Introduction
Ensuring the safety and effectiveness of medical devices is paramount, and this is achieved through a meticulous and multi-faceted approach to testing. Medical device testing encompasses a comprehensive range of methodologies aimed at validating that these devices meet stringent regulatory standards and perform reliably in real-world scenarios. The primary categories of testing include functional, safety, biocompatibility, usability, and software testing, each addressing specific aspects of device performance and compliance.
Functional testing verifies that the device operates as intended, while safety testing evaluates potential risks, including electrical, mechanical, and thermal safety. Biocompatibility testing ensures that the materials used do not cause adverse biological reactions, and usability testing focuses on the device’s user interface to enhance user experience and minimize errors. For devices incorporating software, rigorous software testing is conducted to ensure reliability and security.
Adhering to these testing protocols not only ensures compliance with regulatory standards but also enhances the overall quality and safety of medical devices, contributing to improved healthcare outcomes. The importance of these rigorous testing processes is underscored by real-world case studies, such as the Philips recall, which highlight the critical role of comprehensive testing in ensuring patient safety and avoiding potential risks.
Types of Medical Device Testing
Medical equipment evaluation encompasses a thorough array of methodologies designed to guarantee that instruments comply with rigorous safety, effectiveness, and regulatory criteria. The primary categories of testing include:
- Functional Testing: This ensures the device operates according to its intended use, aligning with performance criteria defined by regulatory bodies like the EU MDR. It confirms that the equipment meets all operational requirements and functions reliably under anticipated conditions.
- Safety Evaluation: Essential for assessing potential risks associated with the apparatus, safety evaluation includes evaluating electrical, mechanical, and thermal safety, as well as ensuring the apparatus complies with international safety standards. 'This evaluation is essential in the benefit-risk analysis, ensuring advantages surpass any hazards throughout the product's lifecycle.'.
- Biocompatibility Evaluation: To ensure the apparatus does not cause harmful biological responses, biocompatibility evaluation involves chemical characterization and other methods tailored to the materials utilized in the apparatus. Adhering to FDA guidelines and consensus standards, this evaluation is essential for instruments that interact with patients.
- Usability Testing: This emphasizes the user's interface, evaluating how easily and safely it can be utilized by intended users in real-world settings. Standards like IEC 62366 guide the usability engineering process, integrating human factors engineering to minimize use errors and enhance overall user experience.
- Software Evaluation: For gadgets incorporating software, this assessment ensures that the software operates reliably and securely. It includes validation and verification processes to detect and mitigate potential software faults, addressing challenges unique to Software as a Medical Device (SaMD).
Following these evaluation procedures not only guarantees alignment with regulatory requirements but also improves the overall quality and reliability of healthcare products, leading to enhanced health outcomes. Real-world case studies, such as the Philips recall, highlight the significance of thorough examination and adherence to prevent potential hazards and guarantee patient safety.
Functional Testing
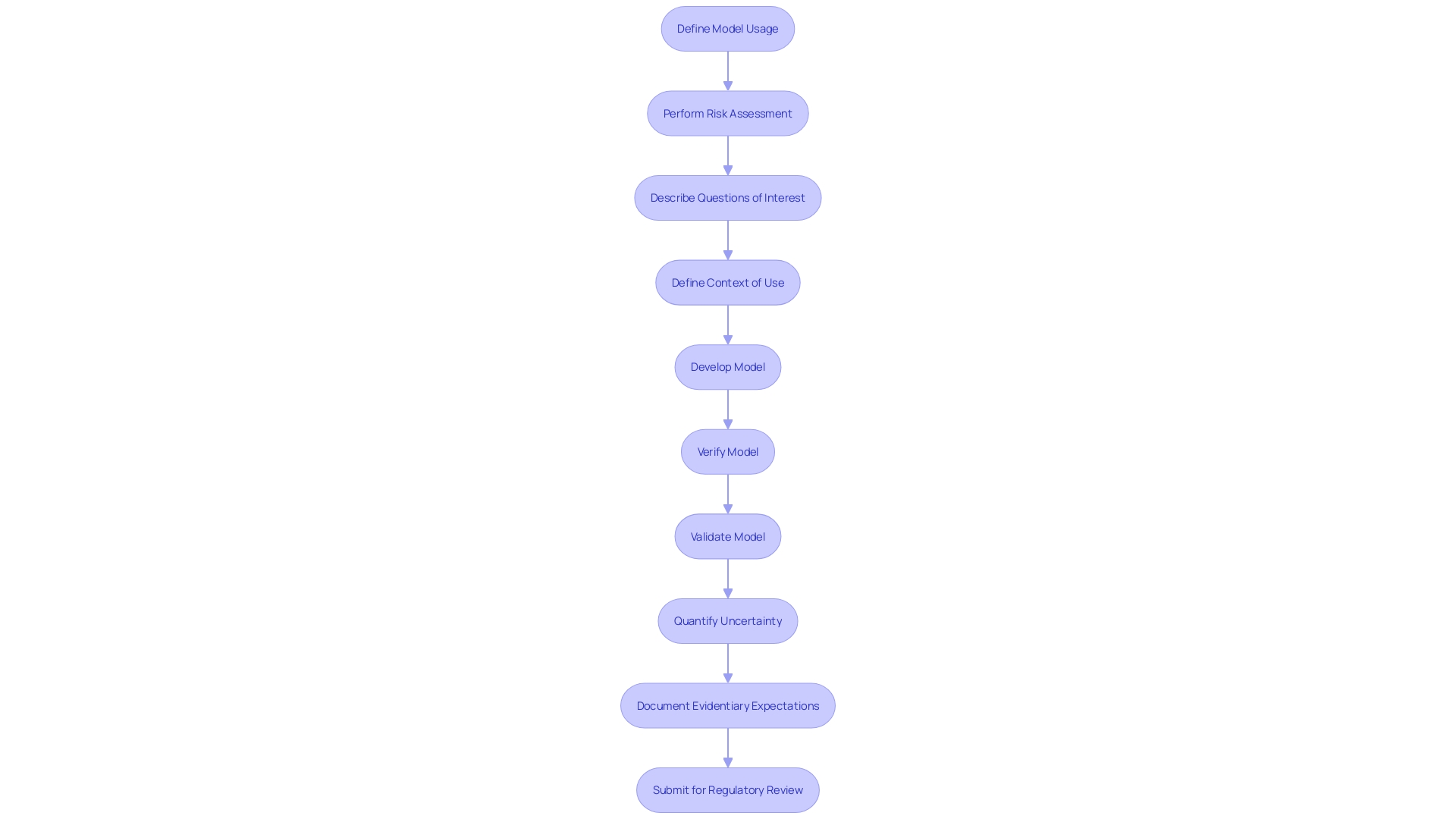
'Functional assessment is essential in determining if a healthcare instrument operates as expected under defined circumstances.'. This type of testing ensures that all operational features are effective and reliable, confirming that the equipment behaves as expected in real-world scenarios. According to the FDA recognized standard ASME V&V 40, assessing the credibility of computational modeling through verification and validation is essential for regulatory submissions. This standard outlines a nine-step process to develop and assess the credibility of computational models, which includes defining how the model will be used and performing a risk assessment.
