Introduction
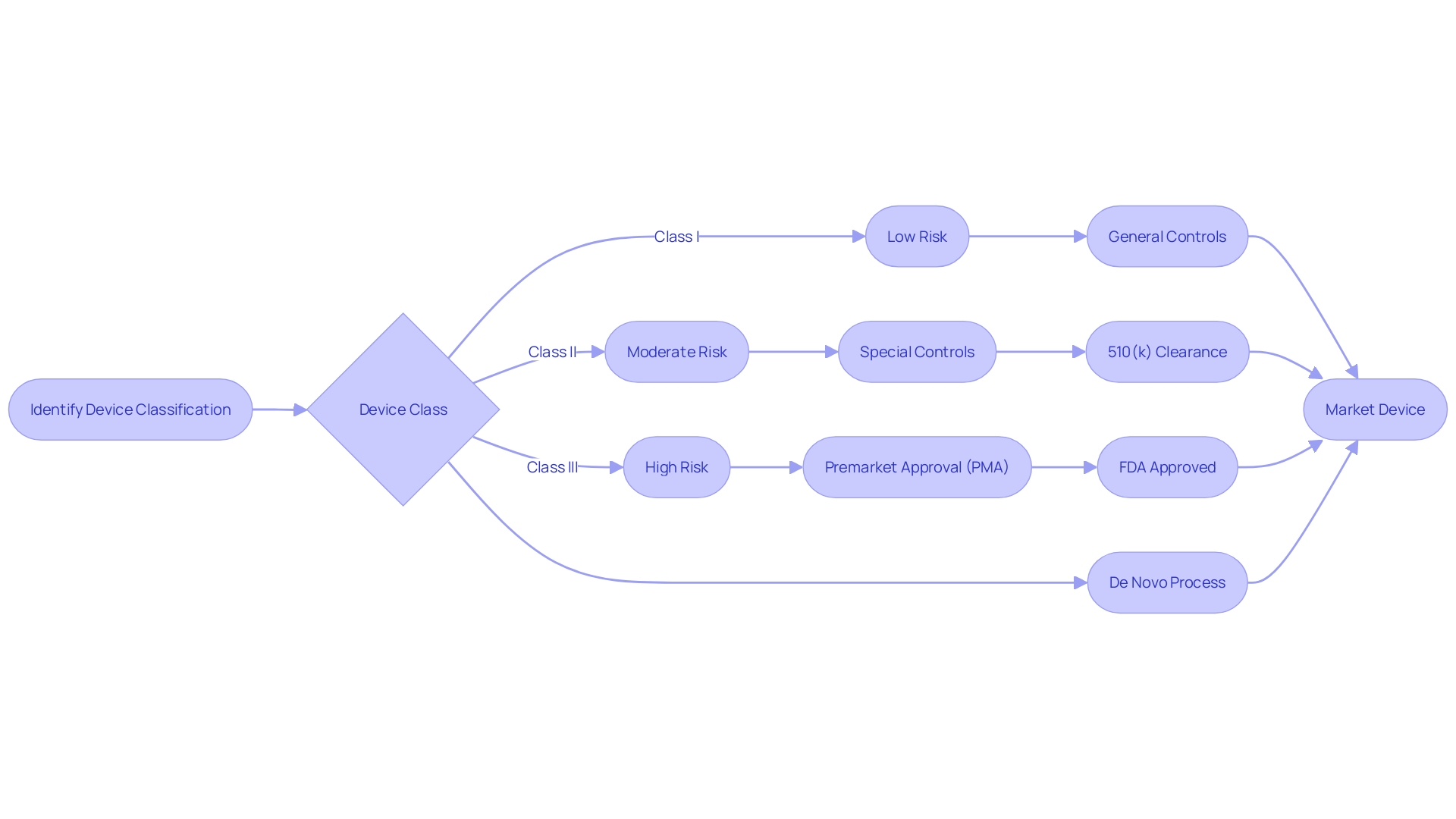
The FDA's device classification and regulatory pathways are crucial for ensuring the safety and effectiveness of medical devices in the United States. Understanding these processes is essential for manufacturers seeking approval for their devices. Medical devices are categorized into three classes based on the level of risk they pose to patients.
Class I devices pose the least risk, while Class III devices pose the highest risk. The classification system plays a vital role in regulatory processes such as the 510(k) premarket notification and Premarket Approval (PMA) pathways. For Class II devices, which involve moderate risk, special controls may be required for regulatory approval.
The FDA's commitment to transparency and patient safety is evident in its adoption of voluntary consensus standards developed by Standards Development Organizations (SDOs). These standards ensure that products, processes, or systems meet the necessary specifications. The FDA actively promotes the use of these standards, enhancing regulatory review efficiency and quality.
The FDA also strives to use consumer-friendly language in its guidelines and publications, making complex information more accessible to the public. Navigating the FDA's device classification and regulatory landscape is crucial for manufacturers, striking a balance between innovation and patient safety.
Understanding FDA Medical Device Classifications
The Food and Drug Administration (FDA) plays a pivotal role in ensuring the safety and effectiveness of medical instruments. Devices are categorized into three classes based on the potential risks they pose to patients, with Class I posing the least risk and Class III the most. This categorization system is a cornerstone in processes, including the 510(k) and Premarket Approval (PMA) pathways.
Class II devices, which involve moderate risk, often require 'special controls' for regulatory approval. For instance, the clozapine test system was recently classified into Class II, with specific controls codified to assure its safety and effectiveness. Such measures not only guarantee patient safety but also expedite access to innovative medical solutions.
The FDA's commitment to transparency and patient safety is evident in the adoption of voluntary consensus standards developed by Standards Development Organizations (SDOs). These standards are established through a method that values openness, balanced representation, and fair treatment. They play a crucial role in conformity assessments—ensuring that a product, process, or system meets the required specifications.
For example, the FDA's Division of Standards and Conformance (DSCA) actively promotes the development and use of these standards. By implementing policies that promote the use of standards, the DSCA enhances the efficiency and quality of review, ultimately fostering innovation and facilitating patient access to novel equipment.
Recent FDA actions, such as the publication of guidelines for clear and neutral presentation of major statements in direct-to-consumer drug advertisements, highlight the agency's dedication to using consumer-friendly language. This approach is designed to make complex information more accessible and understandable to the public.
In summary, comprehending the FDA's classification of equipment and the significance of consensus standards is vital for navigating the regulatory landscape. These components ensure a balance between innovation and patient safety, guiding the 510(k) and PMA procedures towards successful approvals of healthcare equipment.
