Overview
Cost-efficient trial designs in Argentina are poised to significantly enhance research outcomes. By employing adaptive, platform, and basket trial methodologies, these designs introduce a level of flexibility in study protocols and resource allocation that is crucial for modern clinical research. Such innovative approaches are not merely theoretical; they can lead to substantial cost reductions, improved patient recruitment, and more efficient data collection. Ultimately, these advancements facilitate the progression of medical technologies in the region, underscoring the vital role of collaboration and strategic innovation in overcoming key challenges within the Medtech landscape.
Introduction
In the rapidly evolving landscape of clinical research, innovative trial designs are reshaping how therapies are developed and evaluated, particularly in regions like Argentina.
- Adaptive trial designs
- Platform trials
- Basket trials
These methodologies are at the forefront, offering unprecedented flexibility and efficiency in testing new treatments. These methodologies not only optimize patient outcomes but also significantly reduce costs, making them appealing to Medtech companies. As the demand for patient-centric approaches and real-world evidence grows, researchers are increasingly turning to digital health technologies and strategic partnerships with local CROs to streamline operations and enhance recruitment.
This article delves into the transformative impact of these trial designs and methodologies, highlighting their potential to revolutionize clinical research and improve patient care in Argentina and beyond.
Adaptive Trial Designs
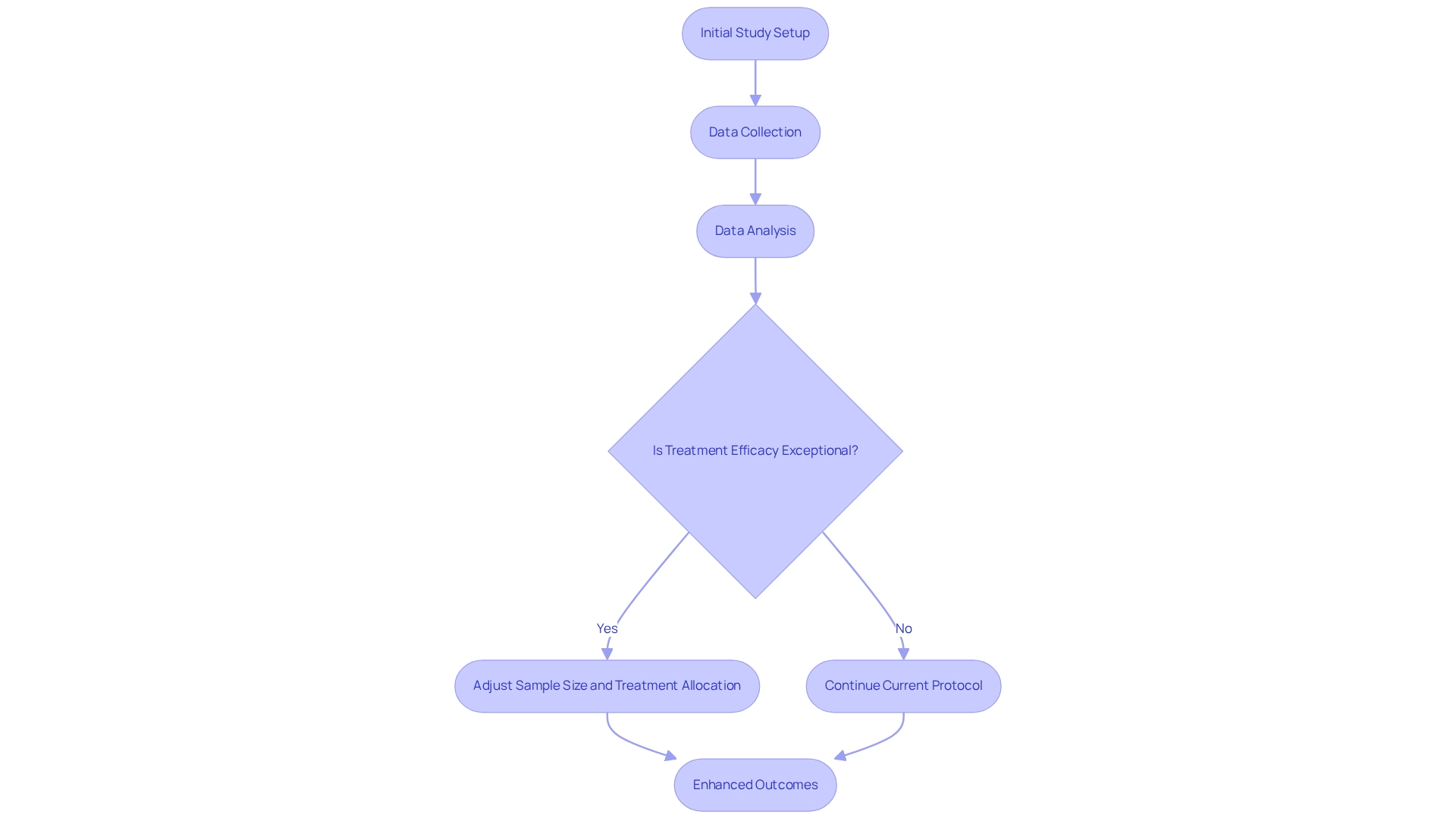
Adaptive study designs are increasingly recognized in clinical research for their inherent flexibility, enabling real-time adjustments to protocols based on emerging data. This adaptability empowers researchers to modify sample sizes, treatment regimens, or endpoints as the study unfolds. For instance, if initial findings reveal a treatment's exceptional