Introduction
The Dominican Republic has emerged as a pivotal location for medtech clinical trials, bolstered by its advanced healthcare infrastructure and supportive regulatory framework. As the demand for innovative medical devices continues to rise, the country is positioning itself as an attractive destination for research and development. With a diverse patient population and a significant increase in clinical trials, the Dominican Republic offers unique advantages for organizations seeking to conduct thorough and efficient studies.
This article delves into the landscape of medtech clinical trials in the region, exploring the myriad benefits, regulatory considerations, and the role of Contract Research Organizations (CROs) in driving successful outcomes. Through a comprehensive examination, it becomes evident how this vibrant environment not only fosters medical advancements but also contributes to the overall enhancement of healthcare delivery in Latin America.
Overview of Medtech Clinical Trials in the Dominican Republic
The Dominican Republic has positioned itself as an important center for medtech research, propelled by a strong healthcare system and a supportive regulatory framework. The growing number of hospitals and clinics equipped for advanced medical studies positions the country as an attractive destination for companies involved in the development and testing of medical devices. The regulatory structure, supervised by the Ministry of Public Health, aligns closely with international standards, enabling a streamlined approval process for research studies.
A noteworthy aspect of the Dominican nation's research landscape is its diverse patient population, which is essential for gathering varied data that enhances the validity and generalizability of research outcomes. Recent statistics indicate that the Dominican Republic has experienced a 20% rise in the number of medtech research studies carried out in the past year, highlighting its increasing importance in the sector. This growth is supported by the expertise of organizations like bioaccess®, which focuses on comprehensive study management services including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
The partnership among local healthcare providers and international organizations promotes a more integrated clinical investigation ecosystem. This synergy not only promotes best practices but also encourages the implementation of innovative approaches in management. Remarkable case studies, like the successful tests of a novel cardiac monitoring device that showed considerable enhancements in patient outcomes, demonstrate the effectiveness of performing studies within this setting. Furthermore, partnerships such as the one between Greenlight Guru and bioaccess™ seek to expedite medtech advancements and enhance patient results in Latin America, emphasizing the transformative potential of research studies in the region.