Utilizing computational modeling and simulation (CM&S) in the creation of medical instruments offers significant advantages. It allows manufacturers to test products virtually before physical development, thus identifying and mitigating potential issues early in the design phase. This approach not only shortens product development cycles but also reduces costs by eliminating the need for extensive physical proof-of-concept tools. Moreover, evidence of performance can be generated by assessing large datasets, which is particularly beneficial for ensuring compliance with stringent regulatory standards such as those set by the FDA, ISO, and ASTM International.
Peter Oberst, Director of Applications at Ascential Technologies, emphasizes the importance of balancing business objectives with technical requirements to create robust solutions. This is especially significant in the realm of healthcare equipment evaluation, where protection, dependability, and adherence to standards are crucial. Furthermore, the transition of healthcare apparatus systems to the cloud presents new obstacles, especially regarding security and scalability, which need to be tackled to guarantee the security and efficiency of both the instruments and their related software.
'The significance of operational assessment cannot be exaggerated, as it plays a crucial role in the clinical evaluation and post-market observation of healthcare instruments.'. By ensuring that a product operates dependably under real-world conditions, producers can offer thorough proof of its safety and effectiveness, ultimately aiding in improved patient results and progress in healthcare technology.
Safety Testing
Safety testing is an essential procedure focused on recognizing and reducing possible dangers linked to healthcare instruments. 'This thorough assessment is crucial, considering that during a recent decade, over 1.7 million injuries and 83,000 fatalities in the U.S. were potentially associated with defective healthcare tools ranging from surgical masks to implantable pacemakers.'. In response, the FDA has initiated the development of a surveillance system to monitor these instruments, although it faces challenges such as securing funding and identifying patient users.
The FDA's approach to risk assessment emphasizes the importance of reviewing existing literature and public information to pinpoint specific risks and appropriate mitigation measures. Clinical information, while valuable, must be examined for relevance, especially when there are changes in the material or production process of the healthcare product.
Dr. Sandra Rothenberg from the Rochester Institute of Technology emphasizes the challenges researchers encounter in obtaining comprehensive information on equipment's significant similarity, which complicates the evaluation of possible risk concerns. In spite of these obstacles, safety evaluation continues to be a crucial element of the healthcare product lifecycle, guaranteeing that items are both secure and effective for patient use.
Biocompatibility Testing
Biocompatibility evaluation is an essential procedure to guarantee that medical instruments engage safely with biological systems. This testing involves a comprehensive evaluation of how the materials used in the apparatus affect the body, aiming to prevent adverse reactions. Utilizing existing literature and public information is essential for identifying specific risks and mitigation measures, as advised by the FDA. 'Manufacturers should consider chemical characterization methods tailored to the materials of the product and adhere to FDA-recognized consensus standards for consistency and efficient review.'.
'Incorporating clinical experience and data from previous similar products can enhance the understanding of biocompatibility, though it is crucial to assess the relevance of such information, especially when there are material changes.'. 'Non-clinical animal studies can provide initial proof of security and performance, but additional tests may be required if negative biological reactions are noted.'. Recent advancements, such as BioInteractions’ Astute coating, demonstrate the ongoing efforts to improve compatibility and patient safety by mimicking natural biological layers, reducing the risk of rejection and thrombosis.
'Submission to regulatory bodies like CDRH or CBER requires a detailed risk assessment summary, addressing identified risks, mitigation strategies, knowledge gaps, and any adverse effects noted during evaluations.'. By adhering to these guidelines, sponsors can create a strong biocompatibility plan, promoting the approval and safe utilization of healthcare instruments.
Cytotoxicity Testing
'Cytotoxicity assessment examines the harmful impacts of medical tools on cultured cells, which is crucial in deciding if an instrument could damage living tissues.'. According to FDA guidelines, sponsors must provide a detailed risk assessment, including explanations of identified risks, mitigating information, and any toxicities observed during biocompatibility tests. This assessment helps ensure comprehensive evaluation and highlights any knowledge gaps. Leveraging existing literature and public information can further assist risk reduction efforts, making the process more efficient and decreasing the need for unnecessary evaluations. Chemical analysis of the apparatus in its final form is also essential for long-term toxicity evaluation, supported by assessments of the apparatus geometry and surface properties.
Irritation Testing
'Irritation testing is crucial in evaluating whether healthcare instruments induce localized inflammatory responses when in contact with skin or mucosal surfaces.'. This evaluation guarantees the safety of materials utilized in equipment that interacts with patients. For instance, the Guinea Pig Maximization Sensitization Test (GPMT) and the NaCl Extract Test are standard procedures in nonclinical studies. These tests, conducted under FDA directives, validate the scientific credibility of data submitted for Investigational Device Exemption and Premarket Approval applications.
Materials utilized in medical instruments can have diverse interactions with tissues. As noted by experts, “All these devices are made of various materials. These materials are diverse and can interact differently with the tissue where they are placed.” This biocompatibility is context-dependent; a material suitable for a short-term application might cause adverse effects if used long-term.
