Overview
The article compares Medtech clinical trial locations, highlighting Europe and Latin America as regions that offer distinct advantages for conducting trials. It emphasizes that Europe benefits from a robust regulatory framework and diverse populations, while Latin America provides faster timelines and cost efficiencies, making both regions attractive for sponsors aiming to enhance recruitment and optimize operational costs.
Introduction
The selection of clinical trial locations is a critical decision that can significantly impact the success and efficiency of research efforts. As the medtech industry continues to evolve, understanding the geographical advantages of various regions becomes essential for sponsors and researchers alike.
- Europe, with its robust regulatory frameworks and diverse patient populations, presents unique opportunities for accelerated patient recruitment and data collection.
- Meanwhile, Latin America is emerging as a promising landscape, characterized by faster trial timelines and cost-effective solutions, particularly in regions like Barranquilla, Colombia.
This article delves into the pivotal factors influencing the choice of clinical trial locations, including:
- Regulatory considerations
- Patient population diversity
- Cost implications
It also explores the innovative trends reshaping the future of clinical research. By examining these aspects, stakeholders can better navigate the complexities of trial management and ultimately enhance the quality and accessibility of medical advancements across the globe.
Geographical Advantages in Medtech Clinical Trials
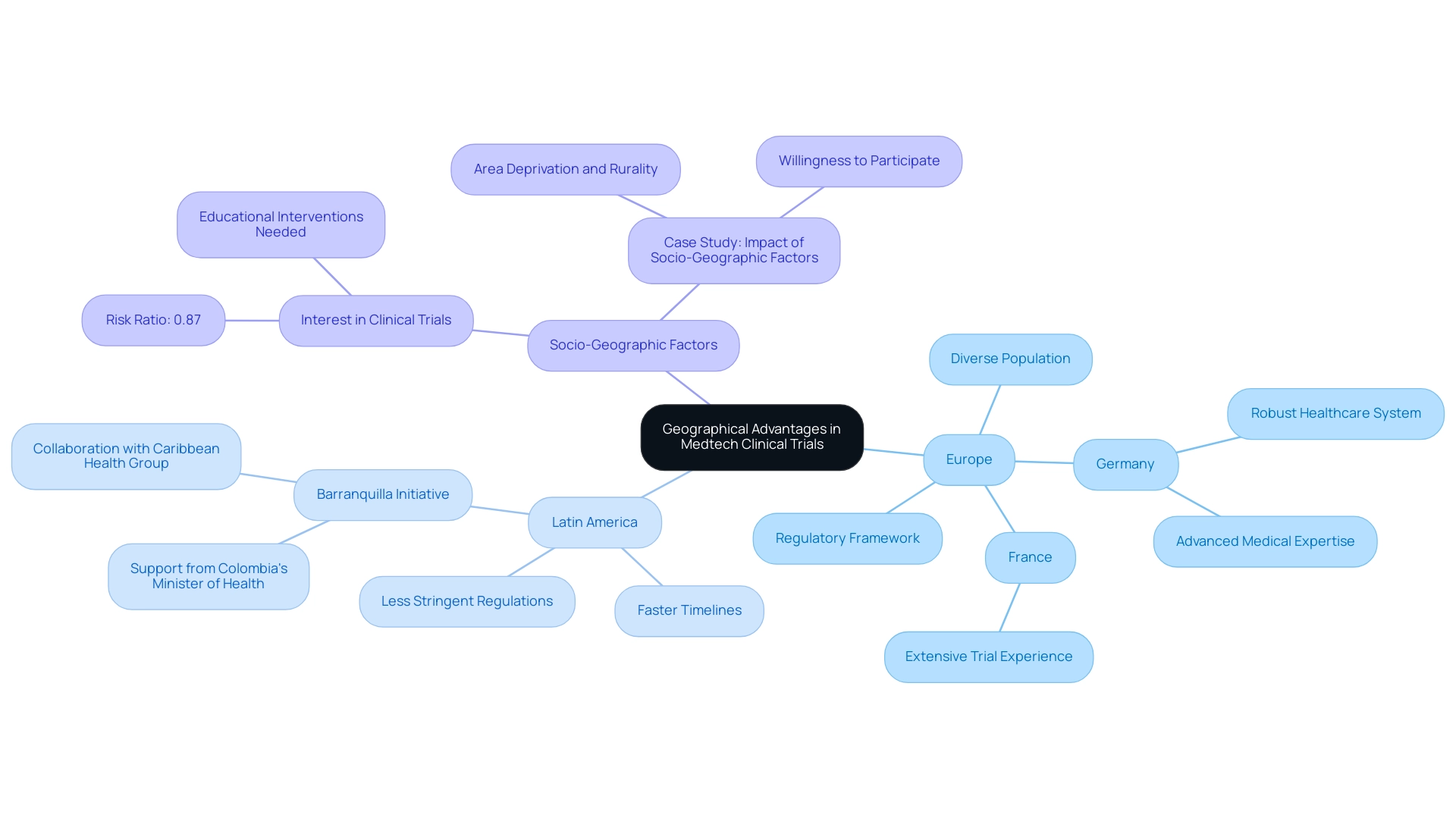
Geographical advantages are essential when selecting Medtech clinical trial locations, particularly in Europe and Latin America, where unique benefits can be observed. Europe is characterized by its well-established regulatory framework and diverse population, significantly enhancing recruitment and accelerating data collection processes. Germany and France are considered highly attractive Medtech clinical trial locations due to their robust healthcare systems, advanced medical expertise, and extensive experience in conducting trials.