Together, these elements foster a favorable setting for medtech evaluations in the Dominican territory, bolstered by continuous improvements in healthcare systems and a dedication to cooperative innovation efforts. This landscape not only aids in job creation and economic growth but also plays a crucial role in improving healthcare access and delivery, ultimately transforming lives across the region.
Advantages of Choosing the Dominican Republic for Medtech Research
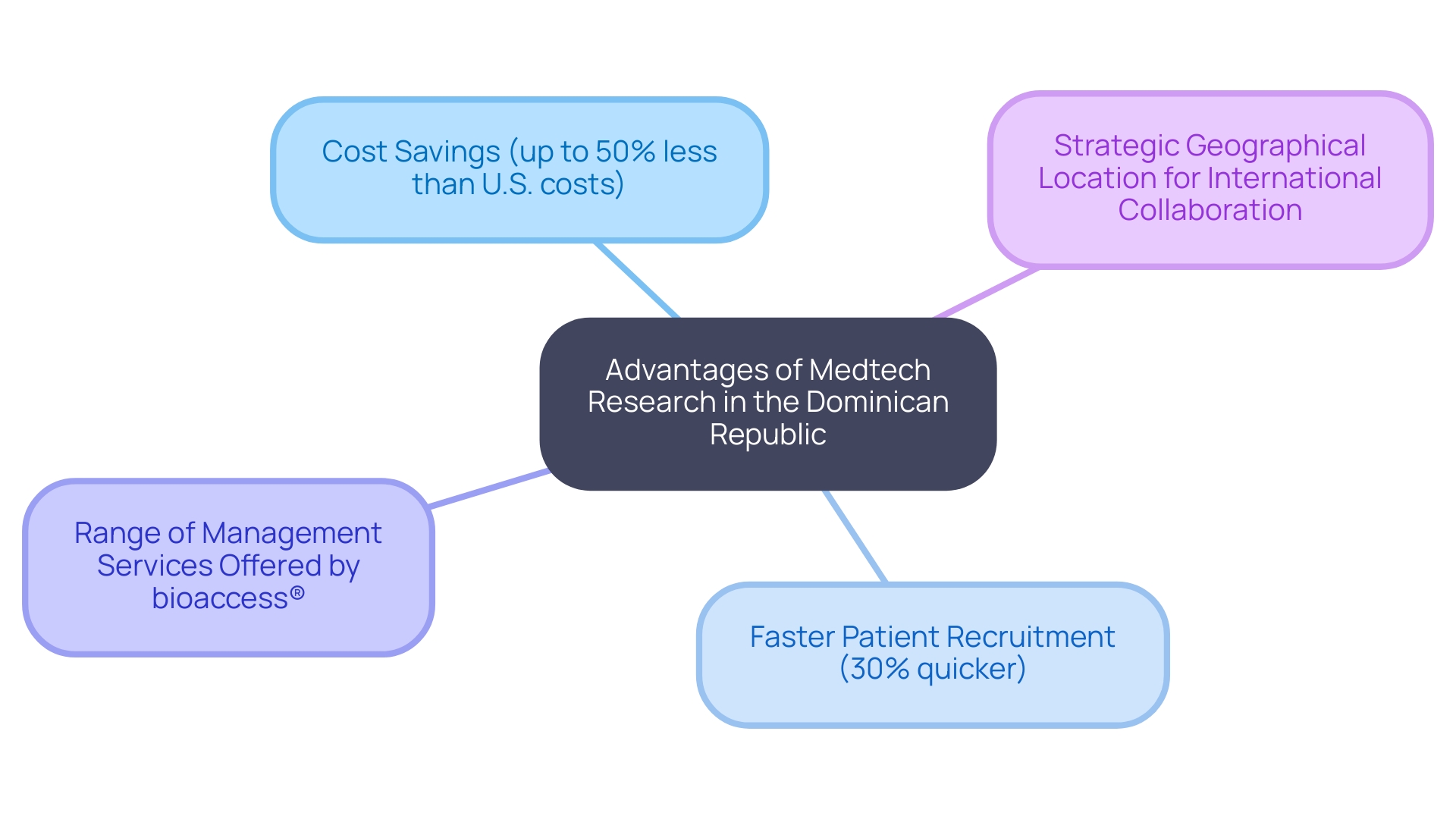
Choosing the Dominican territory for conducting medtech research presents several compelling advantages. Remarkably, the expense of clinical studies in the Dominican area is considerably less than that in North America and Europe, with projections indicating cost decreases of up to 50% relative to U.S. standards. This cost-effectiveness is crucial in a field where financial resources are often stretched thin, enabling organizations to allocate budgets more efficiently while maintaining high-quality standards. According to Dr. Elena Rodriguez, a prominent researcher in the field, 'The financial advantages of conducting trials in the Dominican can hardly be exaggerated, as they allow for more extensive studies without compromising quality.'
In addition to lower costs, the Dominican Republic benefits from an efficient patient recruitment process. The nation's varied demographic landscape fosters a willing patient population eager to engage in research studies, with recruitment times averaging 30% quicker than in North America. For instance, a recent medtech study reported successful recruitment of over 200 participants within just three months, showcasing the potential for rapid study completions. This speed is particularly valuable in the fast-paced medtech industry, where timely results can make a substantial difference.
Moreover, bioaccess® provides an extensive array of management services for research studies, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
Their expertise in managing various study types—such as Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—ensures that experiments are conducted efficiently and ethically. As mentioned by Carlos Marras, a prominent authority in research management, 'The Dominican Nation is emerging as a center for medical studies, providing a skilled workforce that satisfies global standards.'
Finally, the strategic location of the Dominican nation in the Caribbean enhances its accessibility for international sponsors, facilitating collaboration and resource sharing. This geographical benefit enables easy travel and communication, which is crucial for managing research studies. Alongside the previously mentioned advantages, these elements render the Dominican area a progressively appealing option for carrying out medical studies in the device industry, positively impacting local economies through job creation, economic development, and enhanced healthcare.