Recording these findings in a risk evaluation is essential. The FDA stresses a risk-oriented method, advising thorough biocompatibility evaluations to recognize and reduce possible dangers. 'This rigorous assessment process not only ensures patient safety but also aids in navigating the complex regulatory landscape, ultimately facilitating the development and approval of safe and effective healthcare products.'.
Sensitisation Testing
Sensitisation assessment is essential in determining if a medical instrument could provoke an allergic response upon repeated exposure. 'This testing not only assesses the potential for hypersensitivity but also ensures that the components do not release harmful substances.'. For instance, volatile organic compounds (VOCs) derived from the production process of plastic parts in respiratory equipment can pose significant risks, including skin sensitisation and long-term toxicity. These compounds are meticulously analyzed using techniques like gas chromatography-mass spectrometry (GC-MS) to ensure they are below defined toxicological thresholds.
Recognising potential risks is a key component of biocompatibility assessments. Factors such as the item's material, geometry, and surface properties must be considered as they can induce unwanted tissue responses. Furthermore, combining clinical and non-clinical animal studies into the assessment process improves comprehension of safety, although these studies may not always yield comprehensive biocompatibility information.
The effect of allergens, especially in equipment utilized by vulnerable groups, cannot be overstated. For example, peanut allergies are a common and severe condition, often requiring precise diagnostic methods like the oral food challenge test, despite its complexity and inherent risks. Innovations in evaluations, such as the alternative developed by researchers at the University of Bern, which mimics allergic reactions in a test tube, offer higher diagnostic accuracy and reduce the need for riskier procedures.
Integrating thorough assessment and evaluation techniques, including awareness evaluation, is crucial to guarantee the security and effectiveness of healthcare instruments throughout their lifespan.
Systemic Toxicity Testing
'Systemic toxicity testing is an essential aspect of assessing medical instruments, concentrating on their potential to damage vital organs and systems throughout the body.'. 'This comprehensive assessment is essential for confirming the overall safety of a product before it reaches the market.'. 'Utilizing published literature and previous experiences with comparable tools is especially priceless.'. When a new item closely resembles an existing one in terms of material composition and intended use, existing data can significantly streamline the risk assessment process. This approach not only aids in identifying specific risks but also helps in formulating effective mitigation measures.
Chemical analysis plays a pivotal role in this examination, especially when considering long-term toxicity. Evaluating the device in its finished form is crucial, but it is equally important to examine its geometry and surface properties to gain a complete understanding of potential toxic effects. The FDA suggests a comprehensive evaluation of accessible information to prevent repetitive and needless assessments, including animal evaluations. This guidance aims to maximize the use of relevant data, thereby promoting efficiency and consistency in the review process.
For instance, the FDA's guidance on analytical chemistry testing offers a framework for assessing biocompatibility. This includes chemical characterization as a strategy for the overall biocompatibility evaluation of medical equipment. Various techniques may be required depending on the materials utilized or historically established methods for specific instruments. Furthermore, the guidance underscores the importance of following device-specific FDA guidelines or recognized consensus standards that address chemical analysis requirements.
In practical application, such as the UW Translational Center for Microphysiological Systems, advanced platforms using human kidney cells have shown promise. These systems replicate human kidney function more accurately than conventional approaches, enabling more precise toxicity evaluation and the creation of new therapeutic strategies. These advancements underscore the significance of strong systemic toxicity evaluation in guaranteeing the security and effectiveness of healthcare instruments.
Usability Testing
Usability testing in healthcare tools is a thorough assessment of how healthcare professionals and patients engage with the tool, concentrating not only on the user interface but also on the overall user experience. This process involves service blueprinting and stakeholder analysis to ensure that all interactions, whether physical or digital, are seamless and efficient, ultimately enhancing safety and effectiveness. For instance, the user interface specifications prescribed by the US Food and Drug Administration (FDA) are critical. These guidelines necessitate healthcare tools to move from hospital environments to home care with a user-focused design strategy, including intuitive graphical interfaces and ergonomic controls. By comprehending the varied requirements of individuals in various settings—whether in an ICU, NICU, ER trauma bay, or at home—usability evaluations guarantee that healthcare tools are efficient and user-friendly in every situation. As Morven Shearlaw aptly states, "Quality management is about quality, it's not just about proving that you can sell in the market because you've got a certain certification."
Software Testing for Medical Devices
Evaluating software for healthcare tools entails thorough examinations to guarantee that the software elements operate properly and securely. This includes both verification and validation processes. Conventional methods for software evaluation, such as unit examination, integration assessment, and system analysis, are crucial but may not completely tackle the intricacies of contemporary healthcare instruments, which depend significantly on interactions between hardware and software.
A more unified verification strategy is necessary to identify subtle yet critical bugs that may arise from these interactions. The Portable Stimulus Standard (PSS), developed by Accellera, offers a robust solution by enabling the creation of automated, reusable, and portable test scenarios for both software and hardware. This comprehensive approach helps prevent system crashes, security vulnerabilities, and other unwanted effects that could jeopardize patient well-being.
As healthcare instruments become more interconnected with AI/ML technologies, upholding quality assurance and regulatory compliance is an escalating challenge. Automation and quality process enforcement are crucial in ensuring teams follow best practices and proactively address risks. By adopting advanced tools and methodologies, developers can enhance the security and reliability of healthcare software, ultimately improving patient outcomes.
Verification and Validation
Verification ensures that the software meets the specified requirements, confirming that each function operates as intended. On the other hand, validation confirms that the software fulfills its intended use in real-world applications, ensuring that it performs effectively in practical scenarios. Both procedures are essential for adherence, as they assist in recognizing possible hazards and guaranteeing the dependability and security of health equipment. According to Ketryx’s CEO Erez Kaminski, “The complexity of healthcare products and software has increased significantly over the past few decades, leading to challenges in development and compliance.” Ensuring that software undergoes robust verification and validation is essential for meeting rigorous regulatory standards and protecting patient safety. Furthermore, safety is a crucial aspect of creating software for health-related instruments. Any potential communication with a medical device must be secure and encrypted to comply with medical device regulations, often using established cryptographic communication protocols such as Transport Layer Security (TLS).
