Introduction
Contract Research Organizations (CROs) play a crucial role in the advancement of medical device technology. From early-stage research to post-market surveillance, CROs collaborate with manufacturers, regulatory bodies, and healthcare professionals to streamline the progression of medical devices. In a rapidly evolving landscape, CROs navigate market dynamics, regulatory compliance, and ethical considerations to ensure that advancements align with societal values and regulatory standards.
This article explores the benefits of partnering with CROs, the key services they offer, their expertise and capabilities, successful case studies, challenges and opportunities in clinical trials, regulatory compliance, and future trends and innovations in medical device CRO services. As the medical device sector continues to evolve, CROs are at the forefront of embracing cutting-edge innovations to optimize the development process and bring new medical technologies to market efficiently and effectively.
The Role of CROs in Medical Device Innovation
As a hub of innovation in the healthcare sector, Contract Research Organizations (CROs) are crucial in the advancement of technology for healthcare equipment. These entities are intricately involved in the lifecycle of healthcare equipment, from early-stage research to post-market surveillance. They work together with manufacturers, regulatory bodies, and healthcare professionals to facilitate the development of healthcare technology from idea to clinic. With the fast development of healthcare advancements, contract research organizations are progressively essential in navigating the intricate interplay of market dynamics, regulatory compliance, and ethical considerations that shape the field.
The partnership with contract research organizations provides medical device companies with a strategic advantage in expediting the development and market introduction of innovative products. This symbiosis not only fuels technological innovation but also ensures that ethical, legal, and social ramifications are thoroughly considered. The ability of Chief Risk Officers in addressing these concerns is encapsulated in governance frameworks that evolve alongside emerging technologies, ensuring that progress aligns with societal values and regulatory standards.
Recent developments in AI regulation, as announced by the Biden administration, underscore the importance of governance in technology. The projected market growth for AI platforms in healthcare, anticipated to reach $4.3 billion by 2024, further highlights the critical role of Contract Research Organizations in guiding healthcare tools through a rapidly changing landscape. Companies like Greenlight Guru, with industry veterans like Chris, a biomedical engineer with 13 years of experience, lead the way in showcasing how integrated services from concept generation to market launch can optimize the development process, reducing costs and expediting timelines.
In the words of Lisa Hellstrom, Clinical Programme Director at Camurus, 'We need the expertise of contract research organizations.'. But I understand that they are big organizations who see delays, it's not possible for small companies to do all the communications with all the sites ourselves." This sentiment underscores the necessity of CRO partnerships, especially for smaller entities seeking to navigate the intricate and multifaceted healthcare sector. In the ongoing progress of developing healthcare equipment, highlighted by technological advancements such as AI, contract research organizations (CROs) remain indispensable allies in the pursuit of delivering impactful innovations to the market.
Benefits of Partnering with a CRO for Medical Device Development
Working together with Contract Research Organizations (CROs) can provide a strategic benefit for companies involved in the development of healthcare products. These specialized entities bring a wealth of knowledge regarding the complex regulatory environment, which is critical for ensuring that new medical devices meet necessary guidelines and regulations. For instance, the Medical Device Regulation (MDR) presents a challenging legal framework in Europe, requiring compliance for market entry. Companies specializing in clinical research organization are skilled in navigating these intricate requirements, thus facilitating successful certification and compliance.
In addition to regulatory prowess, CROs are equipped with the resources to conduct efficient clinical trials. They simplify the procedure of data gathering and evaluation, which is crucial in establishing the safety and effectiveness of healthcare instruments. This capability is demonstrated by professionals in the field like Etienne Nichols, who use their experience in manufacturing and product development to assist in the development of innovative solutions for combining and delivering drugs.
Furthermore, the capabilities of contract research organizations extend to providing strategic insights throughout the development process. They assist in overcoming the various challenges that arise, optimizing product performance along the way. This is especially important in the context of the constantly changing landscape of healthcare instruments, where technologies like the CardiAQ system are making progress with advanced sensors and AI algorithms that enhance early identification and management of illnesses.