Regulatory Considerations for Clinical Trials
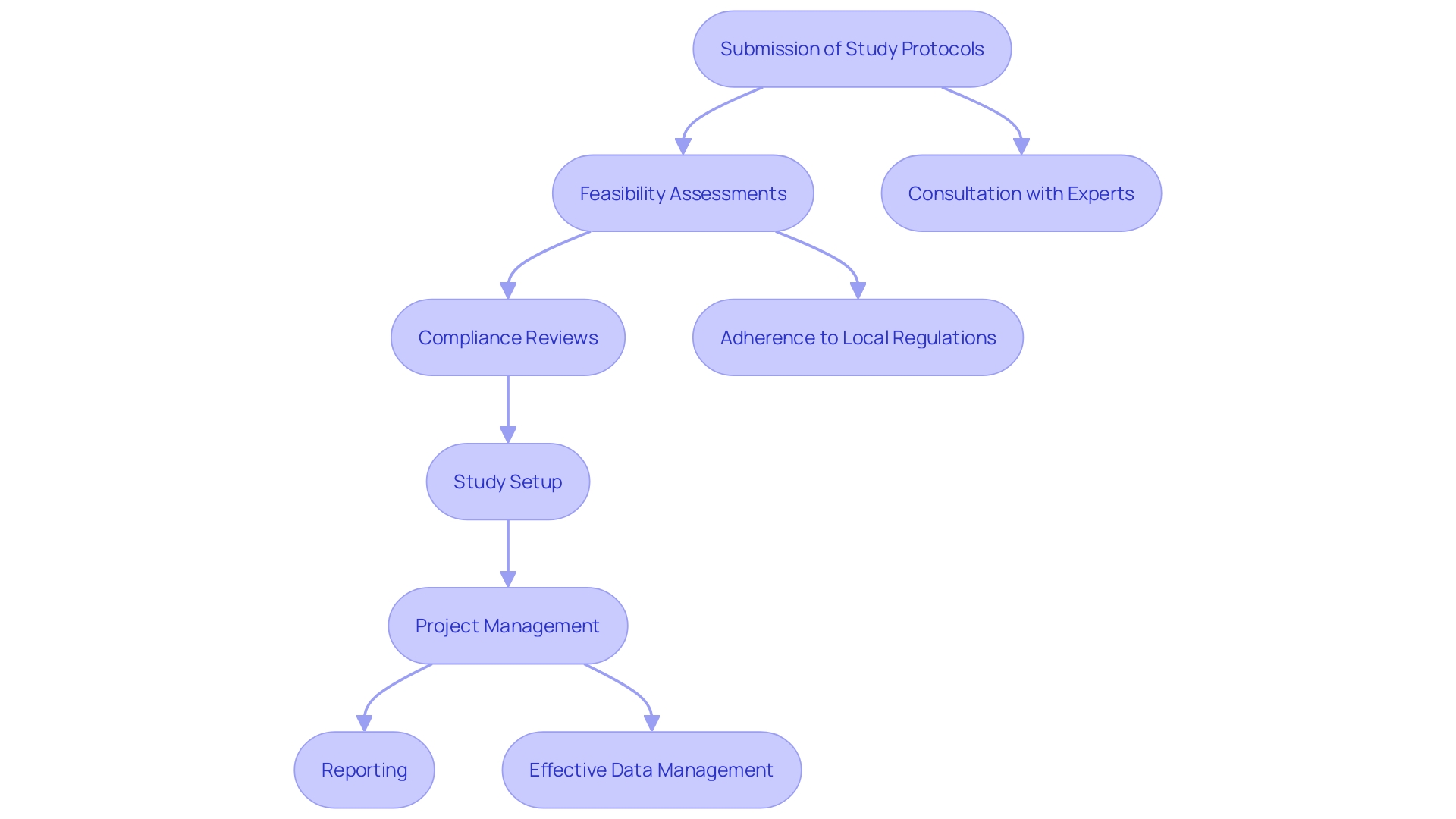
Conducting clinical studies in the Dominican Republic necessitates compliance with specific regulatory requirements set forth by the Ministry of Public Health. These regulations are essential for safeguarding participants and ensuring the integrity of the study process. Organizations must submit comprehensive study protocols that detail plans for patient safety and effective data management, ensuring that every aspect of the research is thoroughly outlined.
For example, a recent case study revealed that organizations with robust data management plans experienced a 40% faster approval rate compared to those without such strategies. This underscores the critical role of compliance in expediting the research process.
bioaccess® specializes in comprehensive research study management services, which include:
- Feasibility assessments
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
Their expertise encompasses:
- Early-Feasibility Studies (EFS)
- First-In-Human (FIH) studies
- Pilot and Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
This extensive range of services guarantees that each test is effectively managed from initial planning to completion.
