices in 2018 alone, reflecting a steady pace of innovation aimed at addressing unmet health needs.
As the Dominican Republic continues to improve its health services, the outlook for device manufacturers and researchers is encouraging. Firms such as Orbicor Technologies, which creates IoMT (Internet of Medical Things) devices, illustrate the transition towards incorporating advanced technology in healthcare practice. Orbicor’s innovative devices, designed to provide real-time health data, highlight the potential for transformative medical solutions in the region.
This encouraging perspective is further backed by the rising use of home healthcare equipment and the integration of these devices with electronic health records and telehealth platforms. Such advancements ensure continuous monitoring and improved clinical decision-making, ultimately enhancing patient care. The Dominican Republic's commitment to developing its healthcare infrastructure, coupled with global trends in medical technology, underscores a bright future for stakeholders in the medtech sector.
Conclusion
The Dominican Republic's healthcare system, comprising both public and private sectors, offers unique opportunities and challenges for clinical research and medical device development. The public sector serves a significant portion of the population, albeit with resource constraints, while the private sector provides advanced services. Addressing issues such as limited resources and access barriers is critical for enhancing service quality and public health outcomes.
The country’s improved regulatory environment and the presence of experienced Clinical Research Organizations (CROs) make it an appealing location for clinical trials. The commitment to inclusive trial design ensures that research findings are reflective of the diverse patient population, reinforcing the credibility of studies conducted in the region.
Despite these advantages, challenges such as regulatory delays and infrastructure deficits persist. Collaborative efforts between local institutions and international partners are essential to overcoming these barriers and developing a robust research ecosystem. Initiatives like ACALconecta highlight the potential for regional cooperation to advance healthcare research.
The growing demand for medical devices, fueled by an expanding healthcare sector and infrastructure investments, indicates a positive outlook for the medtech industry. The integration of innovative technologies in healthcare aligns with global trends, underscoring the importance of continued investment in research and development. As the Dominican Republic enhances its healthcare capabilities, focusing on infrastructure improvements and workforce training will be vital for advancing medical knowledge and improving patient outcomes in the region.




