Introduction
In the intricate realm of medical device regulation, the Clinical Evaluation Plan (CEP) serves as a fundamental strategic document, meticulously outlining the methodologies for gathering, analyzing, and interpreting clinical data. This detailed plan ensures the demonstration of a medical device's safety and performance, providing an essential roadmap that guides the clinical evaluation process. The CEP encompasses various critical aspects, including the type of investigation, rationale for selection, endpoints, and variables.
It also details subject selection criteria, the investigation population size, and the representativeness of this population concerning the target demographic, addressing the inclusion of vulnerable subjects when necessary.
To maintain transparency and ensure unbiased results, the CEP outlines steps like randomization and describes clinical procedures and diagnostic methods. Ethical, legal, and social considerations are integral, especially given the evolving regulatory landscape. Compliance with these regulatory demands is paramount, emphasizing the importance of aligning clinical evaluation plans, reports, and risk management files.
Furthermore, the analysis of post-market surveillance data is crucial for a comprehensive evaluation, helping stakeholders navigate future regulatory challenges. This structured approach ensures that all stakeholders understand the objectives, methodologies, and regulatory requirements necessary for a successful clinical evaluation.
Understanding the Clinical Evaluation Plan (CEP)
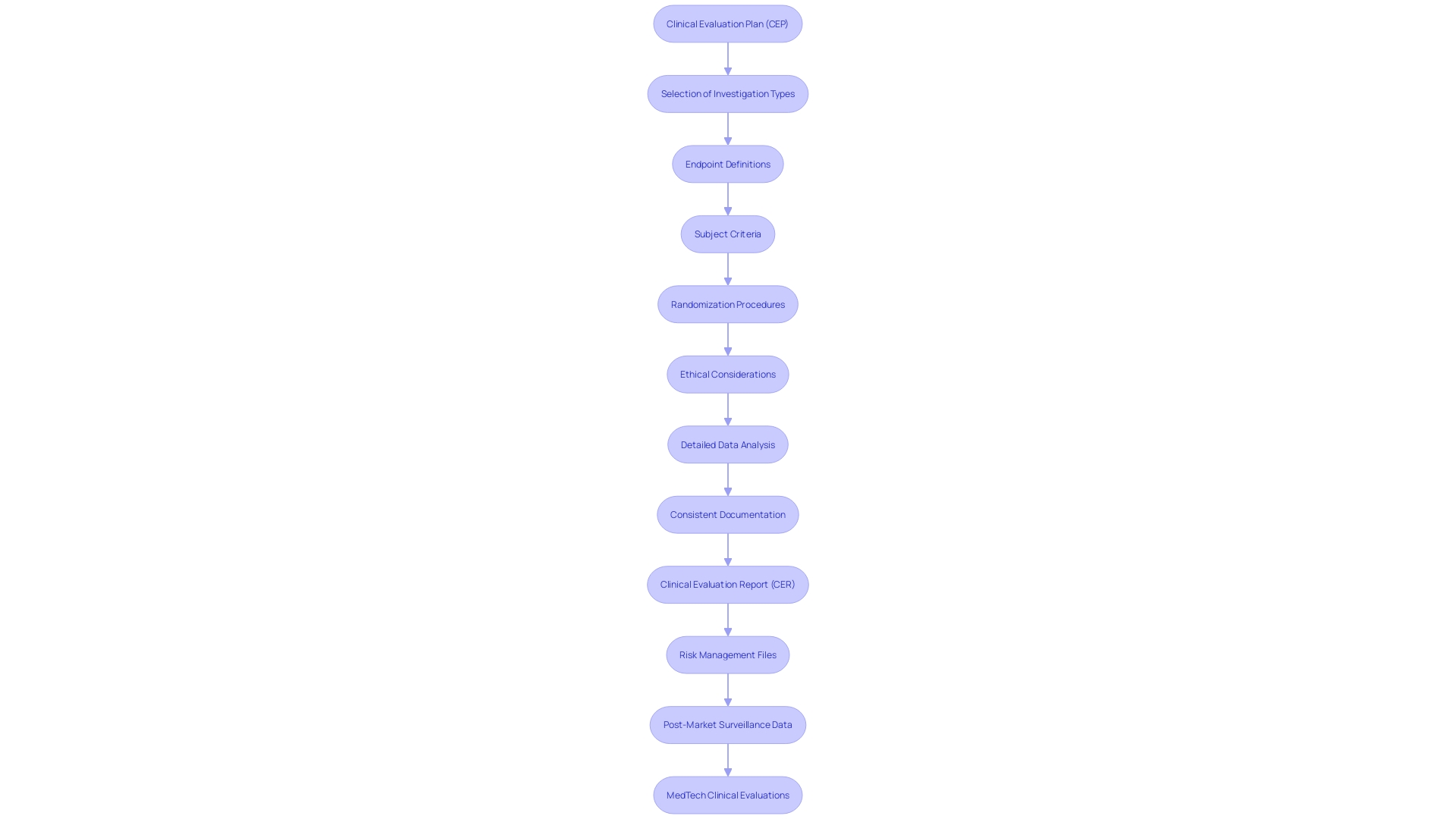
A Clinical Evaluation Plan (CEP) is a vital strategic document that carefully details the approaches for collecting, analyzing, and interpreting research data to show the safety and performance of a medical instrument. This document acts as a vital roadmap, directing the evaluation process by offering detailed information on various important aspects.