efficacy, the study can be recalibrated to allocate a larger number of participants to that treatment group, thereby enhancing outcomes for individuals. This methodology not only boosts testing efficiency but also significantly reduces expenses by minimizing unnecessary patient exposure to less effective therapies.
In Argentina, where regulatory frameworks are rapidly evolving, the implementation of cost-efficient trial designs can facilitate compliance with local requirements while upholding stringent study standards. Recent statistics indicate that such trial designs can yield a 20-30% reduction in overall research costs, a figure corroborated by industry analyses that highlight the financial advantages of these methodologies. Moreover, successful case studies in the region, particularly those employing real-world data collection methods, illustrate how adaptive designs have been effectively utilized to enhance research outcomes, providing valuable insights into treatment safety and efficacy.
As major regulatory agencies in Europe and the US continue to issue supportive guidelines for adaptive designs, the momentum for their adoption in Latin America is poised to increase. Engaging stakeholders and informing them about the benefits of these designs is crucial for their approval and effective application in clinical studies. With bioaccess®'s expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), Medtech companies can leverage these strategies to enhance study efficiency and reduce costs. By effectively employing adaptive study designs, bioaccess® not only improves outcomes but also contributes to cost-efficient trial designs for Argentina, ensuring that resources are allocated judiciously throughout the process. As Philip Pallmann noted, "All authors read and approved the final manuscript," underscoring the collaborative effort essential in clinical research. By fostering a deeper understanding of adaptive study designs, bioaccess® can expedite medical innovations more swiftly.
Platform Trials
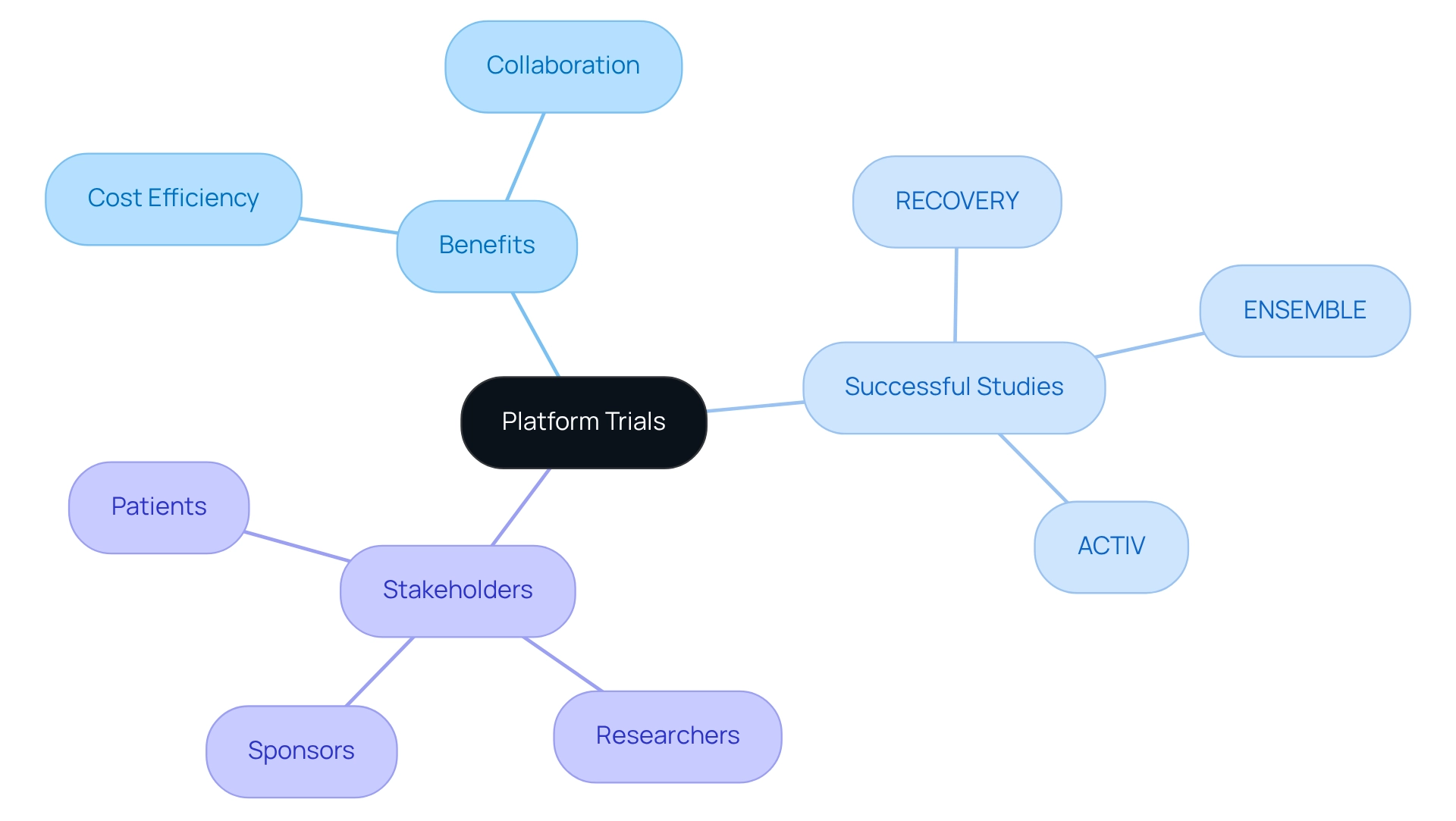