In contrast, Latin America offers unique opportunities for faster timelines, attributed to less stringent regulatory environments and an expanding pool of treatment-naive patients. Notably, the collaboration between bioaccess™ and Caribbean Health Group aims to position Barranquilla as one of the top Medtech clinical trial locations in Latin America, supported by Colombia's Minister of Health. This initiative is set to streamline testing processes at Medtech clinical trial locations, thereby enhancing recruitment efficiency and retention rates, with bioaccess™ bringing over 20 years of Medtech experience to manage Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies effectively.
Moreover, the cost-efficiency of conducting experiments in these areas can result in significant decreases in overall costs, attracting sponsors keen to enhance their return on investment. Recent findings indicated a risk ratio of 0.87 (95% CI, 0.75–1.02) for interest in clinical studies among individuals in lower disadvantaged settings between rural and urban areas, highlighting the need for targeted educational interventions to enhance participation rates. The case study titled 'Impact of Socio-Geographic Factors on Clinical Research Interest' illustrates how socio-geographic factors, such as area deprivation and rurality, influence cancer patients' interest in studies, emphasizing the necessity for targeted approaches to improve access.
As Jesse Russell aptly noted, understanding these geographical nuances is essential for sponsors to strategically select Medtech clinical trial locations that align with their objectives and resources. This thorough method of assessing geographical benefits ultimately leads to enhanced research success rates and improved treatment results among various populations. Additionally, future research is needed to explore the reasons for low participation rates among rural and disadvantaged patients and to develop interventions to overcome these barriers.
Emerging Trends and Innovations Shaping Clinical Trial Locations
The terrain of Medtech clinical trial locations is undergoing a transformative change, fueled by emerging trends and innovations, especially the growth of decentralized studies (DCTs). This method allows researchers to perform studies from a distance, greatly broadening the geographical scope beyond conventional Medtech clinical trial locations. Such a shift is especially advantageous in underserved regions, facilitating patient participation from diverse demographics and Medtech clinical trial locations.