What is 510(k) Premarket Notification?
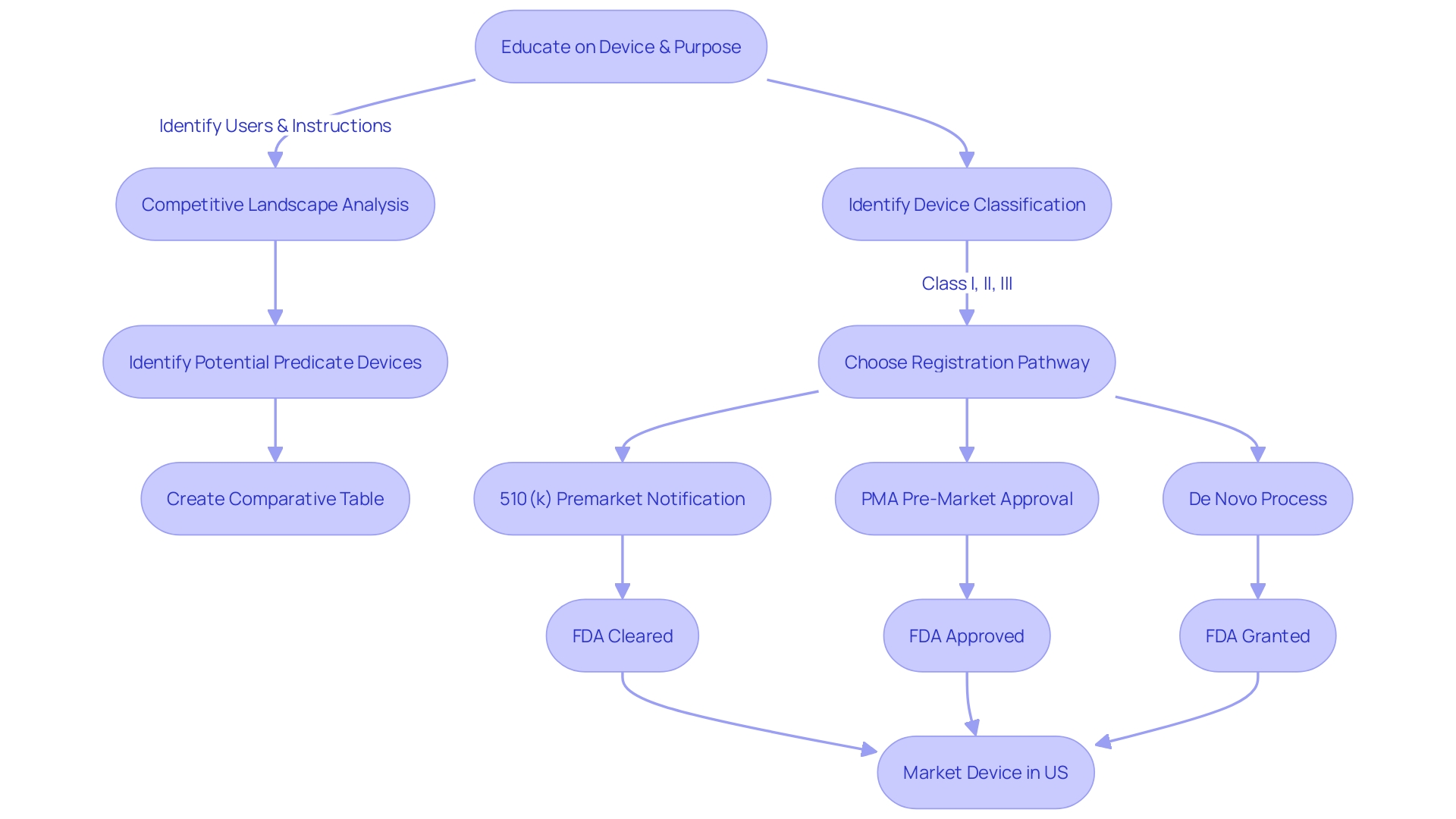
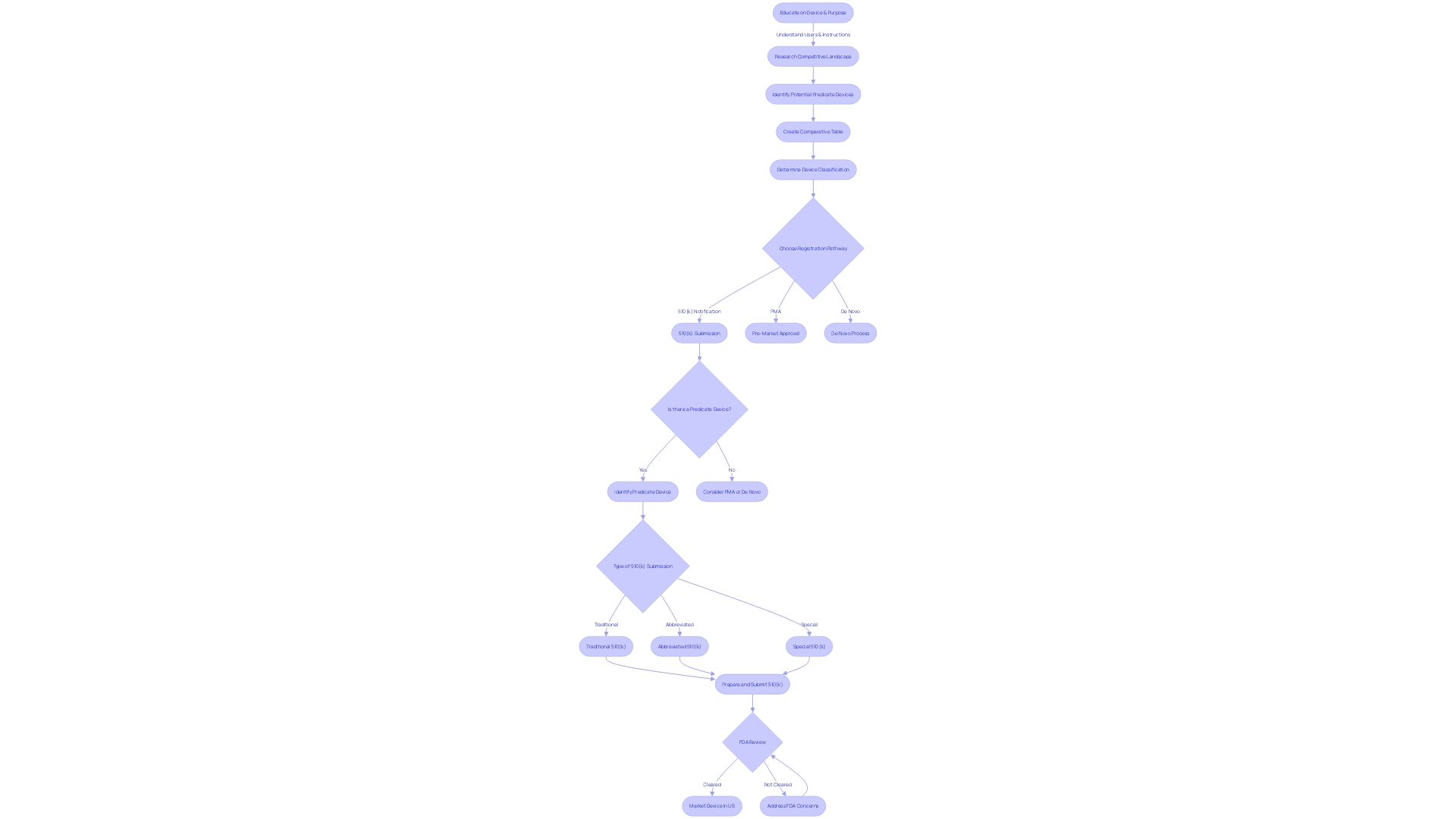
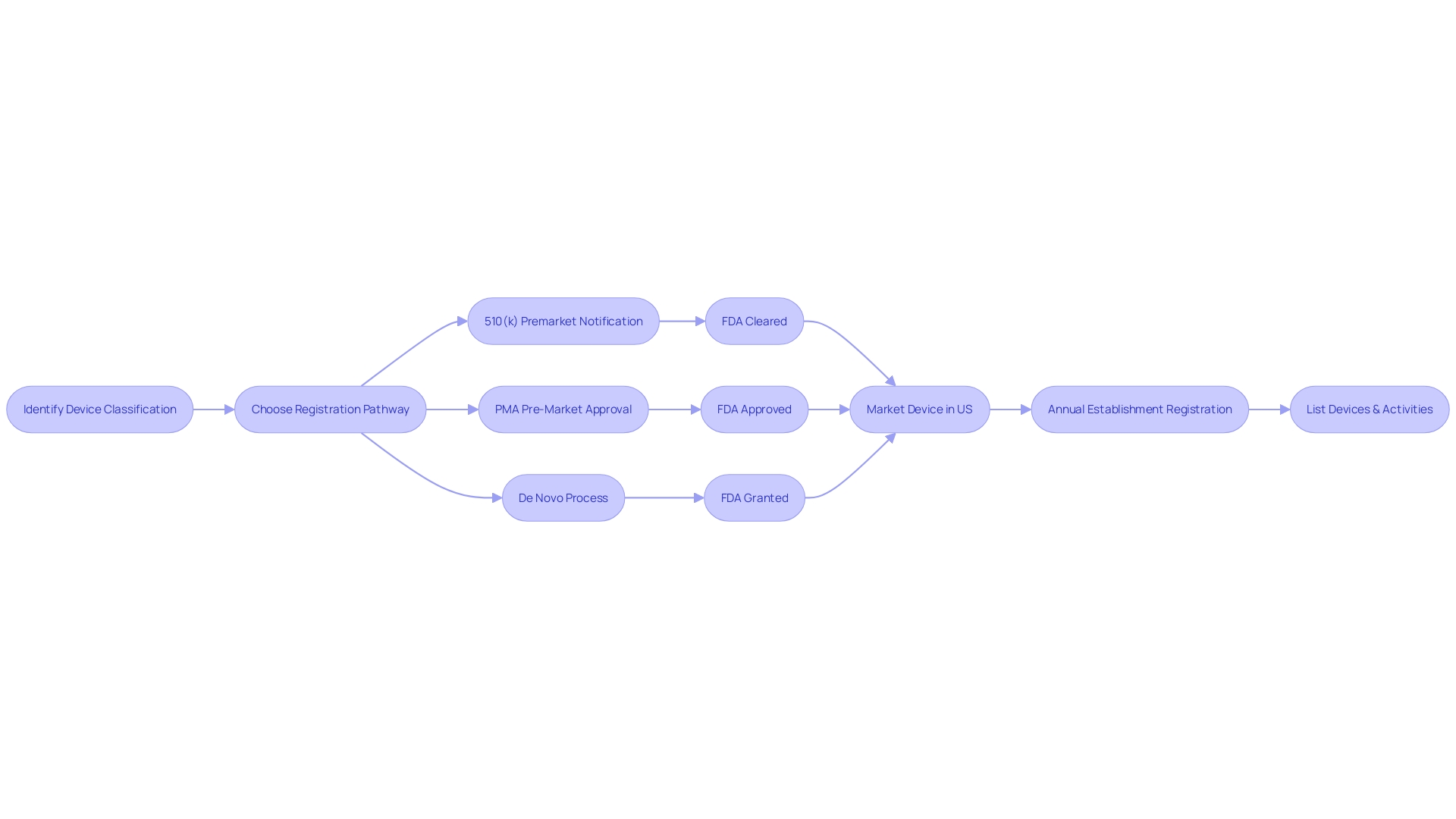
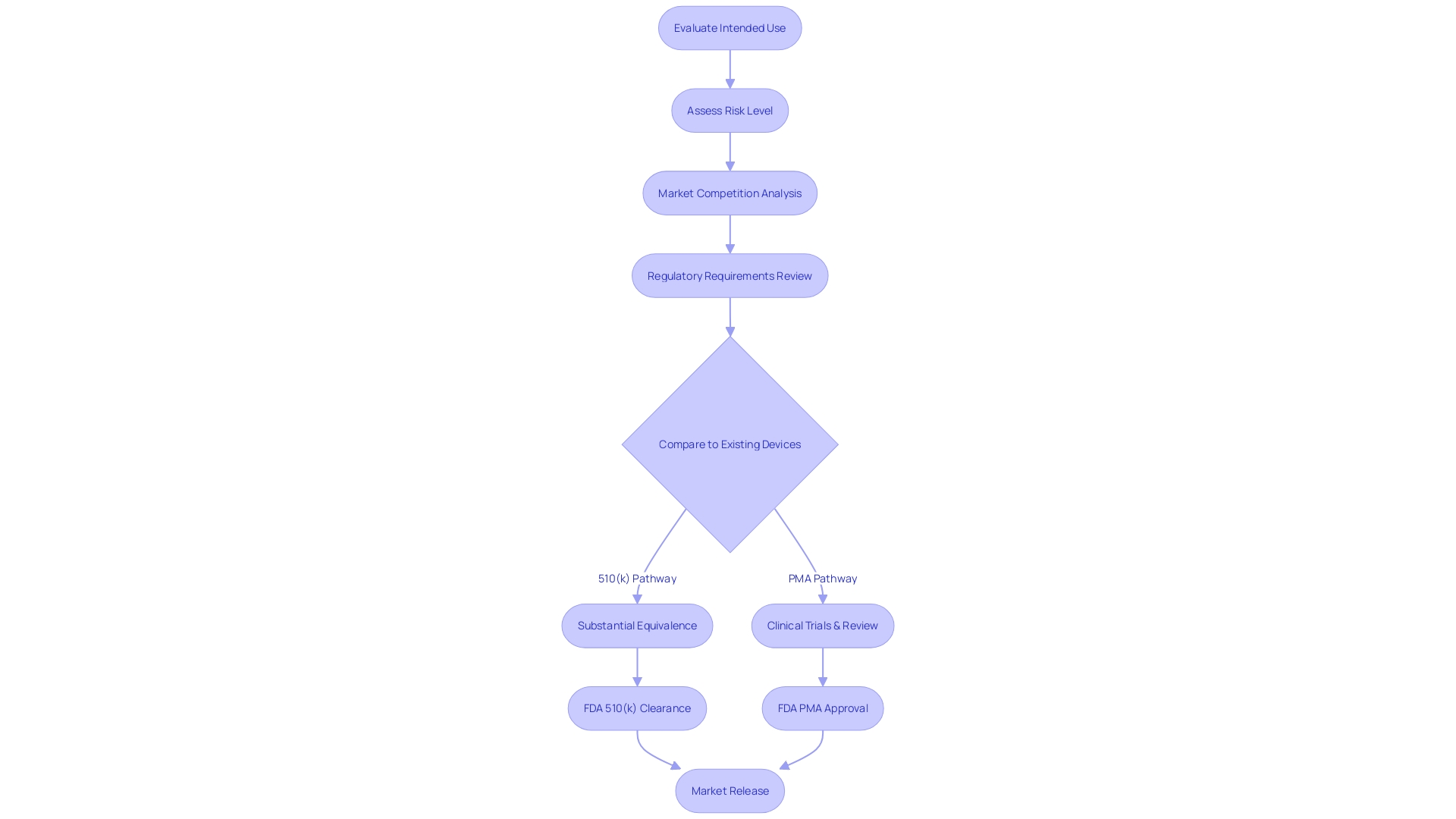
Navigating the 510(k) premarket notification is a crucial step for manufacturers aiming to demonstrate that their new product is substantially equivalent to an already legally marketed item (predicate item) in terms of its intended use, technological characteristics, and performance. The categorization of medical equipment by the FDA into one of three categories, each indicating a distinct level of patient vulnerability, is the inception in this procedure. Determining the accurate categorization guides the selection of the registration pathway, which could be the 510(k) for Class I or II devices, Pre-Market Approval (PMA) for Class III, or the De Novo route for new items that do not fit into an established classification.
To legally market a device in the U.S., it must receive FDA Clearance, Approval, or, in the case of the De Novo pathway, be Granted. The words 'Cleared' and 'Approved' are