Platform studies represent an innovative framework that facilitates the simultaneous evaluation of multiple interventions under a single master protocol. This methodology proves especially beneficial in oncology, where various therapies are frequently developed for the same indication. For instance, a platform experiment can assess different drugs targeting a specific cancer type, enabling swift comparisons of their efficacy and safety profiles.
In Argentina, the implementation of cost-efficient trial designs can significantly enhance collaboration among researchers and sponsors, streamlining patient recruitment and accelerating data collection. By leveraging a shared infrastructure, these studies can yield substantial cost reductions, positioning cost-efficient trial designs as a compelling choice for Medtech firms aiming to optimize their clinical research expenditures.
Notably, successful platform studies such as ENSEMBLE, RECOVERY, and ACTIV have illustrated the effectiveness of this approach in addressing urgent public health challenges. For example, the RECOVERY study facilitated the rapid evaluation of various therapies during the COVID-19 pandemic, underscoring the adaptive design's versatility and responsiveness.
Furthermore, Brian Moore, VP at NICCA USA, Inc., noted, "The caliber of studies they have conducted for us has been outstanding," highlighting the practical efficacy of platform tests. Additionally, with bioaccess®'s expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), the complexities and successes related to regulatory approvals for platform assessments can be navigated effectively.
This comprehensive medical study management approach not only enhances outcomes but also contributes to job creation, economic development, and healthcare improvement in the region. As the landscape of medical studies evolves in 2025, the advantages of platform experiments, supported by bioaccess®'s tailored services, will remain crucial in advancing medical technology and improving outcomes for individuals.