The CEP includes the type of investigation, along with a rationale for its selection, and defines its endpoints and variables. It provides comprehensive details on the medical device and any comparators used in the study, ensuring a thorough analysis. Additionally, the plan specifies the criteria for subject selection, the size of the investigation population, and the representativeness of this population in relation to the target demographic. It also addresses the inclusion of vulnerable subjects, if applicable.
'To ensure impartial results, the CEP outlines steps such as randomization and describes medical procedures and diagnostic methods related to the investigation.'. Any deviations from standard medical practices are highlighted to maintain transparency.
Ethical, legal, and social considerations are integral to the CEP, given the complex landscape of medical apparatus regulation. Ensuring adherence to changing regulatory requirements is crucial, as pointed out by industry specialists who stress the significance of synchronizing assessment plans, reports, and risk management documents coherently. The examination of post-market surveillance data, obtained from both comparable products and the company's own devices, is also vital for a thorough assessment.
In the dynamic regulatory environment, the CEP not only addresses the immediate needs of the assessment but also prepares stakeholders for future regulatory challenges. By offering a clear and organized method, the CEP guarantees all parties comprehend the goals, techniques, and regulatory criteria essential for a successful assessment.
Components of a Clinical Evaluation Plan
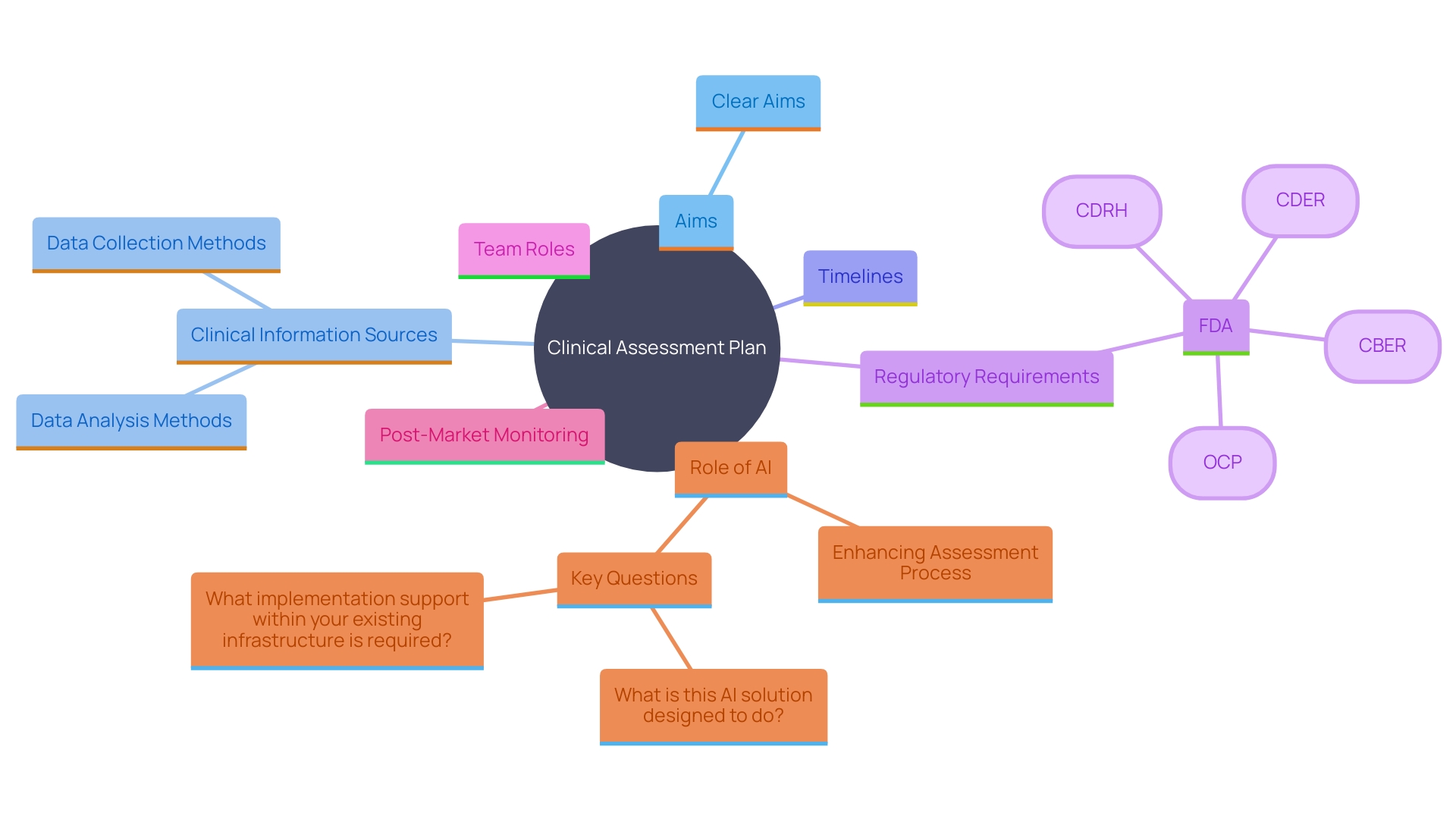