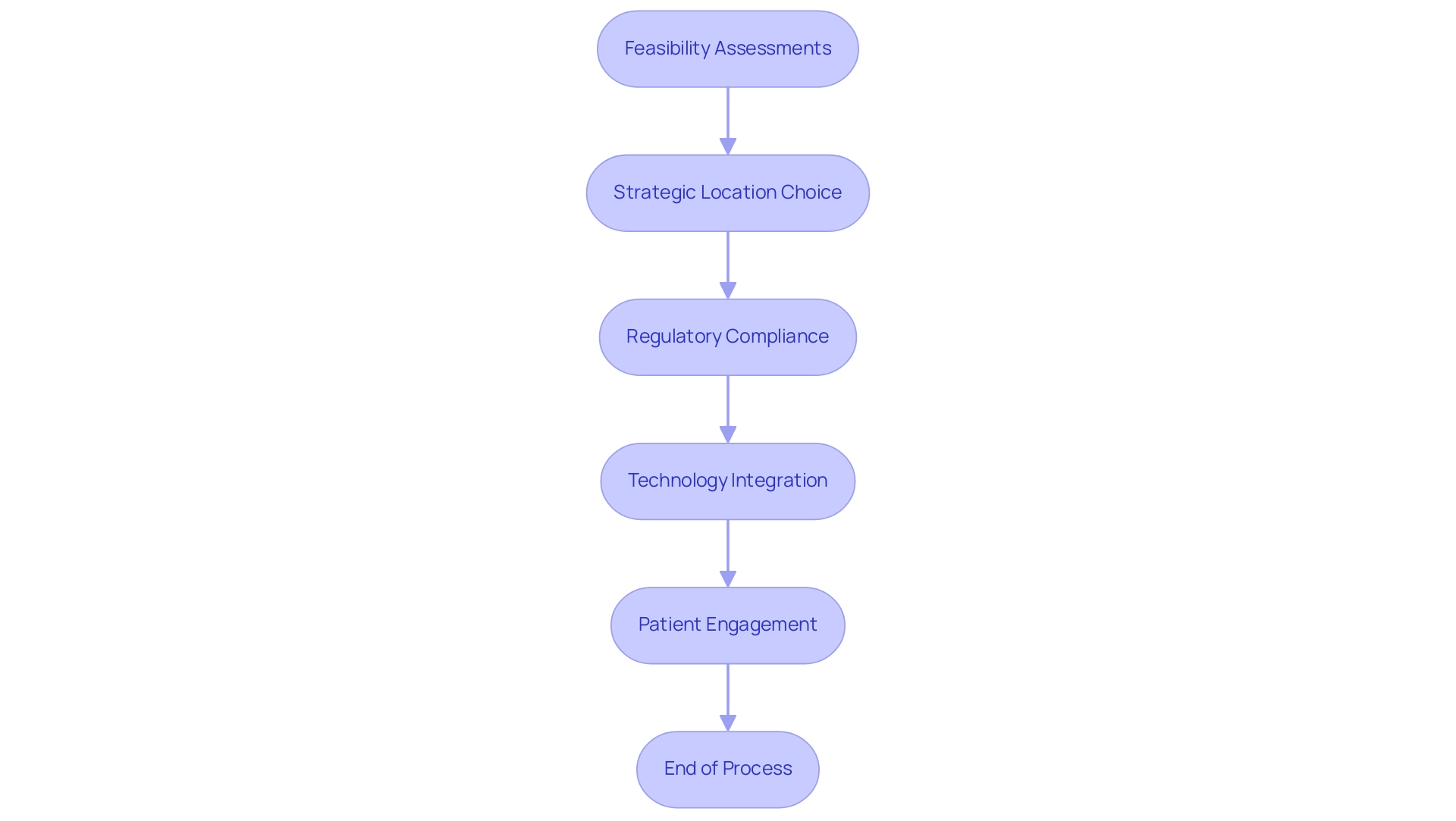
Our extensive management services for studies facilitate this evolution through:
- Feasibility assessments
- The strategic choice of study locations and principal investigators (PIs)
- Detailed evaluations and feedback on study documents to ensure adherence to national requirements
We also concentrate on setup processes at Medtech clinical trial locations, which include:
- Obtaining necessary approvals from ethics committees and health ministries
- Managing import permits for investigational devices, thus optimizing the overall setup
The TIN seeks to learn from multisite studies to evaluate the effectiveness of these decentralized methods, offering essential insights into their influence on clinical studies and local economies, including job creation and healthcare enhancement.
Additionally, advancements in data analytics and artificial intelligence are transforming the processes of selecting Medtech clinical trial locations. These technologies offer essential insights into participant demographics and site performance, enhancing the efficiency of selecting suitable Medtech clinical trial locations. For instance, the CARE4Kids study exemplifies the use of technology, employing MyCap to send reminder notifications to participants while allowing for remote entry of patient-reported outcomes (Pros), thus illustrating the practical application of these innovations through effective project management and monitoring.
However, as the adoption of DCTs increases, privacy and security challenges in remote research processes must be addressed, particularly concerning the transmission of sensitive personal information. This aspect is crucial to ensuring that advancements do not compromise patient data security. Our services include thorough reviews and feedback on study documents to comply with regulatory standards, which is essential for navigating these challenges.
Regulatory changes are also pivotal in this transition. The European Medicines Agency (EMA) has increasingly supported innovative study designs at Medtech clinical trial locations across multiple countries, thereby streamlining processes and promoting cross-border collaboration.