Basket Trials
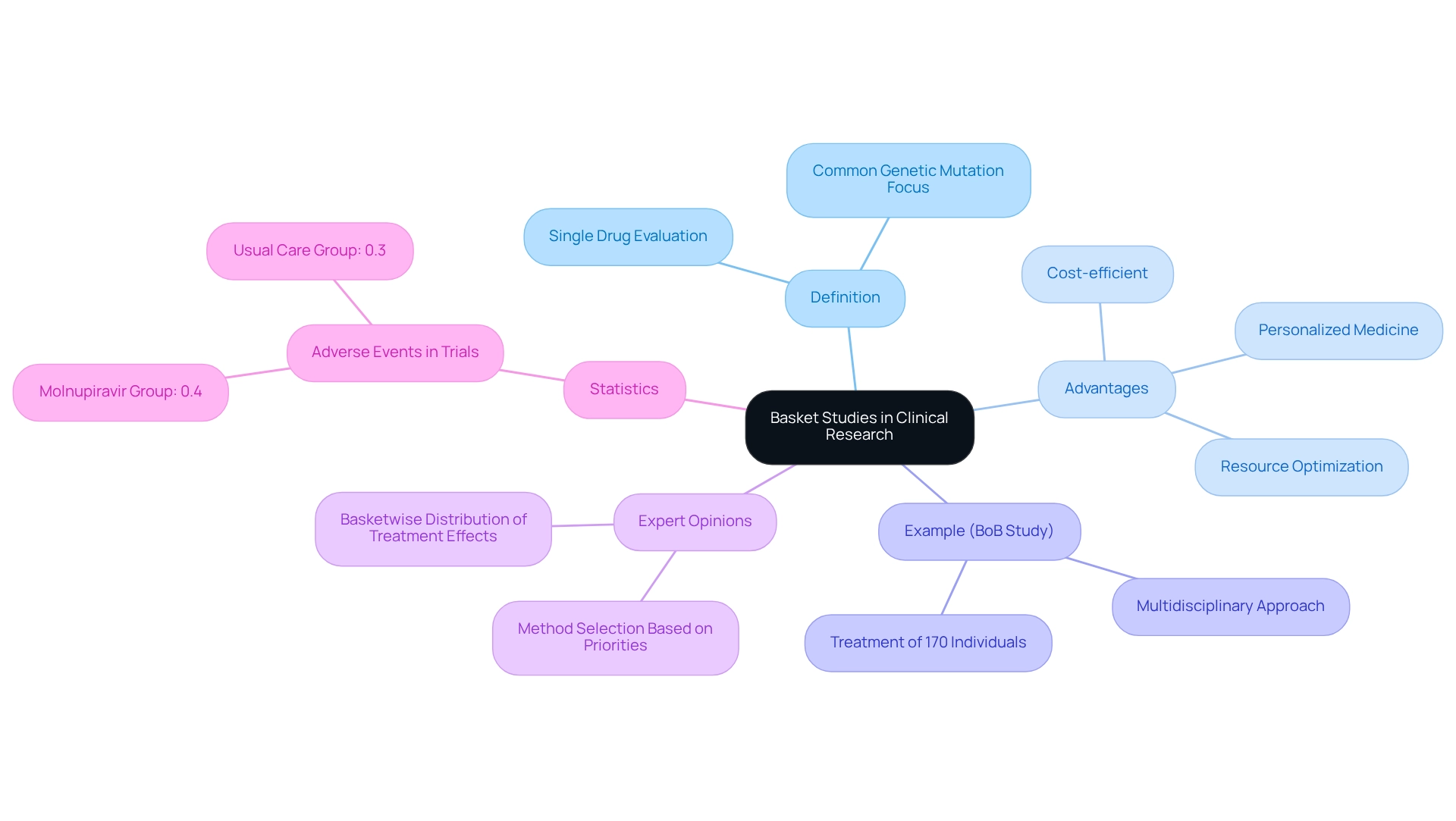
Basket studies represent a groundbreaking method in clinical research, allowing for the evaluation of a single investigational drug or treatment across various disease types that share a common genetic mutation or biomarker. This design is particularly advantageous in oncology, where targeted therapies can effectively address multiple cancers exhibiting similar molecular characteristics. For example, a basket study may assess a new targeted treatment in individuals with diverse tumor types, all of which display a specific mutation.
In Argentina, the healthcare system is progressively adapting to innovative treatment methods, with the adoption of cost-efficient trial designs, such as basket studies, serving as a strategic initiative to accelerate the advancement of personalized medicine. These studies not only enhance individual outcomes but also align with cost-efficient trial designs for the region, thereby optimizing resource distribution and managing expenses effectively. The success of basket experiments in Argentina is underscored by recent initiatives that have demonstrated their capacity to streamline the clinical research process.