Risk Management in Software Testing
Risk management in software evaluation is vital to ensure that potential issues are identified and addressed before they become critical. With the rapid pace of innovation, software applications must evolve quickly to avoid obsolescence, placing immense pressure on quality assurance (QA) teams. Efficient risk management includes not only recognizing possible risks related to software failure but also executing strategies to reduce these risks during the development and evaluation stages.
AI-driven evaluation has emerged as a powerful tool in this landscape, offering enhanced test coverage and efficiency. According to a recent World Quality Report, only 56% of organizations have achieved satisfactory test coverage in their software evaluation processes. AI-driven evaluation solutions can significantly enhance this, with some reports indicating an increase in test coverage by up to 85%. These solutions automate test case creation and execution, minimizing human error and ensuring more comprehensive and accurate evaluation.
For instance, companies that have adopted AI-powered evaluation tools have reported up to a 40% reduction in assessment duration and a 60% decrease in the number of bugs identified in production. This not only speeds up the evaluation process but also enables QA teams to concentrate on more complex and strategic activities. Moreover, a survey by Capgemini discovered that organizations utilizing AI-driven evaluations reported a 30% decrease in assessment costs and a 25% rise in evaluation efficiency.
In the banking sector, where the stakes are especially elevated due to strict regulatory demands and the necessity to safeguard sensitive information, effective risk management in software evaluation can avert disastrous security breaches and financial setbacks. For example, M&T Bank, a leading U.S.-based commercial bank, recognized the importance of establishing organization-wide Clean Code standards to support the maintainability and performance of its software.
Overall, the transformation of software evaluation from a perceived financial liability to a source of substantial cost savings and return on investment underscores the importance of modern risk management strategies. By leveraging advanced tools like AI-driven testing, organizations can not only meet the demands of rapid innovation but also ensure the delivery of high-quality, reliable software products.
Documentation and Traceability
'Keeping detailed records and traceability is essential for preserving the integrity of software development, particularly in strictly regulated sectors such as healthcare equipment.'. By treating documentation with the same importance as code, known as Documentation as Code (DaC), teams can ensure that user manuals, troubleshooting guides, and API documentation remain accurate and up-to-date with every change in the software. This approach minimizes the risk of outdated or incorrect information, thus enhancing accountability and compliance with regulatory requirements.
Incorporating automated systems to update documentation alongside software changes can significantly reduce errors and manual workload. For instance, by integrating end-to-end tests with documentation, every user interface adjustment or workflow modification is instantly mirrored in user guides. This method not only streamlines the process but also ensures that documentation accurately reflects the current state of the software.
The importance of maintaining high-quality documentation cannot be overstated. Inconsistent documentation can damage a brand's reputation, as seen in cases where manual updates become neglected under tight deadlines. An efficient documentation system that updates in real-time can prevent such issues, maintaining the product's credibility and reliability.
Statistics from the industry highlight the effectiveness of automated documentation systems. For example, automated tests can verify changes within minutes, a task that would otherwise take days if done manually. This capability is critical for maintaining operational excellence, as emphasized by industry leaders who stress the necessity of quick recovery and consistent availability of software services.
In summary, by integrating documentation into the development lifecycle and automating updates, organizations can ensure compliance, enhance accountability, and maintain the high quality necessary for success in regulated environments.
Regulatory Frameworks for Medical Device Testing
Regulatory structures are crucial for guaranteeing that healthcare instruments fulfill rigorous quality and effectiveness criteria. The UK Medicines and Healthcare products Regulatory Agency (MHRA) has recently announced a comprehensive plan for new regulations, prioritizing patient safety and aiming for completion by 2025. 'This framework is created to tackle the swift progress in health technology, including implantable tools and healthcare AI, by providing more patient-focused and globally aligned standards.'.
The importance of strong regulations is highlighted by the concerning statistics from the U.S. Food and Drug Administration (FDA), which reported over 1.7 million injuries and 83,000 deaths possibly associated with defective medical equipment over a decade. These incidents highlight the critical need for a reliable surveillance system, which the FDA is currently developing despite challenges in funding and patient identification. Moreover, industry experts emphasize that merely meeting standards does not guarantee the creation of safe and effective products.
'Evidentiary expectations for 510(k) implant products, as outlined by the FDA, demand rigorous clinical data to substantiate claims of security.'. This approach aims to mitigate risks and enhance patient outcomes, ensuring that products on the market do not just pass minimum standards but also deliver real-world safety and efficacy. 'The changing regulatory environment, propelled by rising demands, is influencing the creation and market introduction of healthcare products, urging producers to embrace more efficient and compliant methods.'.
FDA Guidelines
The FDA's guidelines for the evaluation and authorization of healthcare equipment in the United States are extensive and meticulously detailed. These guidelines are largely influenced by the ASME V&V 40, a recognized standard that provides a framework for assessing the credibility of computational models through verification, validation, and uncertainty quantification (VVUQ). This standard outlines a nine-step process for developing and assessing the credibility of computational models and simulations (CM&S) for regulatory submissions, including defining how the model will be used and performing a risk assessment.
Moreover, the FDA's guidance addresses evidentiary expectations for 510(k) implants, emphasizing that meeting the standard does not inherently ensure the safety and effectiveness of the product. Mike Drues, a leading expert in the healthcare equipment sector, remarks, "Just because you're meeting the standard, that just means that you're passing... That doesn't necessarily mean that you're creating a safe and effective product."
The FDA also provides explicit recommendations for the use of clinical data in premarket notification [510(k)] submissions. This guidance is part of the agency's broader commitment to collaborating with industry to enhance patient access to safe, effective, and high-quality healthcare tools. The FDA's function in safeguarding community health goes beyond health instruments to encompass human and veterinary medications, vaccines, biological products, food quality, cosmetics, dietary supplements, and tobacco oversight.
For those interested in the intricate details of these processes, the FDA offers resources such as the Federal Register Documents webpage, which includes the historical record of all FDA recognition determinations. Furthermore, the CDRH Learn modules offer additional information on the use and recognition of consensus standards, ensuring that healthcare professionals have access to comprehensive and current guidance.