Moreover, the contribution of contract research organizations in facilitating successful exits for medical device startups cannot be underestimated. Business development leaders acknowledge the importance of strategic partnerships, acquisitions, or alliances, which organizations specializing in contract research can help negotiate, leading to potentially lucrative exits for startup ventures.
The value of CRO partnerships is further emphasized by the success of companies like Everly Health, which has teamed up with over a hundred enterprise clients to enhance disease detection and prevention using advanced diagnostics and remote care. This shows how contract research organizations can have a crucial impact on advancing healthcare outcomes and innovations.
To summarize, partnering with a CRO offers companies in the field of medical devices not only regulatory guidance and trial management, but also strategic business insights that can lead to the successful launch and scaling of technologies in a highly competitive and regulated industry.
Key Services Offered by Medical Device CROs
Contract Research Organizations (CROs) specializing in healthcare equipment are crucial partners in the healthcare sector, offering comprehensive services that span from the initial concept of a tool through to its final use in clinical settings. An important part of their work is the implementation of user-centered design, guaranteeing that healthcare instruments efficiently fulfill the particular requirements and preferences of different stakeholders, including patients, clinicians, and hospital staff. This approach emphasizes the experiences of all users, including caregivers, support staff, and laboratory technicians, adapting solutions to enhance how these individuals engage with healthcare advancements.
Contract research organizations play a crucial role in coordinating and overseeing the complex aspects of clinical trials for medical devices. Their expertise includes crafting robust study designs, developing meticulous protocols, and selecting optimal sites for trials. They adeptly handle patient recruitment and enrollment, a process that is increasingly important as digital technology reshapes healthcare practices and private practices vie for competitive edges. By utilizing virtual care platforms and other innovations, organizations can enhance patient management and care quality.
Data collection and analysis are other key services provided by CROs, offering insights essential for navigating the market's challenges and seizing its opportunities. They ensure regulatory support and quality assurance, maintaining strict adherence to ethical standards and compliance requirements. Their role extends to the successful launch of new products, demonstrating a proven ability to guide healthcare instruments from ideation to market entry.
Leaders in the industry recognize that successful companies in the healthcare sector, and by extension their CRO partners, often exhibit similar characteristics, such as determination and belief, which are crucial in attaining favorable results such as acquisitions, IPOs, or strategic partnerships. In a fast-growing market for healthcare advancements, which is expected to achieve substantial revenue figures in the future, organizations that excel in combining software and hardware components are especially well-positioned to satisfy the growing need for groundbreaking healthcare solutions. Their contributions are essential to the progress of healthcare technology, impacting the lives of numerous individuals through the products they assist in realizing.
Expertise and Capabilities of Top Medical Device CROs
Leading Contract Research Organizations (CROs) in the healthcare field are at the forefront of facilitating clinical research. Their expertise extends across the intricacies of trials for devices in the realm of medicine, underscored by a seasoned team that includes physicians, biostatisticians, and project managers. These experts possess a comprehensive understanding of the development of healthcare equipment, guaranteeing that every stage of clinical trials is meticulously overseen. The advanced facilities and tools these CROss possess are crucial in the smooth implementation of trials, effective data management, and sophisticated analysis. Advancements in healthcare instrument development are swiftly progressing, as demonstrated by companies like Summer Health, which incorporates generative AI to enhance medical appointment notes, lessening administrative loads and improving patient comprehension. These advancements are not just about technology but also about improving the quality of care, as seen with pulmonary hypertension, a niche yet significant therapy area in medtech. The commitment to improving trials for healthcare equipment is mirrored by professionals in the field, such as Chris, a biomedical engineer with 13 years of experience overseeing clinical studies for Class III products. He embodies the commitment to safety and efficacy in healthcare products, a sentiment shared by professionals who contribute to clinical trials. Their collective efforts are crucial in bringing new, life-changing treatments to market, offering hope to patients and forging a path to a healthier future.