A notable example is the Basket of Baskets (BoB) study, which has successfully treated over 170 individuals by integrating a multidisciplinary Molecular Tumor Board to offer personalized therapy options. This research illustrates how basket studies can expand the range of targeted therapies available to individuals with specific genetic mutations, ultimately facilitating progress in personalized cancer care. The results of the BoB study highlight the effectiveness of this approach, showcasing its ability to deliver customized therapies that enhance patient care.
Expert opinions further underscore the effectiveness of basket studies across various diseases, with oncologists stating that 'the selection of method should be determined based on study priorities and the anticipated basketwise distribution of treatment effects.' As the field of oncology continues to evolve, the current application of basket studies in research is gaining momentum, with ongoing investigations illustrating their significance in improving treatment effectiveness and care for patients. Moreover, statistics reveal that in recent studies, serious adverse events were reported at a rate of 0.4% in the molnupiravir group compared to 0.3% in the usual care group, emphasizing the importance of safety and efficacy in treatment assessments within basket studies.
Utilization of Real-World Evidence
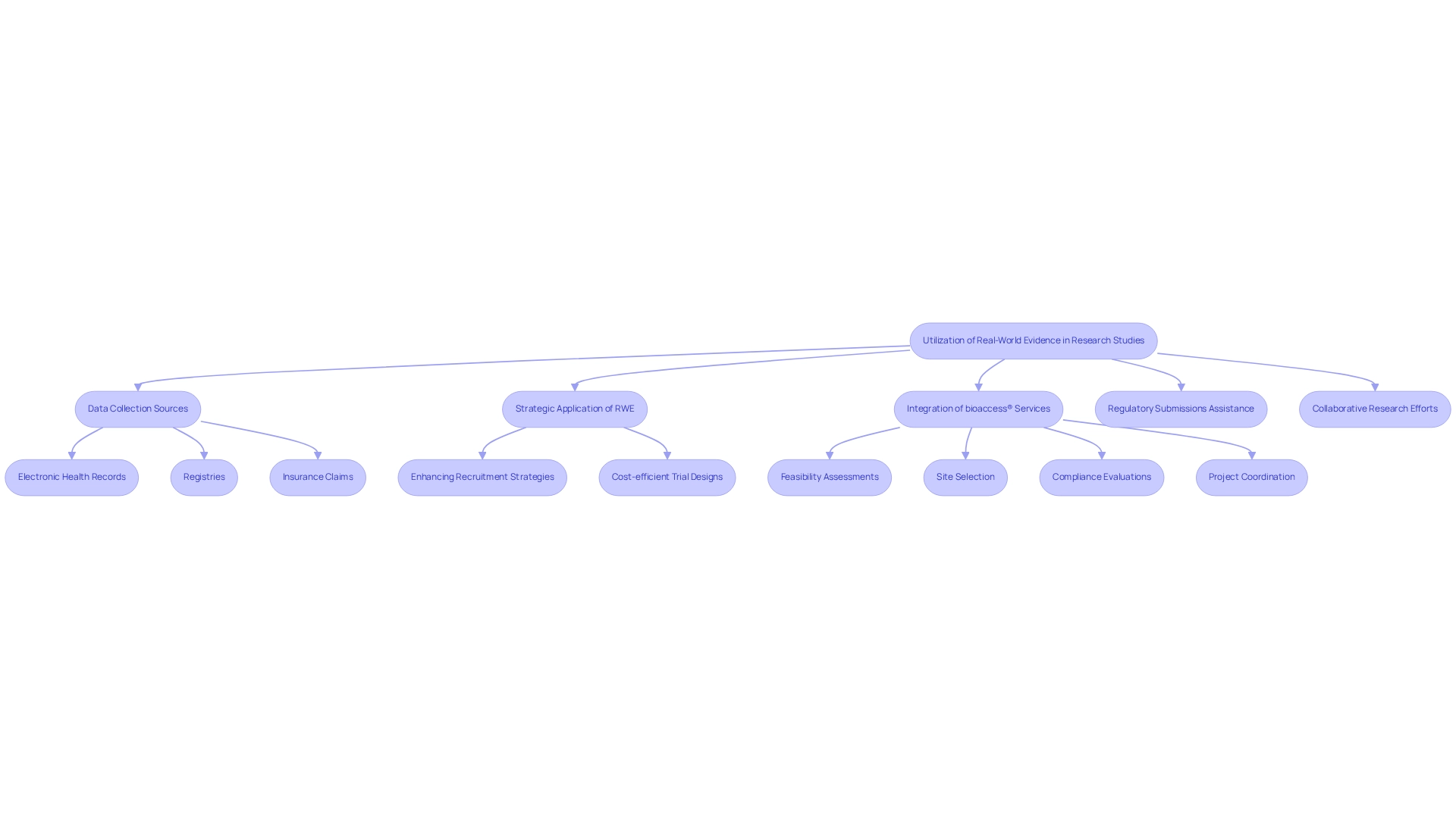
Employing real-world evidence (RWE) in research studies involves the collection of data from unconventional sources such as electronic health records, registries, and insurance claims. This methodology offers a comprehensive perspective on treatment performance in everyday medical environments. In Argentina, the strategic application of RWE enables researchers to identify groups of individuals who stand to gain the most from new therapies, thereby enhancing recruitment strategies and facilitating cost-efficient trial designs. With bioaccess®'s extensive study management services—including feasibility assessments, site selection, compliance evaluations, and project coordination—the integration of RWE becomes even more significant.