ISO 10993 Standards
ISO 10993 encompasses a series of standards designed to evaluate the biocompatibility of medical products, ensuring their safety for human use. Adhering to these standards is critical for regulatory compliance and involves a comprehensive risk assessment process. The guidelines propose methodologies for assessing potential risks related to equipment components that come into contact with breathing gases, considering patient toxicity, sensitization, and irritation. For instance, the standard includes a framework for evaluating respiratory devices, similar to the chemical assessment of extractables and leachables from pharmaceutical containers but with additional physical evaluations.
The evaluation process starts with a paper-based review of current data, identifying information gaps, and establishing requirements for additional examination. This approach aims to minimize the need for animal testing, aligning with the FDA's efforts to harmonize regulatory frameworks globally. The FDA's Quality System (QS) regulation, which applies to all finished product manufacturers, underscores the necessity of having objective evidence of meeting these requirements, although the actual work may be delegated.
Additionally, the FDA's Recognized Consensus Standards Database offers a collection of standards that manufacturers can utilize to assert compliance in product submissions. These standards are regularly updated to reflect the latest scientific and technical advancements. Adopting ISO 10993 not only guarantees adherence but also promotes the creation of safe, effective, and high-quality healthcare products, ultimately improving patient outcomes.
Best Practices for Medical Device Testing
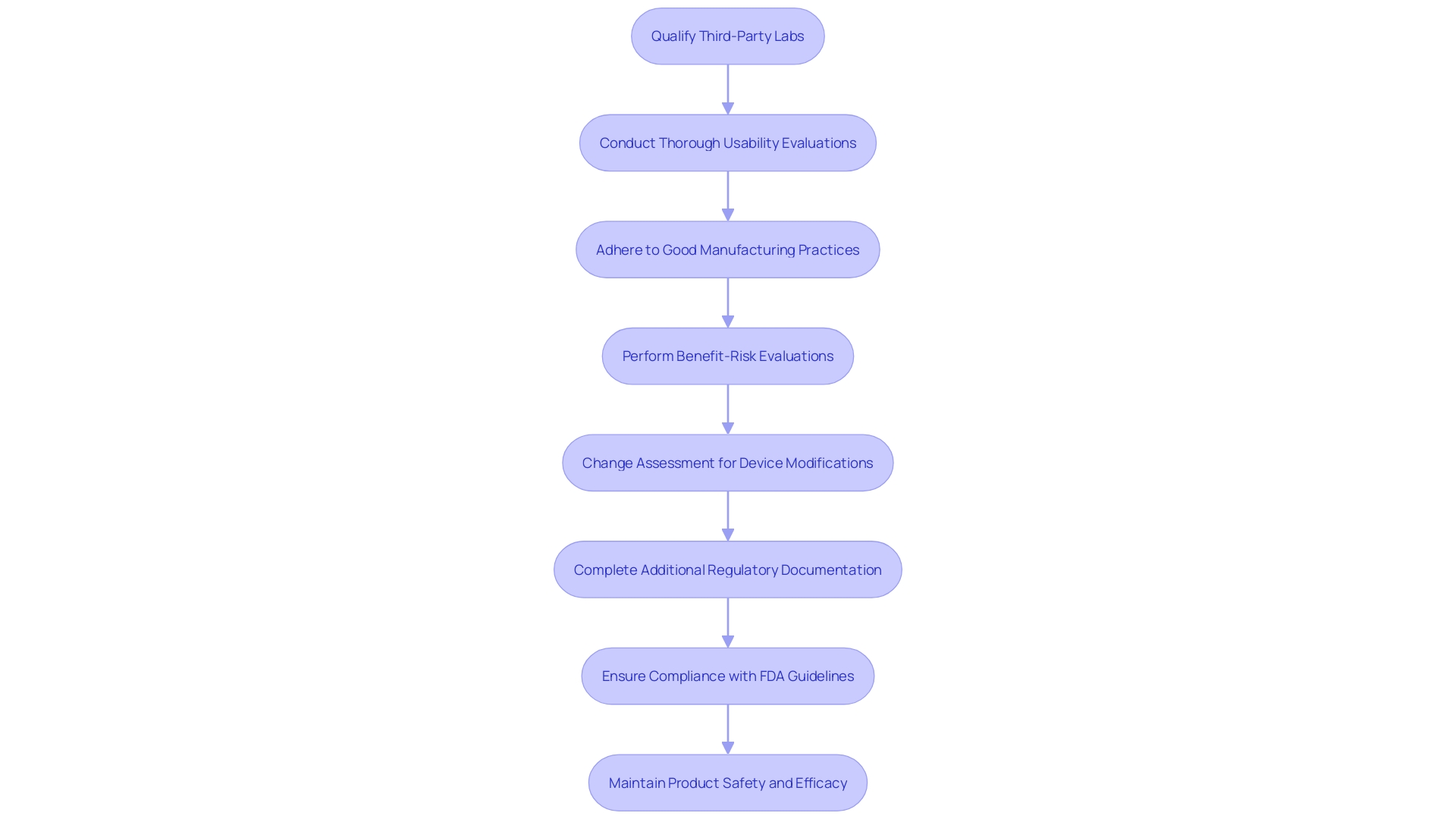
Adopting best practices in healthcare equipment testing greatly improves the dependability and efficiency of the overall procedure. According to the FDA, there has been a troubling increase in submissions containing unreliable data from third-party test labs, particularly in China and India. This surge in questionable data has led to numerous instances where the FDA could not authorize the marketing of medical products, causing direct impacts on sponsors and manufacturers. This also impacts patients and healthcare providers by limiting access to new equipment and potentially disrupting supply chains.
To counteract this issue, it is crucial for manufacturers to take proactive steps. 'Ensuring that third-party labs are thoroughly qualified and closely scrutinizing all assessment data—especially those related to biocompatibility and performance evaluation—are necessary measures to uphold data integrity.'. Numerous mistakes in healthcare instrument evaluation arise from insufficient standardization and inadequate design, which may result in usability problems and product withdrawals. Carrying out thorough usability evaluations during the product creation process can avert such mistakes and improve equipment security.
The complexity of healthcare equipment qualification is further compounded by regulatory constraints and confidentiality concerns. However, adhering to Good Manufacturing Practice (GMP) and international standards such as ISO 13485 can help navigate these challenges. Even though the burdensome nature of these regulations exists, they are crucial for guaranteeing patient well-being and product effectiveness.