Case Study: Successful Collaboration with a Medical Device CRO
An engaging demonstration of the collaboration between healthcare equipment manufacturers and Contract Research Organizations (CROs) is observed through the perspective of a recent partnership. In this case, a healthcare technology company was at the forefront of developing an innovative implantable solution aimed at addressing a particular health concern. The company enlisted a CRO well-known for its expertise in coordinating trials for similar healthcare products. The CRO's role was multifaceted, encompassing the crafting of the study design, the gathering and examination of data, and the critical task of patient recruitment. The culmination of this joint venture was the successful execution of a clinical trial, which highlighted the product's effectiveness and safety. Such a collaborative effort highlights the essential role CROs play in advancing the sector.
Maike Stenull of Johnson & Johnson Innovation encapsulates the essence of this evolution, predicting a healthcare landscape that is increasingly preemptive rather than reactive, leaning on the pillars of interception and prevention. This paradigm shift necessitates multidisciplinary approaches and a reimagining of our healthcare framework. The integration of extensive health data sets also emerges as a critical frontier, as echoed by Stenull, who envisions an era where smart data analytics are seamlessly woven into the fabric of healthcare delivery. Meanwhile, advancements like the Cardiac system demonstrate the progress being made in healthcare technology—integrating high-performance sensors and AI to enhance early identification and management of cardiac conditions, showcasing the development and influence of CRO-led clinical trials in realizing next-generation innovations.
Moreover, the importance of improving the effectiveness of healthcare equipment is emphasized by recent research conducted by the American Heart Association and the European Society of Cardiology, which indicate that cardiovascular diseases are the primary contributors to death in the United States and Europe, respectively. This underscores the necessity for innovative monitoring systems, such as those developed through CRO partnerships, which can offer real-time, precise measurements and ultimately save lives by improving clinical outcomes. As the healthcare industry advances, the strategic partnerships and knowledgeable handling of clinical information facilitated by contract research organizations will be crucial in shaping the future of healthcare technology advancement and patient care.