frequently used interchangeably by professionals in the field, which can result in confusion. Nonetheless, comprehending their differences is crucial as they embody varying degrees of oversight examination and endorsement procedures. The 510(k) pathway, for instance, is typically less rigorous than the PMA pathway and usually does not require clinical trials, which can lead to faster market entry. This was emphasized in the 2018 documentary 'The Bleeding Edge,' which exposed that clinical trials are not obligatory for numerous items cleared through the 510(k) pathway, occasionally leading to patient injuries.
The FDA's commitment to ensuring public health and the safety and effectiveness of instruments means that any public remarks on the regulation process are taken seriously and become part of the public record, highlighting transparency in decision-making. In recent years, there has been a concerted effort to streamline regulatory pathways and enhance cooperation between regulatory bodies and industry players, a movement accelerated by the demands of the COVID-19 pandemic. These initiatives aim to reduce the time for approval for products fulfilling crucial healthcare requirements, especially in growing areas such as digital health and personalized medicine.
Key Features of 510(k) Submissions
The 510(k) submission process is a rigorous pathway requiring manufacturers to present a comprehensive overview of their medical equipment to the FDA. This includes submitting a detailed description of the equipment's intended use and technological characteristics, as well as its performance data and any associated risks. The submission must also include a comparison to an existing, legally marketed item, known as the predicate, to demonstrate substantial equivalence.
During this procedure, manufacturers are responsible for providing a plethora of information, such as the generic and trade names of the equipment, its indications for use, detailed specifications including diagrams or photos, and the functional components or ingredients if the equipment has multiple parts. Each piece of this information serves to establish the apparatus's safety and effectiveness in comparison to the predicate.