Essential elements of a Clinical Assessment Plan (CAP) are crucial for guaranteeing the strength and adherence of the trial assessment process. These components encompass clear aims for the assessment, an extensive list of clinical information sources, thorough methods for information collection and analysis, and a well-defined timeline for the complete assessment process. The CEP must also outline any regulatory requirements, such as those stipulated by the FDA or for CE marking in the EU, and specify the roles and responsibilities of the team members involved.
The importance of a meticulously constructed CEP cannot be overstated. As Dr. Ken Stein of Boston Scientific emphasized, the explosion of medical literature, now doubling every 73 days, presents significant challenges in keeping up with the latest research. A structured approach in the CEP aids in managing this vast influx of information effectively, ensuring that all relevant details are systematically reviewed and incorporated.
Furthermore, the CEP should address the integration of post-market monitoring information, which is often underutilized but critical for continuous safety and performance evaluation. This information, obtained from both comparable products and the company's own equipment, should be examined with the same thoroughness as pre-market information to uncover any potential problems early.
In the dynamic landscape of medical technology regulations, the role of AI is becoming increasingly prominent. AI tools can assist in handling large collections of information, such as those from wearable devices, enhancing the efficiency and precision of analysis. As Stein noted, AI-driven diagnostics have significantly reduced the time needed to detect conditions like atrial fibrillation, leading to earlier interventions and better patient outcomes.
The CEP must also ensure alignment across various documents, including the clinical assessment report and risk management files. 'Inconsistencies in information gathering and analysis strategies can lead to disjointed conclusions, undermining the credibility of the evaluation.'. Therefore, a coherent and detailed CEP is essential for the successful demonstration of a medical instrument’s safety and performance.