As highlighted by P. A. Harris, PhD, > The ideal approach would give research participants options for participation, although this may place additional burden on research coordinators. This sentiment underscores the balance required between enhancing patient options and managing logistical complexities through effective study project management, including regular reporting on study status, inventory, and both serious and non-serious adverse events.
Ultimately, these trends not only optimize efficiency but also foster improved patient engagement and retention, contributing to more successful outcomes. The integration of digital health technologies, including AI-enabled mobile modalities and wearables, is anticipated to improve the precision of study outcomes and endpoints, although challenges persist in managing the diverse datasets generated. These advancements signal a new age in study design, where adaptability and creativity are crucial, further promoting global health enhancement through international partnership and innovation in medtech.
Regulatory Considerations for Clinical Trial Locations
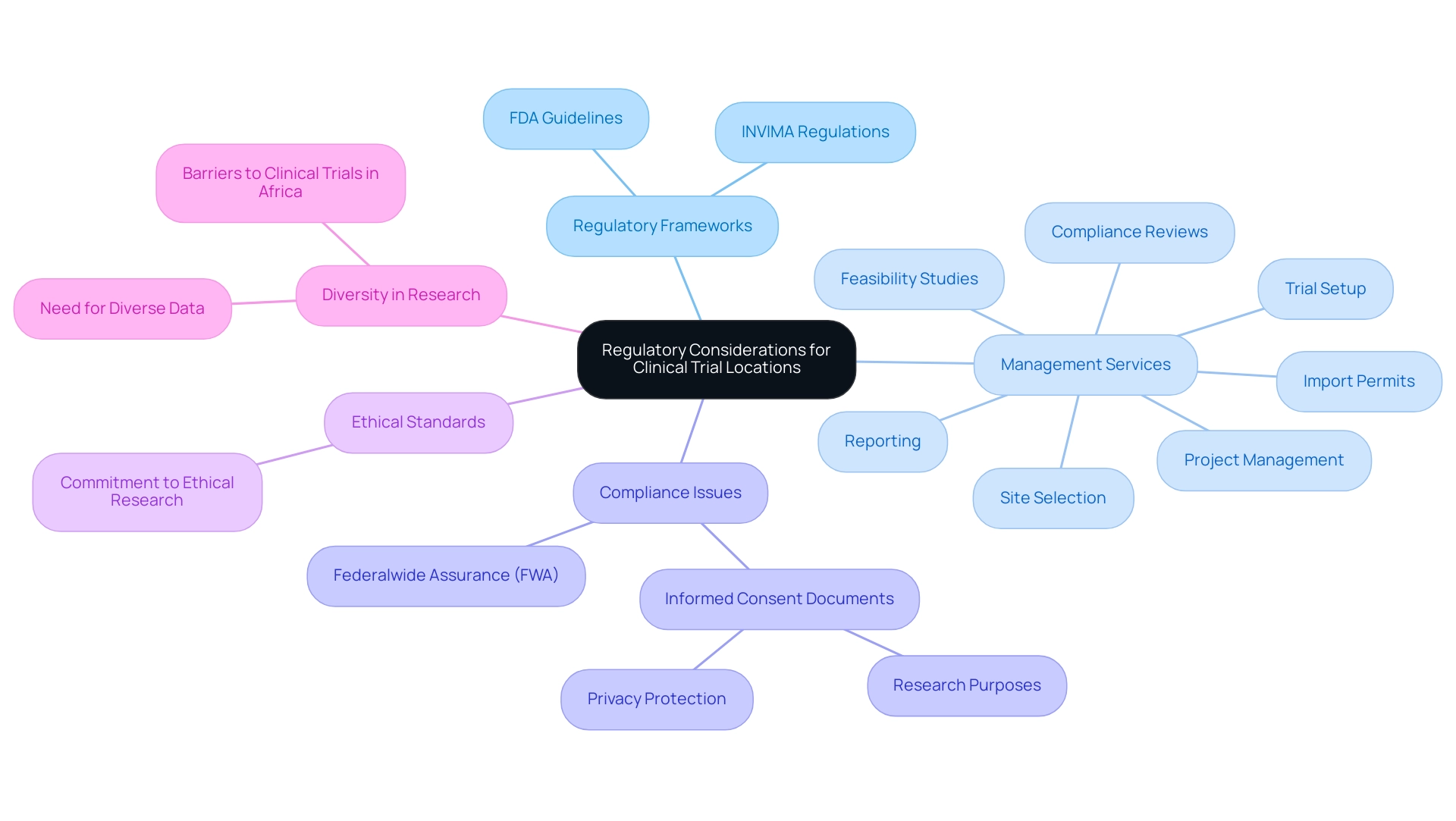
When choosing Medtech clinical trial locations, it is crucial to consider regulatory factors. Each area offers a distinct collection of guidelines that govern the conduct of research. For instance, the United States adheres to stringent FDA guidelines, which, while ensuring participant safety, can lead to prolonged approval times.