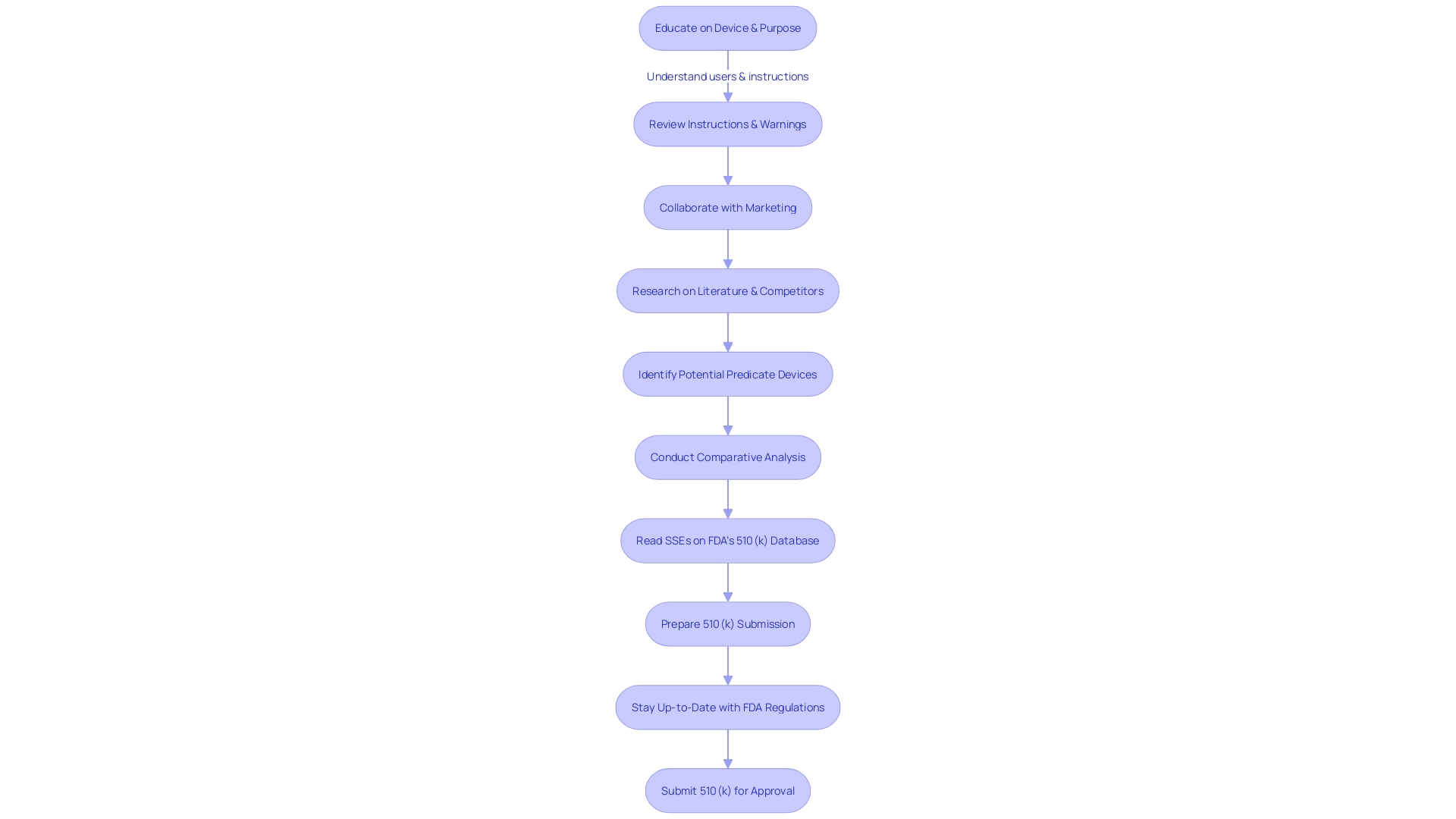
Furthermore, the procedure is vital to guaranteeing that medical professionals, individuals, and other users thoroughly comprehend the device's objective, instructions for use, and safety precautions. The extent of the submission demonstrates the need for comprehensiveness, as emphasized by the founders of Artos through their own experiences with submissions in the life sciences sector. They emphasize that the submissions are not merely bureaucratic paperwork; they are foundational to the FDA's decision-making on how a product can be utilized for patient care.
Understanding the market and competitive landscape is equally essential. Manufacturers must research existing literature, clinical studies, and competitor information to identify potential reference products and construct a comparative analysis table. This practice is not only a regulatory requirement but also a strategic measure to understand the positioning of the new technology within the current market.
The significance of the 510(k) procedure is highlighted by its function in the early identification and prevention of diseases. For example, Everly Health Solutions utilizes advanced diagnostics to combat chronic kidney disease, an area where early detection is critical. This highlights the importance of the 510(k) procedure in introducing new, innovative healthcare tools that can have significant effects on public health.
In summary, the 510(k) clearance is not only about adherence, but about guaranteeing that instruments entering the market are secure, efficient, and capable of delivering the required results for patient care, thus demonstrating the significant duty manufacturers have in safeguarding public health.
Advantages and Challenges of 510(k) Submissions
Mastering the 510(k) medical equipment approval process is intricate, necessitating a comprehensive understanding of the device's intended user base, which includes clinicians, physicians, dentists, and patients. It is also crucial to be knowledgeable about the instructions for use of the apparatus, as well as any associated warnings and cautions. Collaborating with marketing teams to evaluate the competitive landscape is equally important. By conducting thorough research of literature, clinical studies, and competitor information, companies can identify potential predicate instruments that have the same intended use and technological characteristics as the new product. A comparative analysis is then conducted, forming the basis for the 510(k) submission.
The United States Food and Drug Administration (FDA) categorizes medical equipment into three risk-based categories, where class one and two instruments are subject to less stringent regulatory pathways such as the 510(k) clearance. This procedure includes comparing a new apparatus to a present reference apparatus to determine significant similarity. While this pathway is generally faster and more cost-effective than the Pre-Market Approval (PMA) process required for class three items, challenges can arise. For example, identifying a suitable predicate instrument can be challenging, and companies must stay alert regarding the FDA's changing regulations and requirements to ensure successful clearance.
Recent industry trends have shown a drive towards more streamlined regulatory pathways, a movement accelerated by the COVID-19 pandemic. This is particularly evident in the burgeoning sectors of digital health and personalized medicine, where there is a critical need for rapid approval of innovative medical devices. The FDA, committed to safeguarding public health, consistently updates its procedures and guidance to accommodate these advancements while upholding stringent standards for safety and effectiveness. Therefore, it is crucial for companies to remain up-to-date with FDA guidelines and to organize their 510(k) submissions with clarity and precision to facilitate a seamless approval procedure.
What is Premarket Approval (PMA)?
The Premarket Approval (PMA) is the U.S. Food and Drug Administration's (FDA) most rigorous regulatory pathway for medical instruments, especially those that pose a high risk to patients or are novel without a comparable precedent. Devices falling under this category, such as life-sustaining implantables, undergo a comprehensive review process. The PMA pathway mandates a thorough analysis of clinical data to affirm the equipment's safety and efficacy.