The benefit-risk evaluation is another essential element, ensuring that the advantages of a healthcare instrument surpass its dangers. This assessment supports clinical evaluation and post-market surveillance, maintaining the product's safety and efficacy throughout its lifecycle. Engaging in pilot studies, seeking feedback from regulatory agencies, and defining clear performance criteria are vital steps in this process.
Statistics underscore the importance of detailed data analysis and consistent documentation. Connecting disparities between clinical goals and research information through thorough evaluation and principled choices can further improve the quality and dependability of healthcare instruments.
By following these best practices, manufacturers can not only meet regulatory standards but also ensure that their products perform as intended, ultimately safeguarding patient health and improving clinical outcomes.
Risk-Based Approach
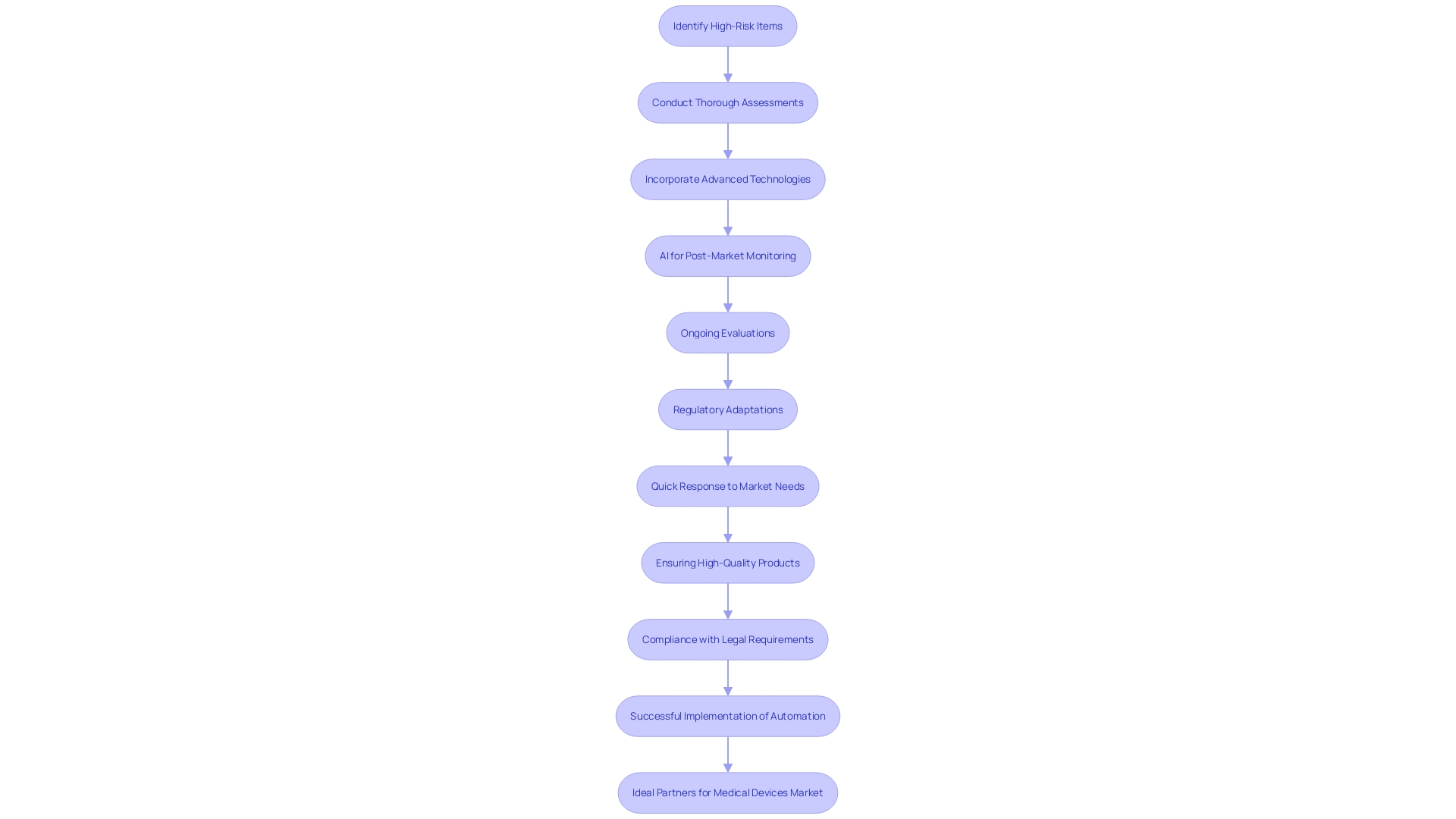
A risk-oriented strategy for testing equipment distributes resources according to the possible dangers linked with various instruments, guaranteeing that high-risk items receive the most thorough assessments. This strategy is crucial considering that over 1.7 million injuries and 83,000 fatalities during a recent decade in the U.S. have been potentially associated with defective medical equipment. Regulatory agencies, including the FDA, are improving their monitoring systems to detect safety concerns more efficiently, beginning with a select number of products and broadening gradually.
The traditional focus on pre-market testing, while essential, is no longer sufficient. 'Manufacturers are now anticipated to participate in thorough post-market monitoring, incorporating advanced technologies like AI, interconnected gadgets, and innovative sensors.'. This shift aims to transform post-market intelligence, which historically relied on voluntary reporting and feedback from users.
Joseph Kotarek from the FDA's Center for Devices and Radiological Health pointed out that reclassifying certain items into class II (special controls) will improve patient access to innovative healthcare products while ensuring their security and effectiveness. This regulatory adaptation underscores the necessity for detailed risk assessments backed by robust data. As Helene Quie aptly stated, 'It's not just about managing risks; it's about ensuring that the benefits are powerful enough to justify those risks.'
In conclusion, the incorporation of digital tools and AI in healthcare instruments, along with strict regulatory standards, is poised to enhance the overall reliability and efficiency of healthcare instruments, thereby ensuring improved patient results.