Identifying and Appraising Clinical Data
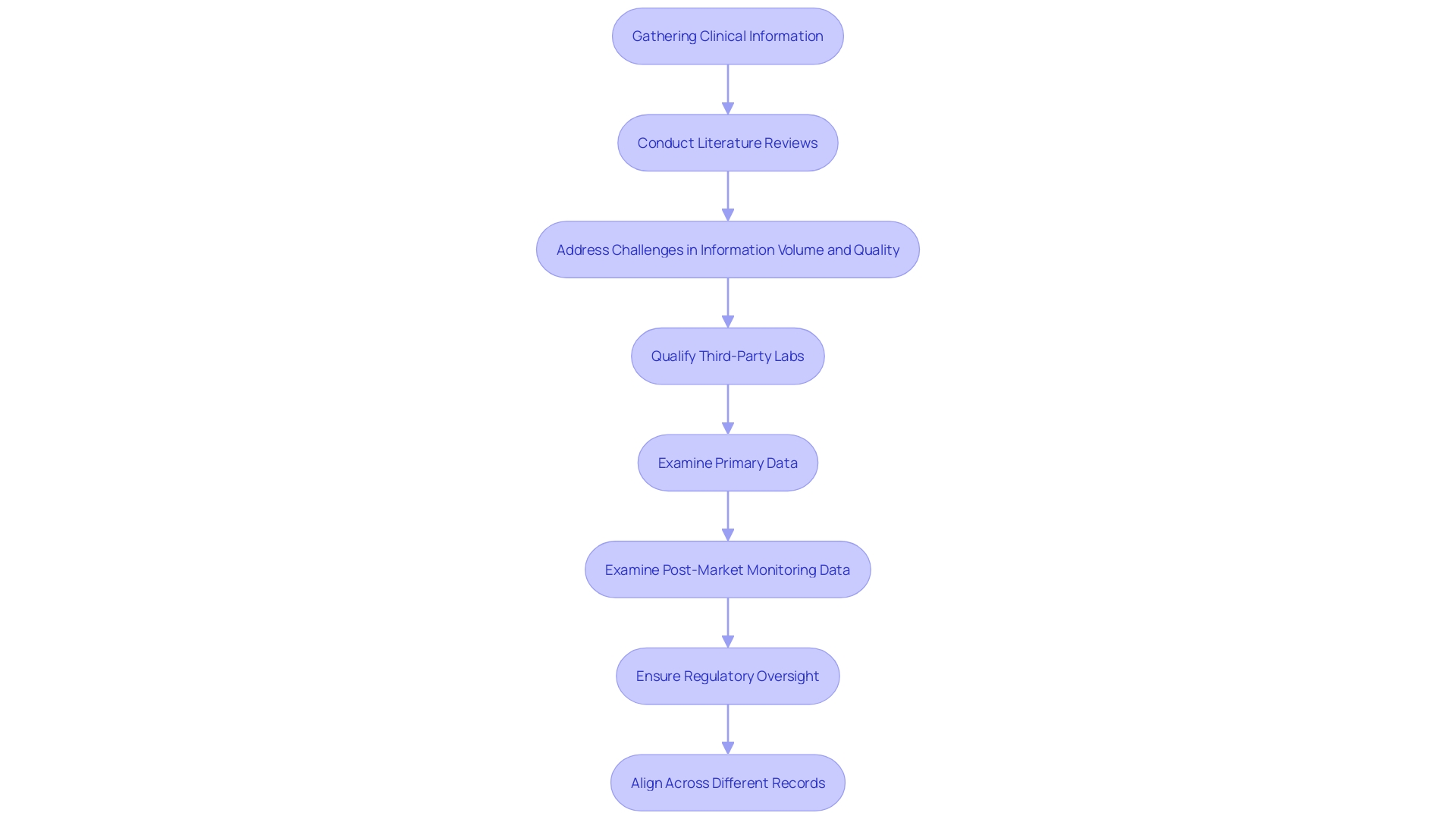
Identifying pertinent clinical information requires a comprehensive approach, including systematic literature reviews and database searches to gather evidence on the safety and efficacy of the device. This process must address challenges such as the volume, variety, and velocity of information needed for reviews. The revised guidance from regulatory bodies stresses the importance for manufacturers and study sponsors to qualify third-party test labs and examine all testing information closely, particularly considering recent rises in submissions containing unreliable information.
Assessing this information entails a thorough examination of its quality, relevance, and applicability to the particular device being reviewed. This ensures that only robust and credible information is included. The thorough examination and display of medical information are essential, as discrepancies and a lack of comprehensive analysis frequently result in gaps between the conclusions made and the actual information provided. Moreover, post-market monitoring information, obtained from both comparable and the company's own products, must be examined with the same rigor as the primary information to provide a comprehensive assessment.
As highlighted by specialists, a persistent issue exists in the coordination of information gathering and assessment approaches across different records, such as the evaluation plan, assessment report, and risk management files. This alignment is essential for a coherent presentation that accurately reflects the information narrative. According to the new framework, strengthening regulatory oversight is key to maintaining patient safety while promoting the introduction of innovative healthcare products that address health inequities and enhance access to care.