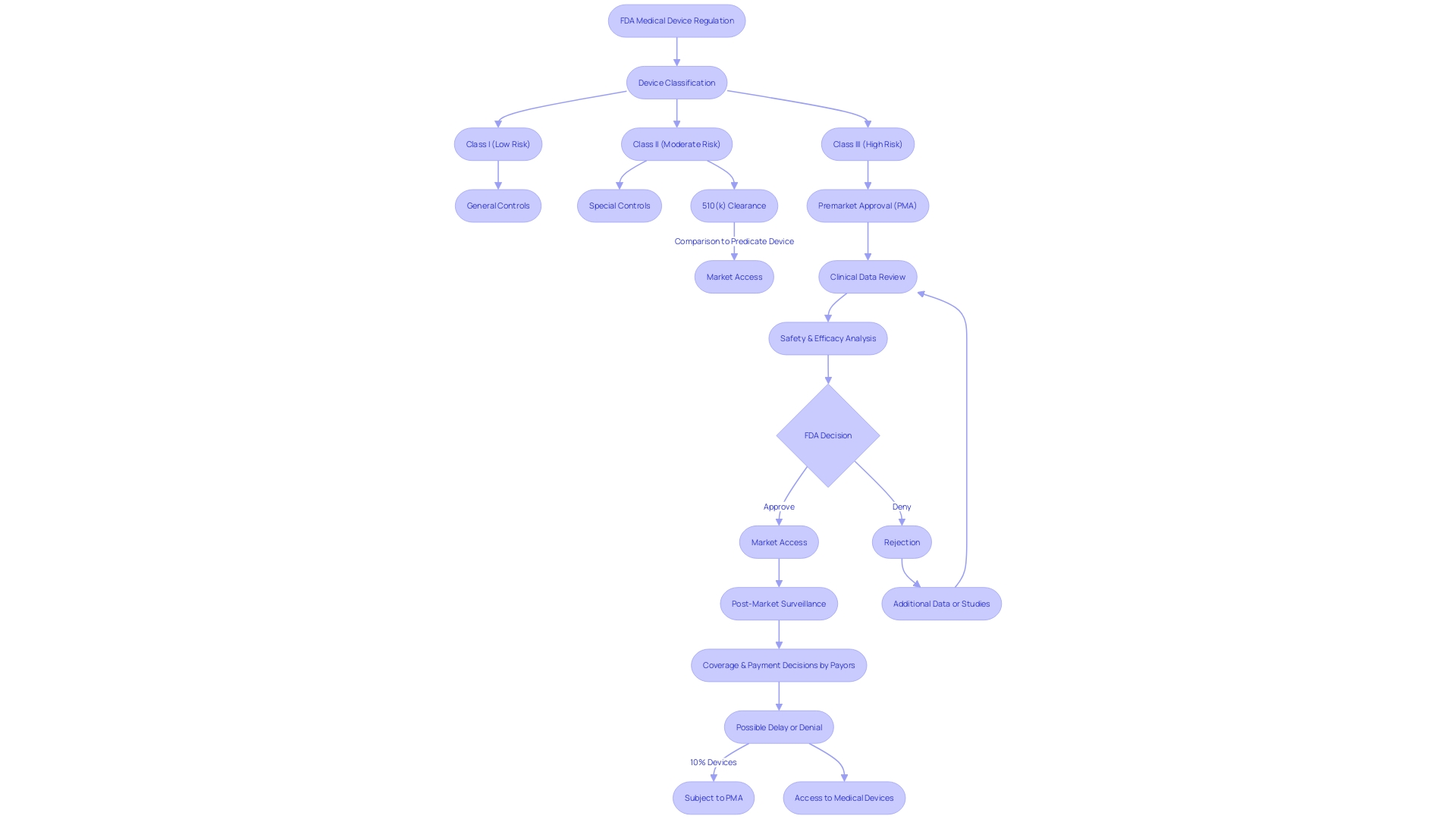
Unlike the PMA, the 510(k) clearance is applicable for class one and two items, which are regarded as lower risk. Such products must show significant similarity to a lawfully marketed model. It is worth mentioning that approximately 10% of devices regulated by the FDA are classified as class three and therefore are subject to the PMA procedure due to their essential role in patient care.
The demand for faster approval processes has increased, especially for products meeting urgent healthcare requirements. The FDA, along with other stakeholders, has been working towards more efficient pathways. This is evident in the realm of digital health and personalized medicine, where rapid innovation is crucial. Although the PMA pathway is rigorous, it is a crucial step in ensuring that high-risk healthcare instruments meet the necessary safety and effectiveness standards before reaching the market.
Key Features of PMA Submissions
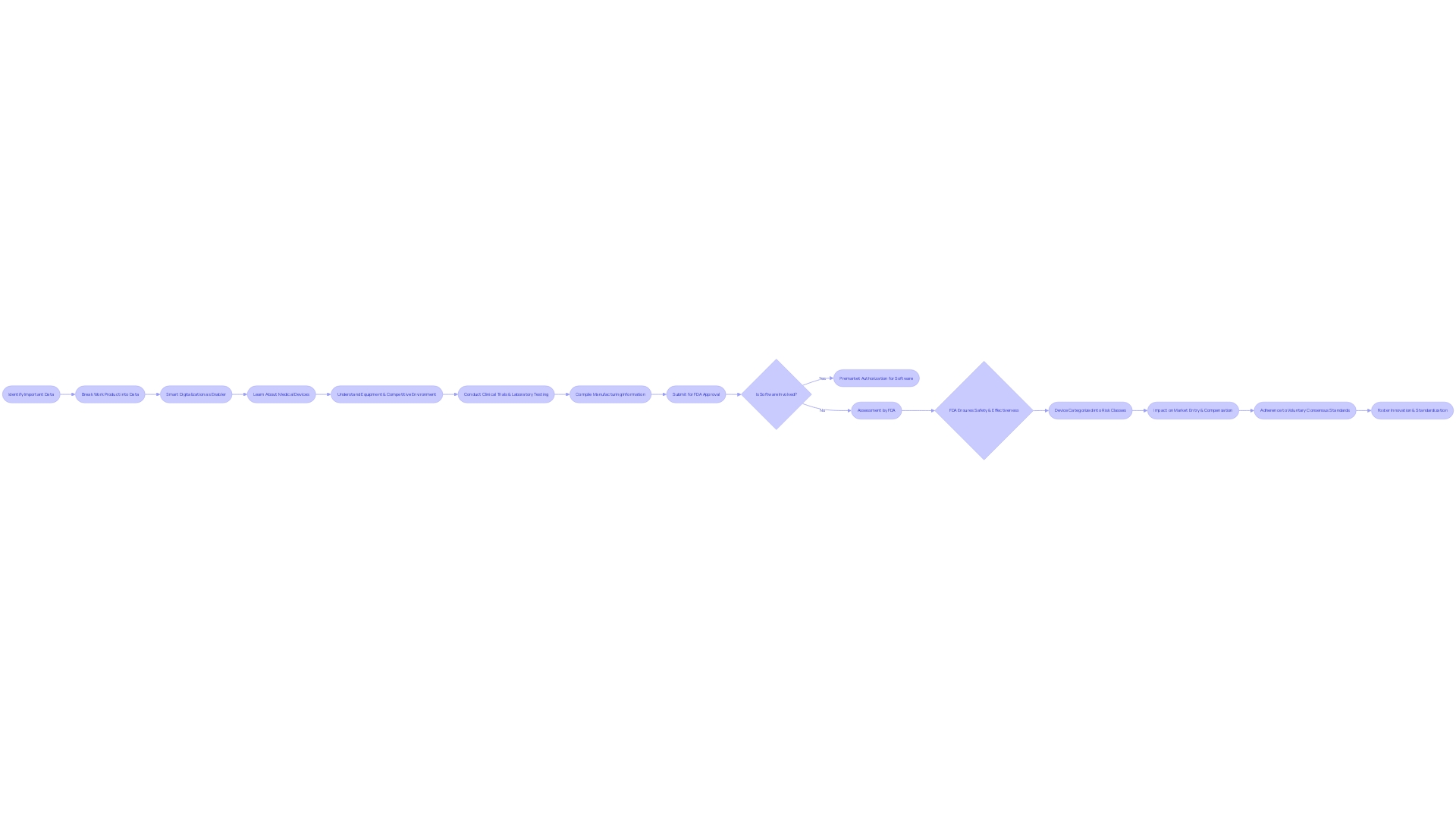
To obtain FDA approval through the PMA route, it is crucial to present a comprehensive range of data, showcasing the safety and effectiveness of the product. This includes data from clinical trials, rigorous laboratory testing, detailed manufacturing information, and any other relevant findings. A case in point is the Impella Connect System, which required premarket authorization for its software functions due to their critical role in monitoring patient-specific medical conditions and issuing timely alarms.