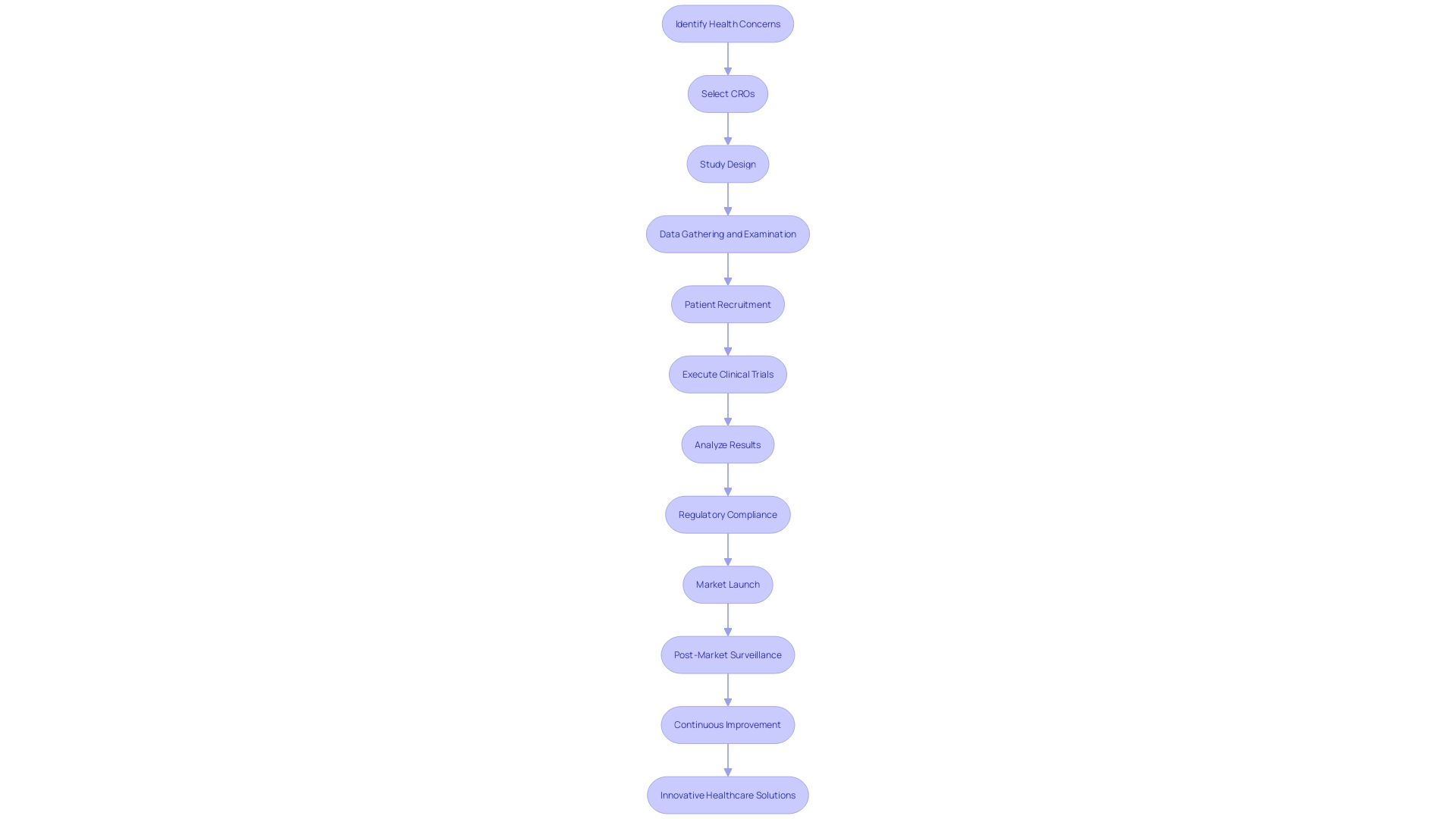
Challenges and Opportunities in Medical Device Clinical Trials
Trials for healthcare products are a crucial stage in the life cycle of product development. They require a meticulous approach to address the multifaceted regulatory landscape, participant recruitment and retention, data management, and patient safety assurance. Embarking on a clinical trial involves a complex interplay between regulatory knowledge and operational execution, where success hinges on thorough planning and adaptive strategies. The complexities of such trials frequently require strategic collaborations with Contract Research Organizations (CROs) that specialize in the field of healthcare instruments. These partnerships can utilize the specialized knowledge of contract research organizations to navigate the regulatory demands imposed by organizations such as the FDA and EMA, which classify products from minimal to significant risk and require different levels of examination. Moreover, as healthcare advancements progress, initiatives such as the University of Rochester Medical Center's implementation of Butterfly POCUS showcase how the incorporation and instruction in innovative diagnostic tools are transforming clinical practices and research methodologies.
Efficient collaboration with CROs can also expedite the data collection and analysis process, addressing one of the main challenges in trials involving devices. The insights from industry reports underscore the importance of readiness to meet regulatory demands, efficiency in document preparation, and the implementation of strategies that align with the evolving regulatory environment. In the present dynamic market, where healthcare specialties are constantly embracing new technologies, as demonstrated by the focused implementation of POCUS in different clinical settings, healthcare equipment manufacturers must stay flexible. The collaboration with a CRO can provide the competitive edge needed to bring innovative and safe products to market successfully. By leveraging the expertise and resources of contract research organizations, companies in the field of healthcare technology can concentrate on optimizing clinical effectiveness, diagnostic precision, and ultimately, patient availability to state-of-the-art solutions.