For instance, Lifebit's federated technology provides secure access to extensive and diverse data from over 100 million individuals, thereby broadening the scope of data available for RWE in research studies. Moreover, RWE plays a crucial role in assisting regulatory submissions by demonstrating the effectiveness and safety of treatments across various demographic groups. This not only accelerates approval processes but also enhances access to innovative treatments, ultimately contributing to more efficient and impactful research studies. As stated, 'an enhanced study workflow doesn’t solely aid sponsors and site personnel—it enables more individuals to engage in studies, which benefits all of us.'
Furthermore, the Lung Cancer Genetics Study Partnership with Lifebit exemplifies the successful application of RWE, aiming to deepen the understanding of lung cancer genetics and potentially leading to improved treatment options. The platform's presence in over 100 nations additionally fosters collaboration among stakeholders in research studies, underscoring the global significance of RWE and the role of bioaccess® in promoting innovation and regulatory excellence within the Medtech industry.
Digital Health Technologies
Digital health technologies, encompassing mobile health applications, wearable devices, and telemedicine, are revolutionizing research by enabling remote monitoring, improving data collection, and enhancing engagement. For example, wearable devices continuously collect health data, providing researchers with real-time insights into patient responses to treatments. In Argentina, the successful integration of these technologies has yielded significant benefits, such as cost-efficient trial designs that streamline operations and reduce expenses. Notably, Phantom Neuro's recent $19M funding to advance human-machine technology underscores the growing interest and financial backing for digital health innovations.
bioaccess® delivers comprehensive management services for research studies, which encompass feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. These capabilities ensure that medical studies are conducted effectively and in compliance with local regulations, a critical factor for the successful integration of digital health technologies. Specifically, bioaccess's experimental setup process involves meticulous planning and coordination with ethics committees and health ministries, while project management includes ongoing monitoring and reporting of study status and adverse events.
Nonetheless, the integration of these technologies presents challenges. Insights from Jones Day emphasize the necessity of addressing legal and regulatory hurdles that may arise in digital health. Additionally, the involvement of external technology suppliers in research studies introduces compliance challenges, as these companies may lack familiarity with research regulations. Effective integration requires diligent oversight to ensure adherence to regulatory standards. Furthermore, professional recommendations, such as those from Cave et al., highlight the potential of leveraging real-world data (RWD) for regulatory purposes, enhancing the credibility of results in medical studies. By facilitating more convenient and accessible participation, digital health tools not only enhance patient recruitment and retention but also contribute to more robust and reliable study outcomes. As the landscape of medical research evolves in 2025, the development of cost-efficient trial designs for Argentina will be pivotal, supported by digital health technologies and bioaccess's extensive services, in improving overall research efficiency.
Feasibility Studies
Feasibility studies serve as essential initial evaluations that assess the practicality of proposed clinical trials. In Argentina, cost-effective trial designs critically evaluate factors such as site capabilities, patient availability, and logistical considerations, all of which are vital given the country's diverse healthcare landscape and varying patient demographics. By identifying potential challenges early, researchers can refine study designs to ensure they are both practical and efficient.
The importance of feasibility studies is underscored by findings from the Clinical Trials Journal, which reveal that studies incorporating comprehensive feasibility assessments achieve higher recruitment rates and enhanced data quality. This proactive approach not only increases the likelihood of success but also effectively manages costs, establishing feasibility studies as a fundamental component of the medical research process.