The complexities of the submission process also require a profound comprehension of the equipment, its utilization, and the competitive environment. According to an industry professional, it is crucial to have an understanding of users, instructions for use, and competitor equipment. This is enhanced by creating comparative tables contrasting the new tool with existing predicate tools to substantiate equivalence.
The FDA's responsibility goes beyond solely regulating devices, as it includes ensuring the safety and effectiveness of a wide range of products. Recently, the FDA released guidelines for direct-to-consumer prescription drug advertisements, emphasizing the clear and neutral presentation of information. This underscores the agency's commitment to transparency and consumer understanding across all areas of public health.
Categorized into three classes according to risk, the PMA procedure is usually reserved for Class III items, which consist of life-sustaining implants such as pacemakers. These instruments constitute approximately 10% of the FDA-regulated healthcare products and undergo a more rigorous evaluation due to their high-risk characteristics.
The PMA procedure is not only concerned with meeting regulations; it also has substantial impacts on market entry and compensation. As discussed during the 2024 MedExec Women Conference, early attention to reimbursement and internal collaboration is crucial for efficient evidence gathering, bridging legal and reimbursement paths, and ultimately ensuring patient access to innovative medical technologies.
Data demonstrates the crucial importance of voluntary consensus standards in the governing structure, with transparency, openness, balance of representation, and fair procedure being fundamental principles. Such standards foster innovation and standardization, facilitating patient access to new equipment while ensuring regulatory quality and global harmonization.
Advantages and Challenges of PMA Submissions
Navigating the journey towards medical equipment approval, especially for high-risk Class III devices, requires a comprehensive understanding of the PMA (Pre-market Approval) process. This rigorous pathway is essential for instruments that sustain or support life, such as pacemakers, which account for roughly 10% of the FDA-regulated devices. In contrast to the 510(k) clearance for Class I and II gadgets that compares new items to existing ones, the PMA pathway has no such predicate comparison and is often the only viable route for innovative products.
The PMA approach is remarkable because it requires substantial clinical evidence to demonstrate safety and efficacy, which in turn ensures patient safety and product quality—top priorities for project managers in the healthcare technology sector. However, it's a pathway marked by complexity and demands considerable investment in both time and resources. Biomedical professionals, such as Chris with 13 years of experience in managing Class III studies, can attest to the intricate nature of PMA studies and the importance of thorough clinical data.
Despite the challenges, the ultimate goal remains clear: improving patient outcomes and quality of life. With the evolution of the industry related to healthcare tools, with advancements in digital health and personalized medicine, there has been a concentrated push towards more streamlined regulatory procedures. Such initiatives aim to reduce the time it takes to approve technologies that address crucial health requirements, a movement accelerated by the worldwide urgency of the COVID-19 pandemic.
The PMA process, while daunting, is a testament to the commitment to patient-first principles, underscoring the importance of human factors in project decision-making. With oversight from agencies such as the FDA and EMA, the industry is constantly adjusting to ensure that the products introduced to the market meet the highest standards of safety and effectiveness.
Comparing 510(k) and PMA: Key Differences
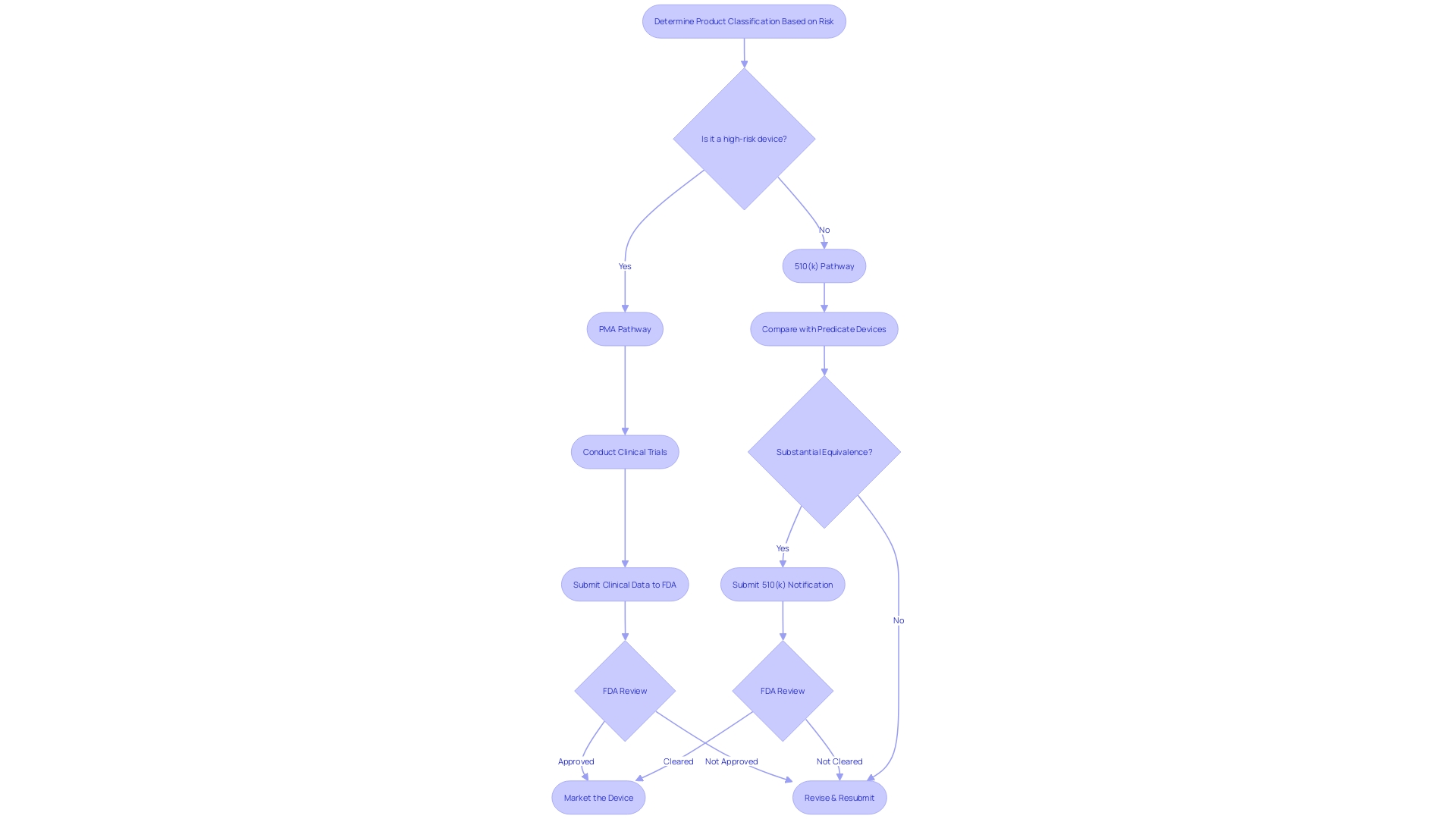
When navigating the regulatory landscape for medical equipment in the United States, comprehending the nuances between the 510(k) and PMA (Premarket Approval) pathways is crucial. The 510(k) process, created for products that are significantly similar to current products (referred to as predicate products), demands a comparative analysis rather than extensive clinical trial data. This involves a thorough review of the competitive landscape, including research literature, clinical studies, and existing marketing materials to identify potential reference products that share the same intended use and technological characteristics.
The PMA pathway, on the other hand, is reserved for high-risk class III equipment that often play a crucial role in sustaining or supporting life. Considering the notable risks linked to these instruments, the PMA procedure requires strong clinical trial data to exhibit safety and effectiveness. The decision between these two pathways depends on the complexity of the apparatus, its classification by the FDA, and the inherent patient risk it carries. The FDA's classification system, which assigns products to one of three regulatory classes based on risk, serves as a starting point for determining the appropriate pathway.