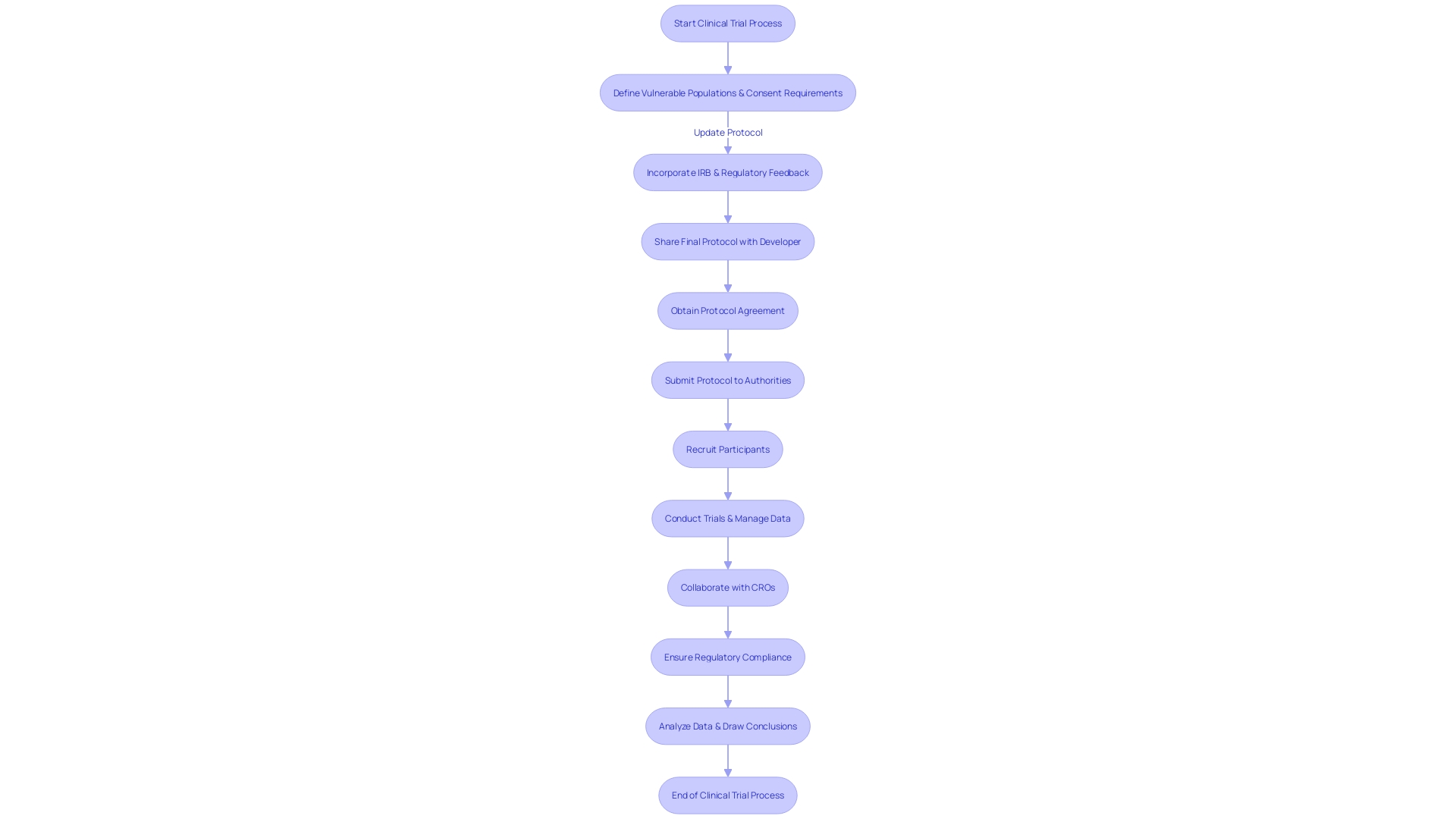
Regulatory Compliance and Ethical Standards in Medical Device Research
Contract Research Organizations play a crucial role in navigating the intricate regulations and ethical considerations in research on healthcare products. In addition to ensuring adherence to guidelines, companies responsible for clinical research must also keep pace with the evolving landscape of technology governance. Their expertise in regulatory frameworks, such as the FDA's rigorous standards for safety and efficacy, is crucial for medical device companies to maintain the integrity of their clinical trials. As exemplified in case studies, the management of emerging technologies poses a range of ethical, legal, and social concerns, requiring a multifaceted strategy by organizations. For example, the FDA recently established guidelines for prescription drug advertisements to improve clarity and impartiality, a step that aligns with the general dedication to consumer-friendly communication and transparency that other organizations must emulate.