As exemplified by ReGelTec's Early Feasibility Study on HYDRAFIL™ for addressing chronic low back pain in Colombia, successful case studies demonstrate the value of well-executed feasibility studies. This study, which involved eleven patients and was conducted remotely, illustrates how tailored approaches can lead to successful outcomes, highlighting the necessity for flexibility in medical investigations. Moreover, understanding the roles of regulatory affairs professionals and project managers is crucial for navigating the complexities of studies in Argentina. As the Medtech sector continues to evolve in 2025, the emphasis on cost-effective trial designs for Argentina will remain vital for optimizing study frameworks and enhancing research outcomes. Bioaccess® offers personalized solutions, including Early Feasibility Studies, First-In-Human Studies, and comprehensive study management services, ensuring that feasibility assessments are tailored to the specific contexts of Medtech companies, thereby reinforcing their commitment to advancing medical devices more swiftly.
Strategic Site Selection
Strategic location choice is vital for the success of research studies, particularly regarding cost-efficient trial designs for Argentina, where healthcare facilities can differ greatly. This procedure includes recognizing and assessing possible research locations according to essential factors like participant demographics, site capabilities, and historical performance metrics. Effective site selection can dramatically enhance participant recruitment; this is crucial given that up to 40% of research sites may experience high dropout rates, potentially compromising the validity of the study and leading to inconclusive results.
By utilizing real-world data and insights from previous studies, researchers can make informed decisions that not only improve study efficiency but also ensure high-quality data collection. This strategic approach minimizes costs associated with underperforming sites and accelerates the timeline for bringing new therapies to market. Moreover, expert insights emphasize that cost-efficient trial designs for Argentina, which are thoroughly researched and context-specific strategies, are crucial for enhancing site selection. This ultimately affects patient recruitment success rates and overall study results. The partnership between bioaccess™ and Caribbean Health Group, revealed on March 29, 2019, illustrates the significance of strategic site selection in establishing Barranquilla as a premier location for research studies in Latin America. Backed by Colombia's Minister of Health, this initiative seeks to improve the research environment, ensuring that locations are well-prepared to fulfill the requirements of contemporary studies.
Particular results from this partnership consist of a notable decrease in recruitment duration and enhanced retention rates, highlighting the efficacy of their strategic method. Furthermore, bioaccess®'s proficiency in overseeing different kinds of studies—including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—underscores the necessity for thorough management services. By leveraging models like those suggested by Medidata, biopharmaceutical companies and CROs can make more data-driven decisions and confidently select high-performing sites to meet enrollment timelines. This comprehensive method not only tackles the challenges of participant involvement but also emphasizes the significance of strategic site selection in achieving successful studies.
Partnerships with Local CROs
Collaborating with local CROs is essential for the success of cost-efficient trial designs in Argentina's clinical studies. These organizations provide invaluable insights regarding the regulatory environment, cultural nuances, and demographics, which are critical for facilitating effective research operations. Local CROs excel in navigating complex regulations, optimizing patient recruitment strategies, and ensuring adherence to ethical standards. This expertise not only streamlines testing procedures but also fosters cost-efficient trial designs, ultimately enhancing research outcomes.
Statistics indicate that 91.8% of studies in Argentina are funded by foreign manufacturers, underscoring the vital role of local collaborations for international Medtech companies. By leveraging the strengths of local CROs alongside bioaccess's extensive management services—including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies—companies can implement cost-efficient trial designs to improve operational efficiency and accelerate the advancement of innovative therapies within a competitive landscape. Furthermore, effective collaborations with local CROs have demonstrated that these partnerships can effectively address common challenges encountered in public healthcare environments, where bureaucracy often impedes research progress. In contrast, private for-profit hospitals, preferred by the majority of CROs, provide a more conducive environment for medical investigation activities, facilitating quicker and more effective execution of studies.
With over 20 years of experience in Medtech, bioaccess is well-equipped to navigate these complexities. Additionally, the research studies market in Argentina is projected to reach USD 506.1 million by 2030, highlighting the significance of cost-efficient trial designs in capitalizing on this growth. As Julio G. Martinez-Clark, CEO, observes, Argentina's burgeoning clinical experimentation sector fosters an environment conducive to scientific advancement and collaborative global initiatives. This collaborative approach, bolstered by bioaccess's expertise, is crucial for ensuring that innovative medical devices reach the market swiftly and effectively. To maximize the benefits of these partnerships, it is advisable to engage with local CROs early in the study design process, allowing for the integration of their insights and the streamlining of operations.