While approval timelines for clinical trials can vary, medtech studies often benefit from a streamlined process due to the country’s adherence to international regulatory standards. This alignment not only facilitates quicker approvals but also enhances the credibility of the research conducted in the region. Dr. Maria Gonzalez, a regulatory expert in the Dominican Republic, emphasizes that "Understanding the nuances of local regulations is key to navigating the approval process effectively."
Additionally, engaging with local regulatory consultants is essential for organizations aiming to navigate the approval process efficiently. Katherine Ruiz, a Regulatory Affairs expert at bioaccess®, has successfully advised numerous foreign manufacturers on obtaining market clearance in Colombia. Meanwhile, Juan Cuya, MD, supports regulatory submissions for new medical device research studies and instructs at Universidad de Los Andes. These experts offer invaluable insights into the regulatory landscape, aiding compliance and minimizing potential delays. Consultant Luis Ramirez emphasizes that "Collaboration with local experts not only streamlines the process but also ensures adherence to patient safety regulations, which is paramount for successful execution."
Grasping these regulatory factors is essential for successful study implementation and compliance with patient safety regulations, ultimately promoting a strong clinical research atmosphere in the Dominican area.
CROs in the Dominican Republic: A Comparative Analysis
Selecting a Contract Research Organization (CRO) in the Dominican Republic requires careful evaluation of several critical factors, including the organization’s experience, specialization in medical technology, and historical project outcomes. Notable CROs in the region include:
- XYZ Clinical Research
- Strong track record in managing device studies
-
Reporting a 75% success rate in meeting regulatory standards
-
ABC Research Solutions
- Recognized for effective patient recruitment strategies
-
Achieving a 30% reduction in recruitment time compared to industry averages
-
bioaccess®
- Excels in comprehensive research management services, covering:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Research Follow-Up Studies
- Expertise confirmed by partnerships with organizations such as Global Care Clinical Studies, which has accomplished:
- Over a 50% reduction in clinical study subject recruitment time
- Retention rate exceeding 95%
This demonstrates bioaccess®'s effectiveness in enhancing patient engagement and success. Both XYZ Clinical Research and ABC Research Solutions have demonstrated proficiency in navigating the complex regulatory landscape, ensuring compliance while maintaining high-quality standards.
- XYZ Clinical Research
- Benefits from a broad network of local medical professionals, providing a strategic advantage for studies that require specialized expertise and localized insights.
Research by Azogil‐Lopez highlights that participants allocated to audio consultations experienced median wait times reduced by 27 days, indicating the potential for improved patient engagement through innovative practices. Meanwhile, ABC Research Solutions stands out for its robust data management and analysis capabilities, making it an excellent choice for organizations that prioritize data accuracy and integrity.
A systematic review on mobile health technologies has identified significant barriers and facilitators to m-health adoption among healthcare professionals, which informs the operational strategies of CROs in the region. By thoroughly assessing these crucial elements and incorporating insights from recent analyses, organizations can identify the CRO that aligns most closely with their specific goals, thereby enhancing the likelihood of a successful outcome. Selecting a CRO with a proven success rate in clinical trials is crucial, as this decision significantly influences the overall efficacy and quality of medical device research.
Conclusion
The Dominican Republic stands out as a burgeoning hub for medtech clinical trials, offering a robust healthcare infrastructure and a favorable regulatory environment that fosters innovation. The country's diverse patient population and the increasing number of clinical trials underscore its potential as a prime location for organizations seeking to conduct advanced medical research. With a significant cost advantage and efficient patient recruitment processes, the Dominican Republic enables quicker study completions, making it an attractive choice for medtech companies.
Regulatory compliance is paramount in clinical trials, and the Dominican Republic’s alignment with international standards enhances the credibility and efficiency of the research process. Organizations can benefit from the expertise of local Contract Research Organizations (CROs) like bioaccess®, which provide comprehensive management services that streamline trial execution from planning through to completion. The successful collaborations between healthcare providers and CROs further illustrate the synergistic environment that promotes best practices and innovative methodologies in medical research.
In conclusion, as the demand for innovative medical devices continues to grow, the Dominican Republic is well-positioned to advance clinical research in the medtech sector. By leveraging its unique advantages, including cost-effectiveness, regulatory support, and a skilled workforce, the country not only contributes to the global medtech landscape but also enhances healthcare delivery in Latin America. Embracing the opportunities presented in this vibrant environment can lead to transformative outcomes for both patients and the industry as a whole.