Comprehensive Validation and Verification
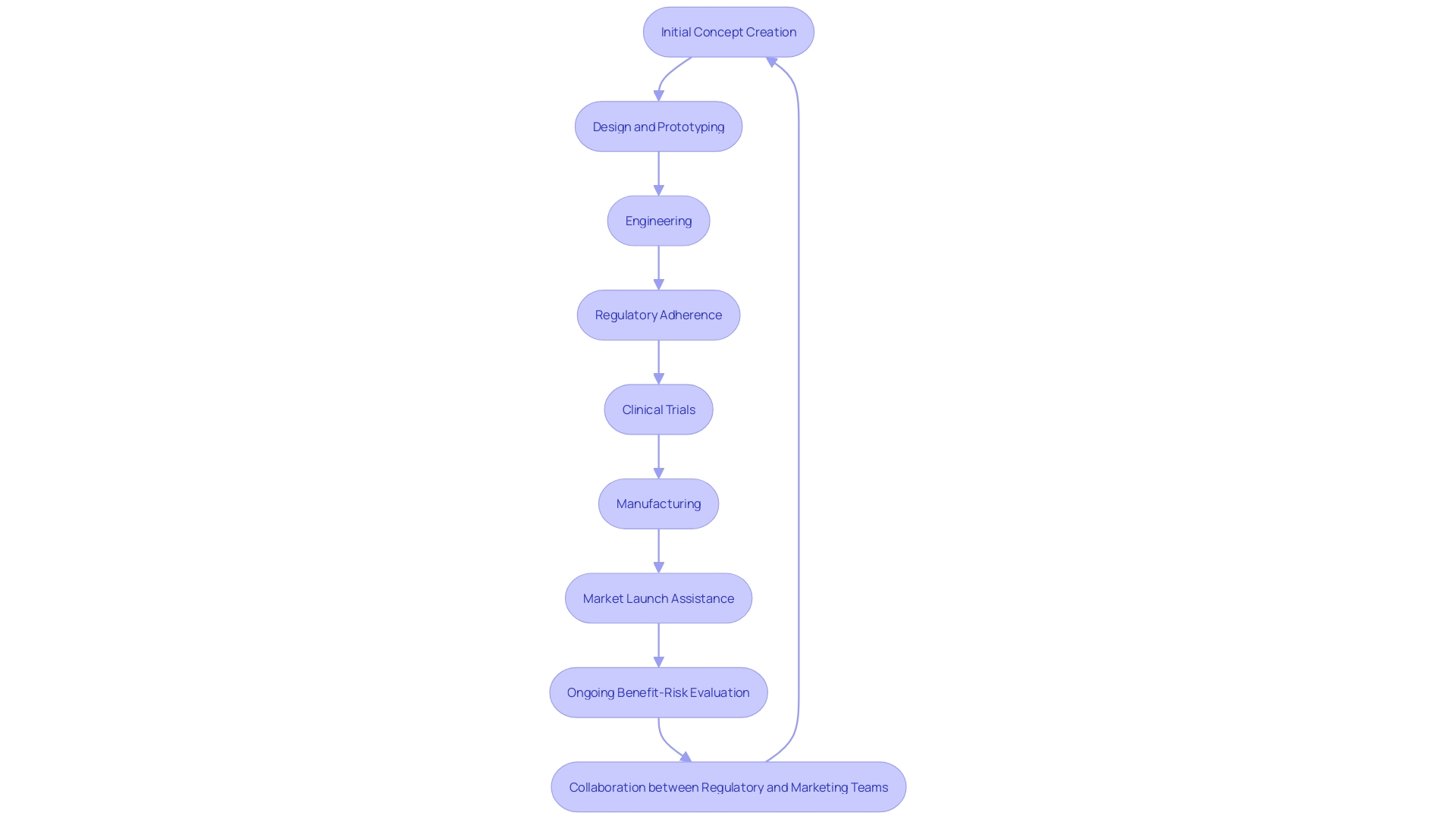
Thorough validation and verification procedures are essential to guaranteeing every element of a healthcare instrument is meticulously examined, leading to superior results and enhanced protection. A skilled healthcare product creator will provide a comprehensive range of services that encompasses the entire lifecycle of product development, including initial concept creation, design and prototyping, engineering, regulatory adherence, clinical trials, manufacturing, and market launch assistance. This thorough approach helps to address the gap often observed between state-of-the-art defined security and performance objectives and the actual data collected during clinical investigations.
The benefit-risk evaluation is an essential part of the design and development process, ensuring that the advantages of the medical instrument outweigh its risks. This assessment plays a significant role in both clinical evaluation and post-market surveillance, continuously ensuring the product's safety and efficacy throughout its lifecycle. A key aspect of this process is defining the right performance criteria and data to support the intended use of the product, which can be complex but is crucial for alignment with regulatory requirements and market expectations.
Clinical benefit and claims must be accurately represented and supported by factual evidence and data, as emphasized by the EU MDR and MDCG. This requires close collaboration between regulatory teams and marketing departments to align the product's market expectations with its actual clinical benefits. Furthermore, integrating clinical experience into the biological assessment process improves the comprehension of biocompatibility, aiding in the creation of safe and effective healthcare products.
In the complex realm of healthcare equipment production, accuracy and excellence are essential. Each phase of the procedure, from preliminary design to ultimate evaluation, must comply with rigorous criteria to guarantee the protection and efficiency of healthcare instruments. The metrology process, which involves scanning multiple parts or long components, detecting minute defects, and providing accurate feedback for adjustments, is central to this journey. 'Following these stringent criteria aids in reducing the concerning pattern noted by the FDA, where untrustworthy information from external testing facilities has led to the inability to grant marketing approval for certain health products.'.
The ongoing initiatives, such as the Innovative Devices Access Pathway (IDAP) pilot, aim to streamline the development of technologies addressing clinical needs without compromising security, quality, and efficiency. These initiatives, supported by significant financial resources, are vital in guaranteeing that new healthcare tools meet the highest criteria of security and effectiveness, ultimately enhancing patient results and progressing healthcare understanding.