Creating a Clinical Evaluation Report (CER)
A well-constructed Clinical Evaluation Report (CER) is crucial in the regulatory submission process, serving as a comprehensive synthesis of medical information and its implications on a device's safety and performance. The CER meticulously consolidates and evaluates clinical information derived from both pre-market and post-market sources, ensuring a thorough assessment. This document requires thorough examination and clear presentation, particularly in the tabular information and references, to support the conclusions drawn.
Given the complexity of medical device regulations, especially with the rise of Software as a Medical Device (Sand), a robust CER must align with the latest regulatory frameworks and compliance processes. Unreliable information display and misalignment between the clinical assessment strategy, the CER, and risk management documents can weaken the integrity of the report. Therefore, it is crucial to maintain consistency and rigor across all documentation.
Moreover, the integration of post-market monitoring information, often underutilized, is essential. 'This information not only enhances the CER but also offers a real-world performance evaluation, which is essential for demonstrating ongoing safety and efficacy.'. By utilizing both primary and post-market information with equal rigor, the CER can provide a more comprehensive risk management perspective.
In the evolving regulatory landscape, ensuring that the CER meets all regulatory requirements is vital for CE marking in the European Union and for navigating global markets. Regulatory professionals and AI-driven tools play a significant role in ensuring compliance and expediting market entry by keeping abreast of regulatory updates and their implications. Thus, the CER is not merely a regulatory necessity but a strategic document that highlights the safety and effectiveness of medical tools, ultimately aiding in public health protection.
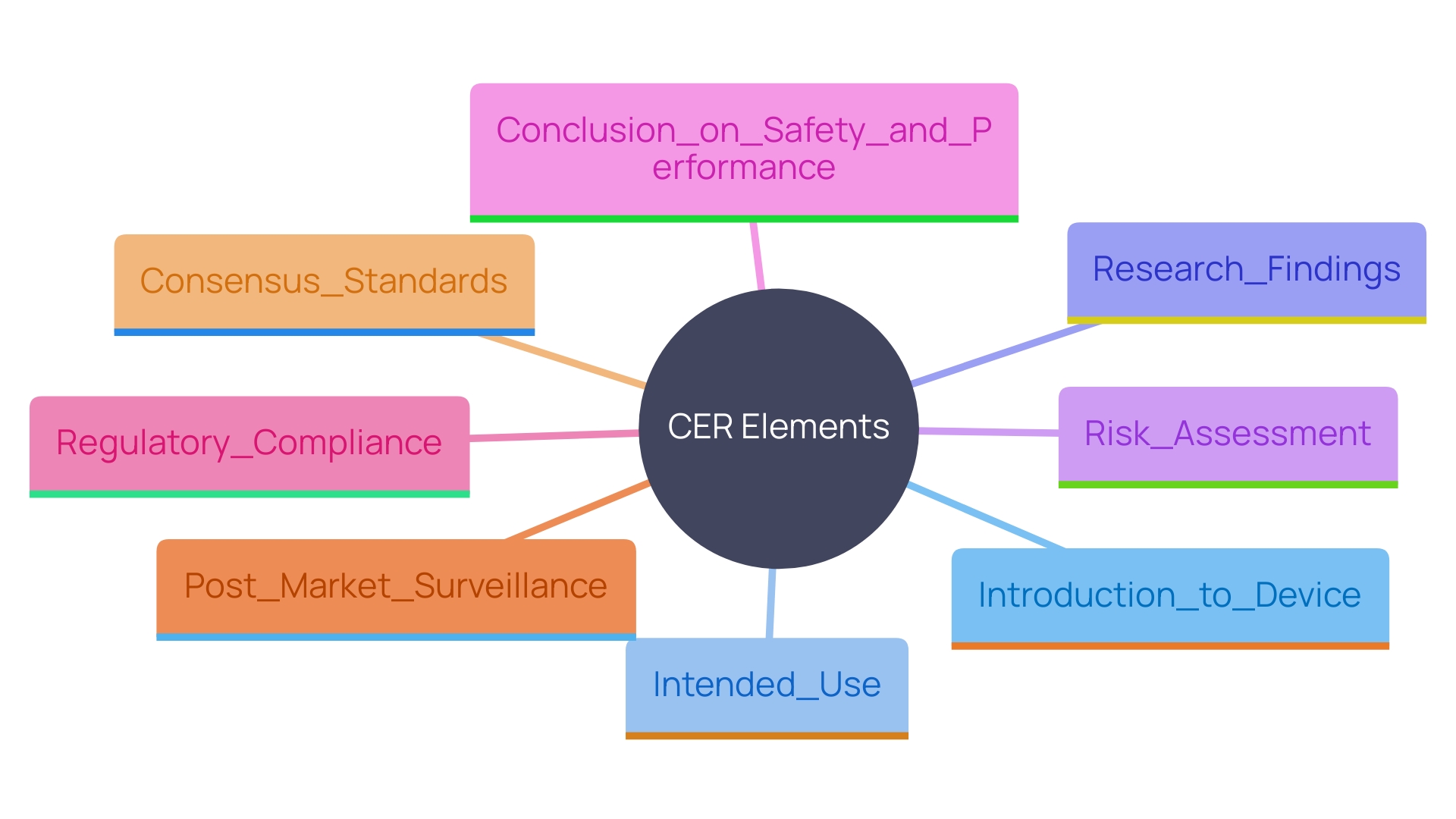
Elements of a Clinical Evaluation Report
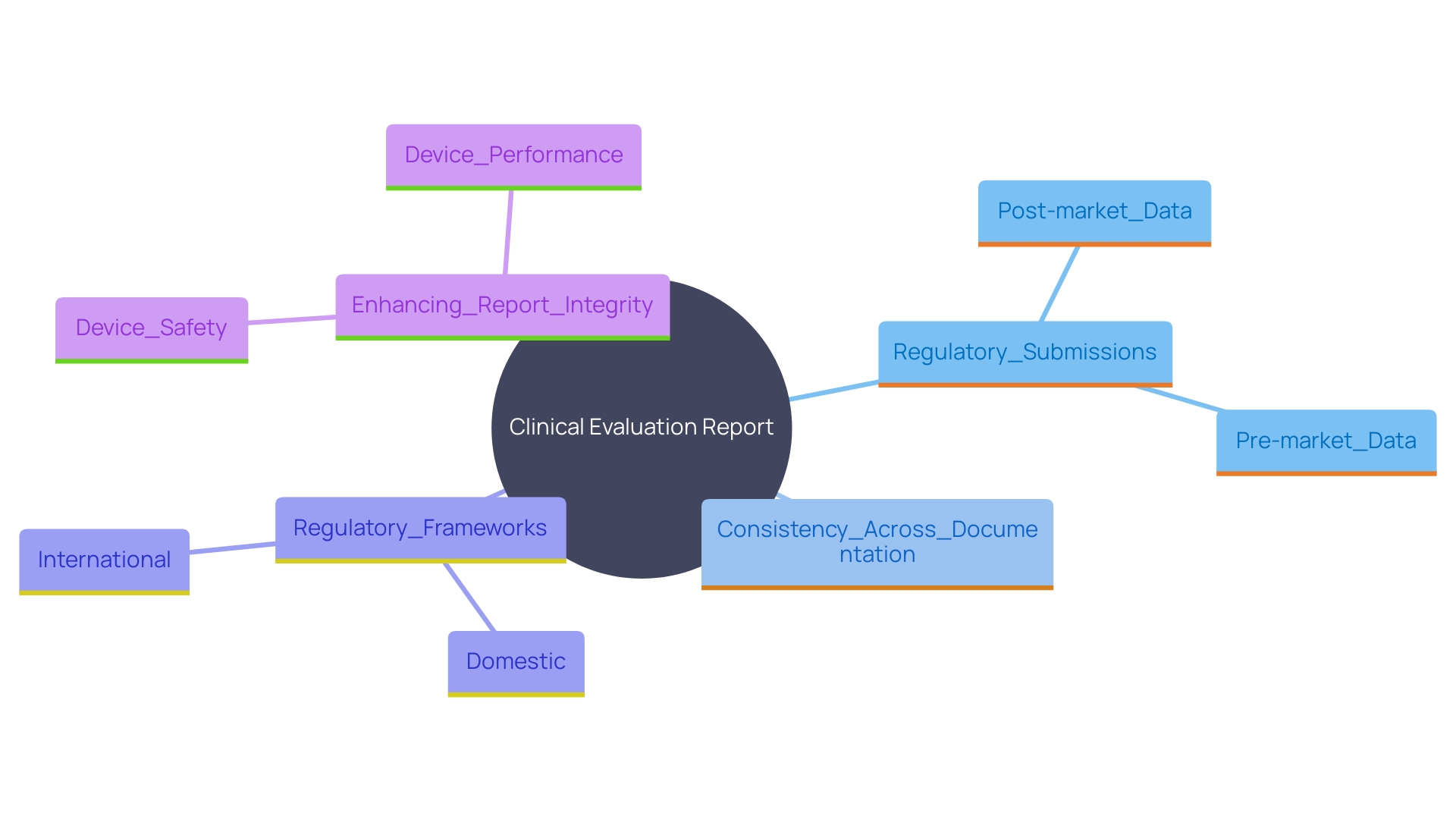
A thorough Evaluation Report (CER) should include several key elements: an introduction to the device, a discussion of its intended use, a detailed analysis of research findings, a risk assessment, and a conclusion regarding the device's safety and performance. Each element must be meticulously supported by collected information and aligned with regulatory expectations. This encompasses pre-market investigation, development, and evidence, along with assessing scientific and medical information related to post-market safety, effectiveness, and performance.