In contrast, Colombia's INVIMA, designated as a Level 4 health authority by PAHO/WHO, supervises the medical device sector and enforces regulations that, while robust, may provide different pathways for initiation. Understanding these regulatory landscapes is crucial for sponsors aiming to navigate compliance effectively at Medtech clinical trial locations to avoid potential delays that could jeopardize study integrity. Moreover, comprehensive clinical trial management services, including:
- feasibility studies
- site selection
- compliance reviews
- trial setup
- import permits and nationalization of investigational devices
- project management
- reporting (study status, inventory, serious and non-serious adverse events)
can facilitate smoother operations within these frameworks.
Notably, between 1970 and 2010, there were significant drug approvals for rare diseases, underscoring the impact regulatory frameworks can have on therapeutic advancements. Additionally, the HHS has advised incorporating specific information in informed consent documents for biospecimen collection, highlighting privacy protection and study purposes, which is essential for regulatory compliance. The Federalwide Assurance (FWA) acts as a commitment by institutions to uphold ethical standards in studies involving human subjects, showcasing the importance of compliance in practice.
As global harmonization efforts advance, notably through initiatives like the RICH E6(R2) guidelines, the prospect of more consistent regulatory environments may emerge, which could influence site selection strategies for Medtech clinical trial locations. Additionally, as Katherine Ruiz emphasizes, the expertise in Regulatory Affairs for medical devices and in vitro diagnostics is critical for navigating these complexities. Lastly, as Mansoor Saleh wisely points out, 'Unless we reinvent the wheel, research data from African sites and the African population will remain absent in published literature,' emphasizing the ongoing need for diverse research data.