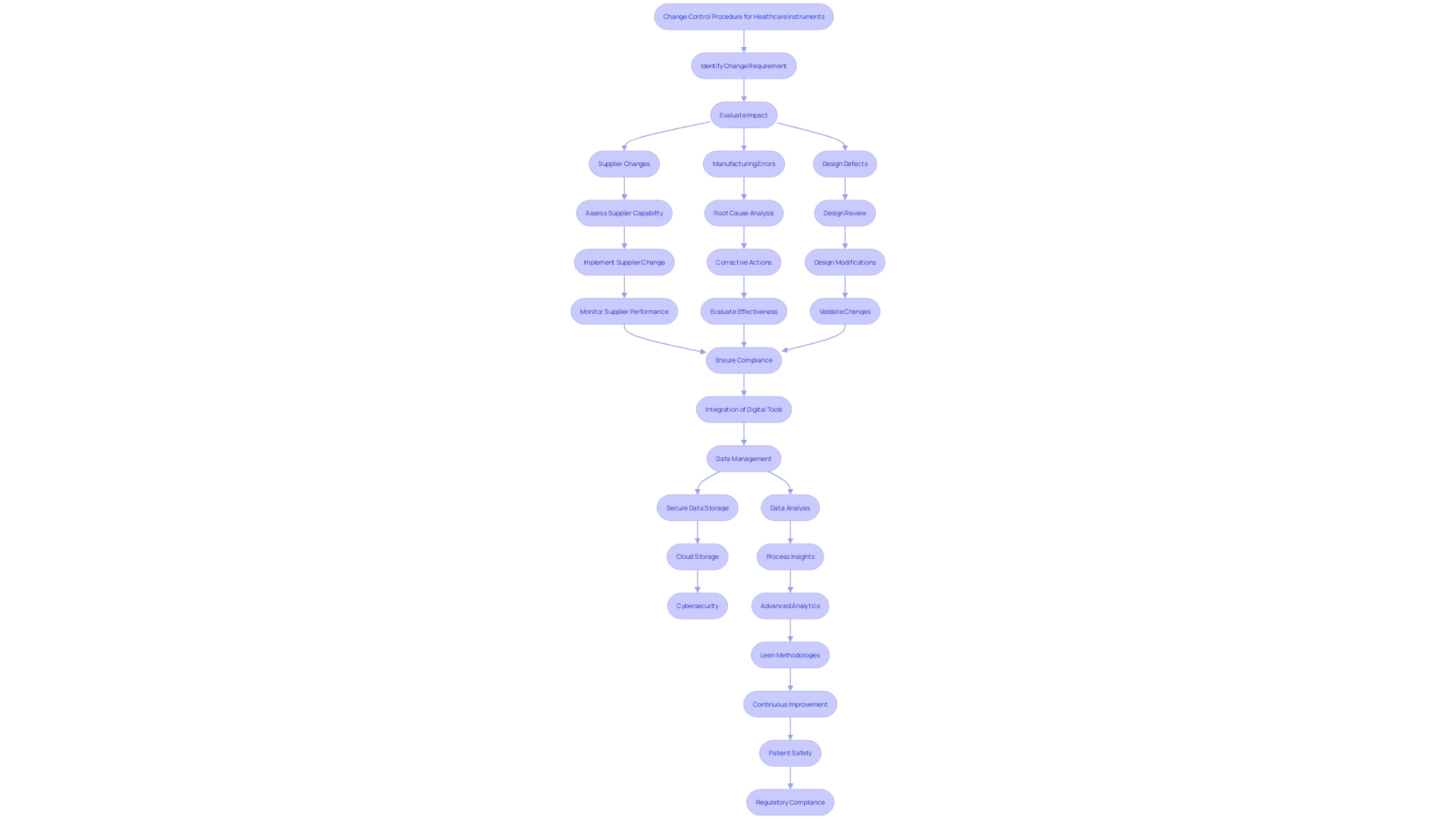
Change Control Process
The change control procedure carefully oversees alterations to healthcare instruments during their entire lifespan, guaranteeing that security and adherence are never jeopardized. This process is critical as it addresses various factors that can lead to nonconformance, such as changes at the supplier, errors in manufacturing, design defects, and poor-quality materials. Handling these changes manually at the serial number level poses a high risk for potential errors, including misidentifying units in different stages of production or shipment.
To mitigate these risks, advanced analytics and digital continuity are implemented across the value chain. For instance, using product data to produce digital work instructions on the shop floor and employing artificial intelligence to analyze quality trends ensures accuracy and compliance at every workstation. This approach not only improves patient safety but also reduces costs associated with regulatory penalties and recalls.
'Recording and addressing nonconformities is an essential aspect of a quality management system for healthcare product producers.'. According to a Grand View Research study, over 50% of manufacturers reported significant non-compliance issues during audits, which highlights the frequent lapses in meeting regulatory standards. These challenges, often arising during the design and process validation stages, underscore the importance of a robust change control process.
Moreover, the implementation of lean methodologies in medical device manufacturing has shown a reduction in product development lead times by 50%, providing cost savings and reducing waste by up to 80% in some settings. This not only enhances market competitiveness but also speeds up the introduction of innovative products, ensuring they meet the highest standards of safety and efficacy.
Conclusion
Ensuring the safety and effectiveness of medical devices is a critical endeavor that requires a comprehensive testing approach. The various types of testing—functional, safety, biocompatibility, usability, and software—each play a vital role in validating that devices meet stringent regulatory standards and perform reliably in real-world conditions.
Functional testing evaluates the operational capabilities of medical devices, confirming they meet performance criteria. Safety testing assesses potential risks, ensuring that benefits outweigh hazards throughout a device's lifecycle. Biocompatibility testing examines how materials interact with biological systems, while usability testing enhances user experience and minimizes errors.
Finally, software testing addresses the complexities associated with digital components, focusing on reliability and security.
The importance of adhering to these rigorous testing protocols cannot be overstated. Real-world case studies, such as the Philips recall, highlight the consequences of inadequate testing and the necessity of comprehensive evaluations to ensure patient safety. As regulatory frameworks evolve to address advancements in technology, the integration of thorough validation and verification processes will continue to be essential in delivering safe and effective medical devices.
In summary, a robust testing framework not only ensures compliance with regulatory requirements but also enhances the quality and safety of medical devices, ultimately contributing to improved healthcare outcomes and patient safety. The commitment to these rigorous standards is paramount in fostering trust and efficacy in the medical device industry.