The importance of a well-structured CER cannot be overstated, particularly in the context of evolving regulatory demands. According to a study of medical device leaders, gaining market approval for new products (40%) and ensuring compliance with regulatory bodies (47%) are top priorities. These tasks have become increasingly challenging due to stringent legislation and the growing volume of literature to review.
A recurring challenge in CER preparation is the thorough examination and presentation of medical information. Discrepancies among records like the assessment strategy, assessment report, and risk management files frequently emphasize a lack of cohesion in information gathering and analysis approaches. This disconnection can lead to discrepancies between the conclusions drawn and the actual information presented. Therefore, it's crucial to maintain a coherent presentation, especially in tables and references.
Consensus standards play a pivotal role in regulatory quality, as they adhere to principles of transparency, openness, and due process. These standards, developed by Standards Development Organizations (SDOs), facilitate regulatory compliance and ensure that the CER meets the stringent requirements set by authorities like the FDA.
'Post-market surveillance information is another essential yet often underused element in clinical assessments.'. 'This information, obtained from both comparable and the company's own offerings, should be examined with the same thoroughness as the primary information to provide a comprehensive view of the product's performance.'.
'The FDA, responsible for safeguarding public health by ensuring the safety and effectiveness of medical products, emphasizes the need for detailed and well-supported Cars.'. As part of its regulatory framework, the FDA requires conformity assessment—a demonstration that specified requirements relating to a product are fulfilled, which includes sampling, testing, and certification.
In summary, a robust CER requires a detailed and coherent presentation of clinical information, adherence to consensus standards, and rigorous post-market surveillance. These elements are essential to navigate the complex regulatory landscape and ensure the successful market entry of medical products.
Regular Updates to the Clinical Evaluation Report
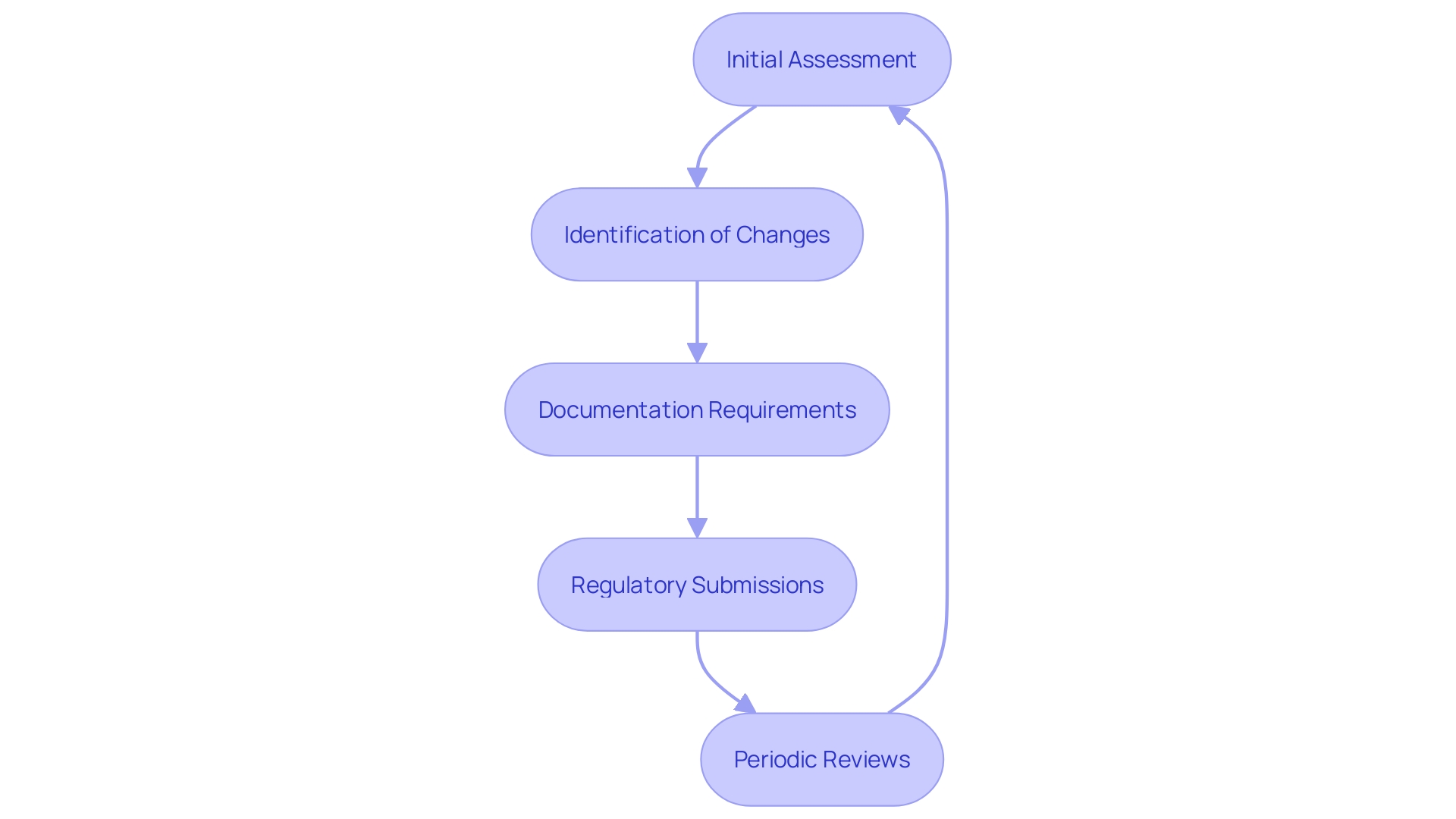
'Clinical assessments are dynamic processes that necessitate ongoing oversight and revisions to the Clinical Assessment Report (CAR) as new information arises or when changes to the apparatus are implemented.'. This ensures that the CER remains up-to-date and accurately reflects the current state of knowledge. For instance, any change in the device's design, whether due to customer feedback, a shift in materials, or manufacturing processes, necessitates comprehensive documentation and possibly a new regulatory submission.