Thus, regulatory considerations must also address accessibility and representation in medical studies across different regions.
Patient Population Diversity and Access
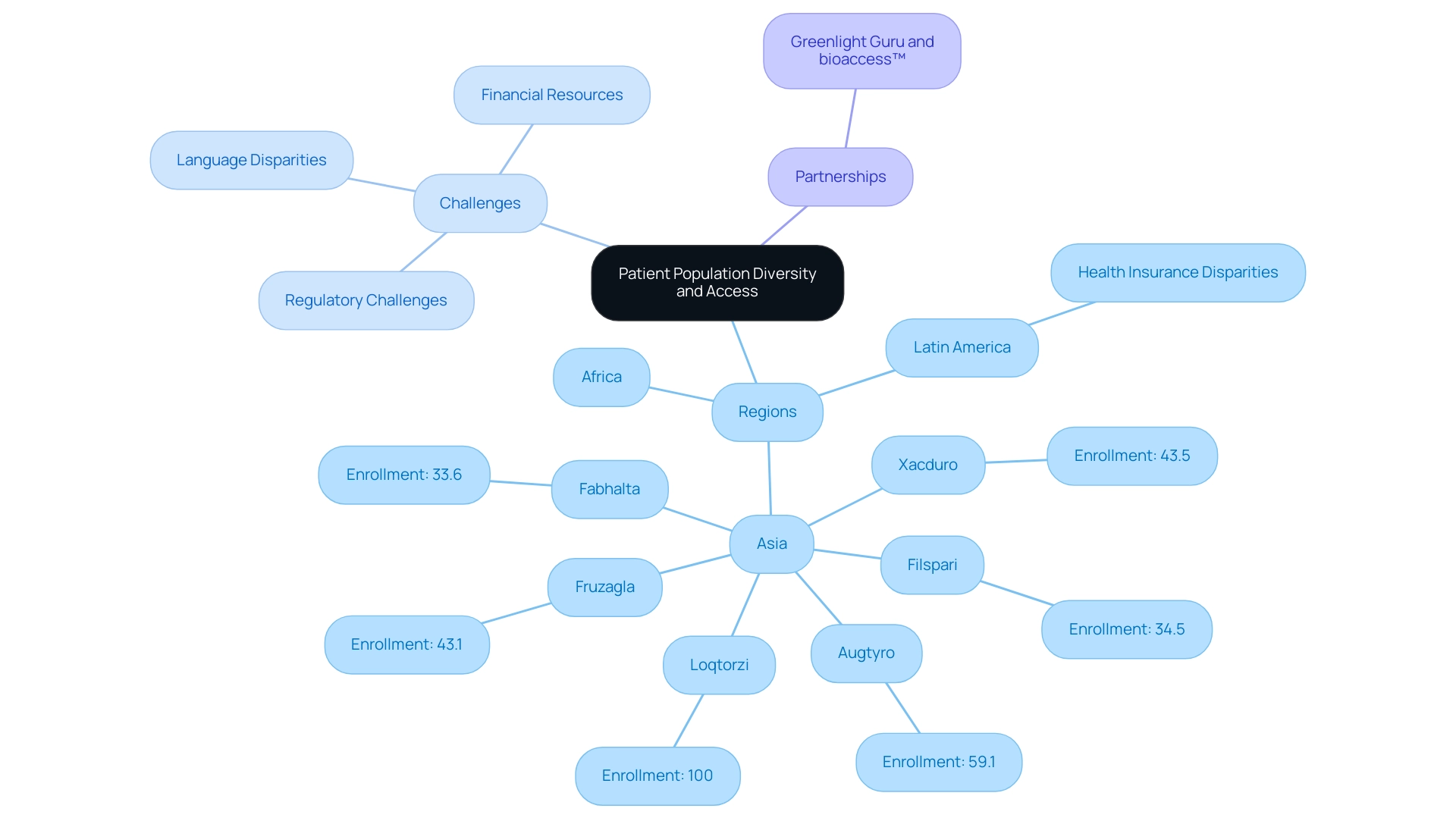
The variety of participant groups in research studies is crucial for guaranteeing that findings are relevant to a broader audience, particularly regarding Latin America’s distinct challenges. Regions like Asia and Africa offer unique opportunities to access diverse demographics that may not be as easily reached in Western countries. Significant studies, such as:
- Fabhalta (33.6% Asian enrollment)
- Filspari (34.5%)
- Xacduro (43.5%)
- Augtyro (59.1%)
- Fruzagla (43.1%)
- Loqtorzi (100%)
showcase the potential for substantial Asian representation in research.
In Latin America, overcoming obstacles like regulatory challenges, language disparities, fragmented resources, and the absence of CRO corporate frameworks is crucial for enhancing patient recruitment and promoting cooperation between local hospitals and American research sponsors. Additionally, the limited financial resources faced by US Medtech companies further complicate these challenges. By utilizing culturally aware methods, Medtech firms can involve local communities to improve recruitment and retention rates, thus resulting in more thorough health outcomes.
The successful partnership between Greenlight Guru and bioaccess™ acts as a framework to speed up Medtech advancements and studies at Medtech clinical trial locations in Latin America, underscored by PAVmed's first-in-human research in Colombia. As JG, a JNCI associate editor and co-author, observes, 'Diversity in research studies is not merely a metric; it is crucial for the advancement of effective therapies for all groups.' Interacting with varied groups of individuals, while tackling the unique issues of Latin America, is crucial for progressing medical research and guaranteeing fair access to potentially life-saving studies.
A solution-driven approach is crucial to bridge the gap between innovation and execution in this region.