It is crucial to highlight that although the 510(k) procedure can occasionally expedite the accessibility of healthcare instruments, it does not consistently necessitate clinical trials, which has resulted in examination and apprehension concerning patient safety. This was emphasized in the 2018 documentary 'The Bleeding Edge,' which uncovered cases where apparatuses cleared through the 510(k) pathway caused patient harm. Experts in regulation and key players in the industry have been promoting the use of more efficient pathways and improved cooperation to accelerate the time it takes to approve products, especially those that address unmet medical needs in fields such as digital health and personalized medicine.
In brief, the decision between 510(k) and PMA pathways requires a strategic evaluation of the classification of the product, the amount of evidence accessible, and the requirements for establishing substantial equivalence or safety and efficacy. The FDA's ongoing efforts to ensure the clarity and accessibility of information related to regulations, as evidenced by their recent rules on direct-to-consumer advertisements, reflect the importance of transparency and consumer understanding in the approval process.
Choosing Between 510(k) and PMA: Strategic Considerations
Understanding and maneuvering through the rules and requirements for approving medical equipment necessitates thoughtful evaluation of different elements. Manufacturers must critically assess the intended use, inherent risk level, existing market competition, and regulatory demands of the product. The financial and temporal investment required for the approval procedure is also a critical determinant, with the 510(k) pathway often being more cost-effective and timely than the Pre-Market Approval (PMA) pathway.
Acquiring a strong comprehension of the apparatus and its competitive surroundings is vital, as indicated by the practice of contrasting the new equipment to analogous, previously sanctioned devices. Developing a thorough side-by-side chart assists in this examination and is a crucial stage toward pinpointing a suitable predicate apparatus that is in line with the FDA's 510(k) procedure.
The 510(k) approval system has been examined for its capacity to expedite devices without necessarily necessitating clinical trials, as emphasized in the 2018 documentary 'The Bleeding Edge.' This has led to debates about the sufficiency of safety and effectiveness data for equipment approved via this route.
Nevertheless, successful navigation of the approval process can lead to various forms of positive business outcomes, including acquisitions, IPOs, licensing agreements, or strategic partnerships, all of which have been historically beneficial for medical device startups. Engaging with regulatory experts and conducting a thorough risk analysis are prudent steps to ensure a well-informed decision when choosing between the 510(k) and PMA pathways, ultimately contributing to the company's potential for a successful exit.
Conclusion
The FDA's device classification and regulatory pathways are crucial for ensuring the safety and effectiveness of medical devices in the United States. The classification system categorizes devices based on risk level, guiding regulatory processes like the 510(k) premarket notification and Premarket Approval (PMA) pathways. The FDA's commitment to transparency and patient safety is seen in its adoption of voluntary consensus standards from SDOs, enhancing regulatory efficiency and quality.
The 510(k) process is vital for manufacturers seeking device approval, requiring substantial equivalence to a legally marketed device. The choice between the 510(k) and PMA pathways depends on factors like device classification, evidence availability, and regulatory requirements. The PMA pathway is the most rigorous, ensuring safety and efficacy for high-risk devices.
Navigating these pathways demands comprehensive device information, including intended use, technological characteristics, performance data, and associated risks. Thoroughness is crucial for establishing safety, effectiveness, and user understanding.
Understanding the FDA's device classification and regulatory landscape is essential for manufacturers to balance innovation and patient safety. By successfully navigating these processes, manufacturers contribute to improving public health.
In conclusion, the FDA's device classification and regulatory pathways are vital for ensuring device safety and effectiveness. Manufacturers must consider risk levels, evidence availability, and regulatory requirements when choosing between the 510(k) and PMA pathways. Providing comprehensive device information is crucial.
By navigating these processes effectively, manufacturers contribute to innovation and patient safety, ultimately enhancing public health.