Amidst fast advancement, contract research organizations have a crucial part in promoting uniformity and guaranteeing that healthcare instruments comply with the strict regulations established by authorities such as the FDA and MHRA. As MedTech leaders grapple with an intricate regulatory environment, these organizations provide the expertise needed to navigate this terrain, ensuring that patient safety remains at the forefront. The MedTech Regulatory Challenge underscores the importance of rigorous quality and regulatory standards as the bedrock of trust in healthcare. Furthermore, industry reports underscore the escalating regulatory demands and the strategies employed by professionals to maintain compliance and efficiency. These insights are priceless for regulatory organizations as they aid companies in aligning with current and future regulatory priorities, ensuring both innovation and patient safety are maintained.
Future Trends and Innovations in Medical Device CRO Services
As the sector for medical equipment continues to rapidly evolve, Contract Research Organizations (CROs) that specialize in healthcare devices are at the forefront of embracing cutting-edge innovations to enhance the development process. These CROs are increasingly integrating artificial intelligence (AI) and machine learning to refine data analysis methods, implementing virtual and remote clinical trials to overcome geographical limitations, and generating real-world evidence to support clinical outcomes. These advancements are changing the landscape of developing healthcare tools by improving the efficiency of clinical trials, enhancing the recruitment and retention of patients, and accelerating the overall process of introducing new healthcare technologies to the market.
With the integration of AI, as reported by GlobalData, there is a marked trend towards leveraging technology to not only assist but anticipate the needs of healthcare professionals. In this vein, the use of larger catheters in the brain for mechanical thrombectomy has been highlighted as an area lacking randomized data, yet real-world evidence points to significant effectiveness. This refers to the wider concept of how real-world data and AI are influencing the future of development in the field of healthcare technology.
The implementation of the AI Act is poised to have a greater impact on the sector, with companies involved in the creation or management of healthcare equipment getting ready for comprehensive obligations, which encompass substantial data disclosures. This has been underscored by the heightened regulatory focus on the processing and use of personal data by digital health companies, following the Federal Trade Commission's (FTC) increased enforcement activities.
Furthermore, the healthcare technology market is experiencing significant growth, as stated in a worldwide projection anticipating the United States to generate the highest revenue of US$215.80 billion by 2024. Contract research organizations (CROs) that focus on healthcare equipment play a vital role in this expansion, delivering crucial services in diverse fields, such as In Vitro Diagnostics (IVD) and healthcare equipment.
The transformation is also evident in the educational sphere, where programs like the one at the School of Medicine and Dentistry have embedded Point-of-Care Ultrasound (POCUS) into their curriculum, reflecting the integration of technological advancements into healthcare training. This shift towards a more technologically adept healthcare workforce aligns with the increasing demand for innovation and efficiency in medical device development, setting the stage for cross to continue driving progress in this dynamic industry.
Conclusion
In conclusion, Contract Research Organizations (CROs) play a crucial role in advancing medical device technology. They collaborate with manufacturers, regulatory bodies, and healthcare professionals to streamline device progression and ensure compliance with societal values and regulatory standards.
Partnering with a CRO offers several benefits for medical device companies. CROs provide regulatory expertise, streamline clinical trials, and offer strategic insights throughout the development process. Their specialized services, including user-centered design, trial management, and data analysis, contribute to bringing innovative medical technologies to market.
Top medical device CROs possess expertise in managing clinical trials and leverage advanced technologies to enhance care quality. Successful collaborations between medical device companies and CROs highlight the indispensable role CROs play in propelling the sector forward.
Challenges in medical device clinical trials, such as navigating complex regulations and ensuring patient safety, can be addressed through partnerships with CROs. CROs also play a vital role in ensuring regulatory compliance and upholding ethical standards in medical device research.
Looking ahead, CROs are embracing cutting-edge innovations to optimize the development process. Integration of artificial intelligence (AI) and machine learning, virtual and remote trials, and real-world evidence generation enhance efficiency and accelerate bringing new technologies to market. CROs remain at the forefront of driving progress in the rapidly evolving medical device sector.
Discover the benefits of partnering with bioaccess™ for your medical device development needs.