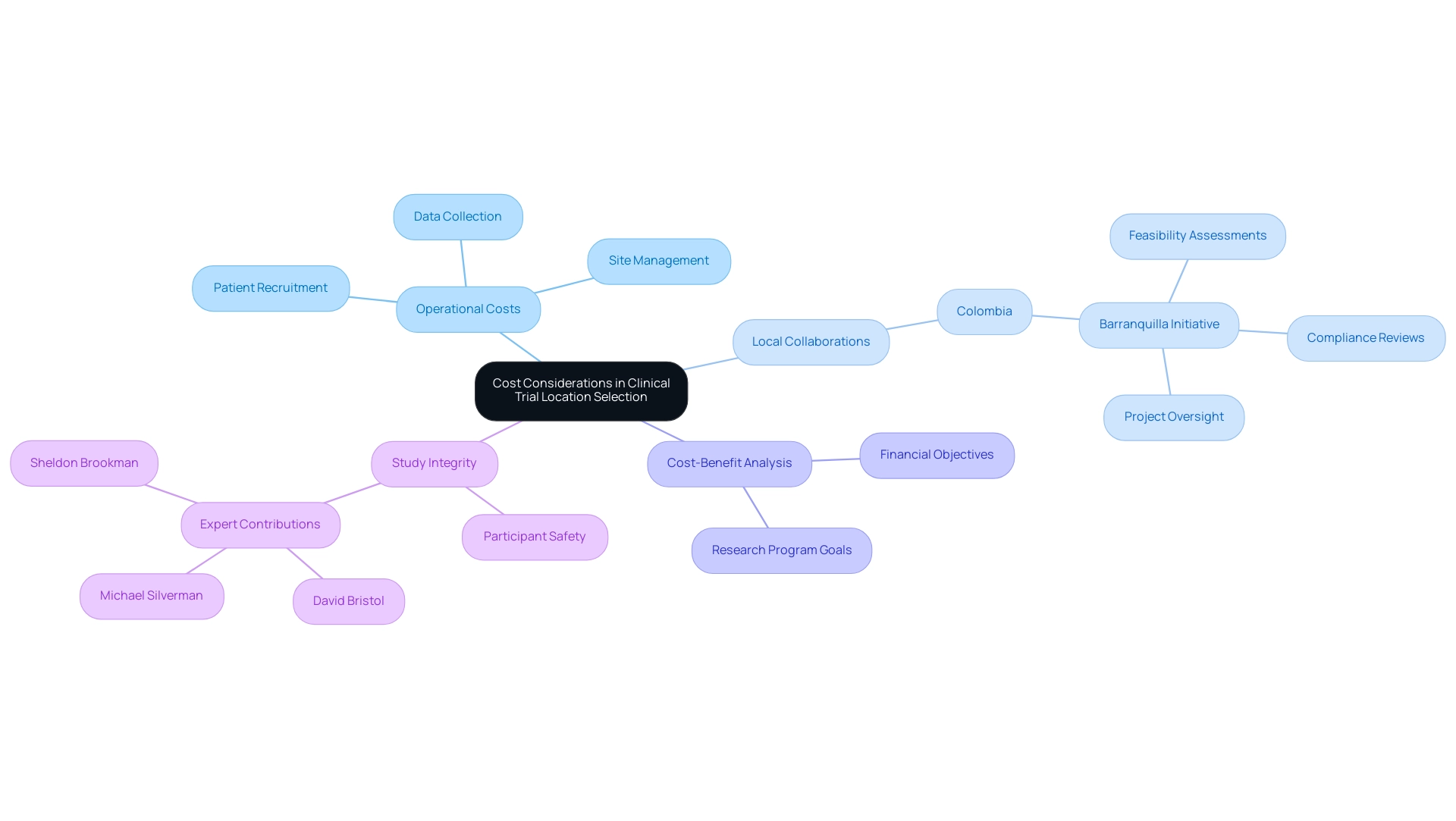
Cost Considerations in Clinical Trial Location Selection
Cost factors play a crucial role in choosing Medtech clinical trial locations, as they can significantly impact budgets and the distribution of resources. Regions such as India and Eastern Europe are increasingly favored by sponsors due to their significantly lower operational costs. For instance, expenses related to patient recruitment, site management, and data collection are often more economical in these areas compared to Western Europe or North America.
However, as bioaccess™ and Caribbean Health Group work together to establish Barranquilla as a premier location for medical studies in Latin America, it is clear that local efforts can also stimulate economic development and job creation. Backed by Colombia's Minister of Health, this collaboration seeks to improve medical study infrastructure through thorough management services for evaluations, encompassing:
- Feasibility assessments
- Compliance reviews
- Setup
- Project oversight
This ensures adherence to regulatory standards. A comprehensive cost-benefit analysis is essential to ensure that the selected location not only meets financial objectives but also aligns with the overarching goals of the research program.