Periodic reviews play a crucial role in identifying any emerging risks or performance issues. This ongoing assessment is paramount, given that the volume, variety, and velocity of information required for these evaluations continue to expand. Effective post-market surveillance is essential, yet often underutilized, in analyzing information rigorously from both similar and the company's own products. This data must be meticulously analyzed and presented coherently to avoid discrepancies between conclusions and the actual data.
Moreover, as legislation becomes more stringent, the global medical equipment industry's challenges grow, emphasizing the need for expertise in Regulatory Affairs. According to recent studies, gaining market approval and ensuring regulatory compliance are top priorities, with 47% of medical equipment leaders identifying these as significant challenges. Therefore, maintaining an aligned and detailed clinical evaluation process is critical for the safety, efficacy, and performance of medical devices.
Conclusion
The Clinical Evaluation Plan (CEP) is vital for ensuring the safety and performance of medical devices through a structured approach to data collection and analysis. It encompasses essential components such as clear objectives, detailed methodologies, and adherence to regulatory requirements. By outlining the investigation type, subject selection criteria, and ethical considerations, the CEP serves as a comprehensive roadmap for stakeholders involved in the clinical evaluation process.
In addition to the foundational aspects of the CEP, the integration of post-market surveillance data is critical for ongoing assessment and evaluation. This data should be treated with the same rigor as pre-market data, allowing for a complete understanding of the device's real-world performance. The importance of a coherent and well-aligned Clinical Evaluation Report (CER) cannot be overstated, as inconsistencies across documentation can compromise the credibility of the evaluation.
Regular updates to the CER are necessary to reflect new data and modifications to the device, ensuring that it remains relevant in a rapidly evolving regulatory landscape. Continuous monitoring and periodic reviews are essential for identifying emerging risks and maintaining compliance with stringent regulations. As the medical device industry faces increasing challenges, a meticulous and structured approach to clinical evaluation is paramount for safeguarding public health and enhancing patient outcomes.