While the appeal of lower expenses is enticing, it is crucial that these savings do not jeopardize the integrity of the study or compromise participant safety. As David Bristol, an independent consultant to ERG, pointed out, 'We also wish to recognize the contributions of our subject matter experts... to shaping our interview questions and the decision-making criteria for the study.' Recent insights from industry experts emphasize that leveraging operational advantages, such as those in Colombia, can lead to significant cost savings while fostering international collaboration and enhancing services at Medtech clinical trial locations, evidenced by GlobalCare Clinical Trials’ success in reducing recruitment time by over 50% and achieving 95% retention rates.
Conclusion
Selecting the right geographical locations for clinical trials is a decisive factor that can greatly influence the success of medtech research. The analysis of regions such as Europe and Latin America reveals distinct advantages, including:
- Robust regulatory frameworks
- Diverse patient populations
- Cost-effective solutions
All of which are crucial for efficient trial management. Europe offers a well-established environment for patient recruitment and data collection, while Latin America, particularly Colombia, is emerging as a viable option for faster trial timelines and lower costs, supported by innovative collaborations.
As the landscape of clinical trials evolves, the rise of decentralized clinical trials and advancements in technology are reshaping site selection and patient engagement. These innovations not only optimize trial efficiency but also enhance the accessibility and diversity of patient populations, fostering more representative research outcomes. The importance of understanding regulatory considerations cannot be overstated, as they dictate the feasibility and integrity of trial operations across different regions.
In conclusion, stakeholders in the medtech industry must carefully evaluate these geographical advantages and emerging trends when planning clinical trials. By strategically selecting locations that align with regulatory requirements, patient demographics, and cost considerations, sponsors can enhance trial success rates and ultimately improve treatment outcomes for diverse populations. This comprehensive approach is essential for advancing medical research and ensuring equitable access to breakthroughs in healthcare.
Frequently Asked Questions
Why are geographical advantages important for Medtech clinical trial locations?
Geographical advantages are essential because they enhance recruitment and accelerate data collection processes, particularly in regions like Europe and Latin America, where unique benefits can be observed.
What makes Europe an attractive location for Medtech clinical trials?
Europe has a well-established regulatory framework, a diverse population, robust healthcare systems, advanced medical expertise, and extensive experience in conducting trials, making it highly attractive for Medtech clinical trials.
What are the benefits of conducting Medtech clinical trials in Latin America?
Latin America offers faster timelines for trials, attributed to less stringent regulatory environments and an expanding pool of treatment-naive patients, which can enhance recruitment efficiency and retention rates.
How is Barranquilla being positioned as a Medtech clinical trial location?
The collaboration between bioaccess™ and Caribbean Health Group aims to enhance Barranquilla's status as a top Medtech clinical trial location in Latin America, supported by Colombia's Minister of Health, focusing on streamlining testing processes.
What is the significance of cost-efficiency in Medtech clinical trials?
Cost-efficiency in conducting trials in regions like Europe and Latin America can lead to significant decreases in overall costs, attracting sponsors who are keen to enhance their return on investment.
What does the case study titled 'Impact of Socio-Geographic Factors on Clinical Research Interest' highlight?
The case study illustrates how socio-geographic factors, such as area deprivation and rurality, influence cancer patients' interest in studies, emphasizing the need for targeted approaches to improve access and participation rates.
What role do decentralized studies (DCTs) play in Medtech clinical trials?
DCTs allow researchers to conduct studies remotely, broadening the geographical scope and facilitating patient participation from diverse demographics, especially in underserved regions.
What services are provided to facilitate decentralized clinical trials?
Services include feasibility assessments, strategic selection of study locations and principal investigators, and detailed evaluations of study documents to ensure adherence to national requirements.
How are data analytics and artificial intelligence transforming Medtech clinical trial processes?
These technologies provide insights into participant demographics and site performance, enhancing the efficiency of selecting suitable Medtech clinical trial locations.
What challenges must be addressed with the adoption of decentralized trials?
Privacy and security challenges related to the transmission of sensitive personal information must be addressed to ensure patient data security during remote research processes.
How is the European Medicines Agency (EMA) influencing Medtech clinical trials?
The EMA supports innovative study designs across multiple countries, streamlining processes and promoting cross-border collaboration in clinical trials.
What is the anticipated impact of digital health technologies on clinical trials?
The integration of digital health technologies, including AI-enabled mobile modalities and wearables, is expected to improve the precision of study outcomes, although challenges in managing diverse datasets persist.