Patient-Centric Trial Designs
Patient-centric study designs prioritize the perspectives and preferences of participants throughout the research process. This methodology encompasses flexible scheduling, remote monitoring, and the integration of digital health technologies to boost patient engagement. Notably, in 2022, the use of virtual elements in assessments surpassed levels seen in the years preceding the pandemic, underscoring the critical role of digital health technologies in enhancing patient-centered methods.
In Colombia, particularly in Barranquilla, where bioaccess™ and the Caribbean Health Group collaborate to establish the city as a premier location for clinical studies, adopting a patient-focused strategy can significantly enhance recruitment and retention rates. This approach has resulted in over a 50% reduction in recruitment time and 95% retention rates.
By actively engaging individuals in the study design process, researchers can develop investigations that align more closely with participant expectations, leading to improved outcomes and heightened satisfaction among contributors. Evidence indicates that studies incorporating participant feedback not only foster greater involvement but also yield more efficient and scientifically valid outcomes.
Furthermore, the establishment of healthcare education programs is essential for equipping individuals for meaningful participation, ensuring their contributions are evidence-based and aligned with scientific objectives. As highlighted by Barger et al., communication through web conferences and emails has significantly improved study design and execution, emphasizing the necessity for effective communication techniques.
Insights from the case study titled 'Future Directions for Patient Engagement' suggest that creating standardized guidelines for patient participation is crucial for encouraging meaningful involvement in medical studies. This ultimately supports the objective of positioning Barranquilla as an appealing center for research in Latin America.
Regulatory Alignment Strategies
Regulatory alignment strategies are essential for ensuring compliance with both local and international regulations in clinical research. In Argentina, where regulatory frameworks are rapidly evolving, it is imperative for researchers to stay updated on the latest guidelines and requirements related to cost-efficient trial designs. By implementing robust regulatory strategies, researchers can streamline the approval process, minimize delays, and enhance the credibility of their findings. As Gurudath Gurjal emphasizes, "Achieving regulatory alignment is vital for clinical study success." This proactive approach not only enables more efficient operations but also cultivates trust among stakeholders, including regulatory agencies and individuals receiving care. Effective regulatory alignment is crucial for advancing innovative therapies and improving patient outcomes in the Medtech sector.
bioaccess® offers extensive management services for studies that encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
In 2023, Argentina represented 0.4% of the global research market, with forecasts suggesting substantial expansion by 2030. As the fastest-growing regional market in Latin America, Argentina's evolving regulatory environment presents both challenges and opportunities for researchers in the medical field to utilize cost-efficient trial designs. Remaining aware of regulatory compliance data, such as the recent rise in approval rates for clinical studies, is essential for successfully navigating this landscape. By leveraging expert insights and case studies on successful regulatory strategies, including the streamlined processes adopted by leading Medtech firms like bioaccess®, researchers can enhance their trial designs and contribute to the advancement of medical technologies. This includes managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), which are critical for the success of innovative medical devices.
Conclusion
The evolution of clinical research in Argentina is profoundly shaped by innovative trial designs, including adaptive, platform, and basket trials. These methodologies provide exceptional flexibility and efficiency, enabling researchers to adjust protocols in real-time based on emerging data. By optimizing patient outcomes and minimizing costs, they present a compelling proposition for Medtech companies navigating the complexities of clinical trials in a rapidly evolving landscape.
The utilization of real-world evidence and digital health technologies significantly enhances the effectiveness of these trial designs. By harnessing data from electronic health records and patient registries, researchers can refine recruitment strategies, ensuring that trials accurately reflect real-world scenarios. The integration of digital tools also promotes patient engagement, making participation in clinical trials more accessible and aligned with patient needs.
Strategic partnerships with local CROs are vital in this context, as they offer essential insights into regulatory requirements and cultural nuances. Such collaborations not only streamline trial operations but also bolster the likelihood of successful outcomes. Furthermore, an emphasis on patient-centric designs ensures that trials resonate with participants, leading to improved recruitment and retention rates.
As the clinical trial market in Argentina continues its upward trajectory, embracing these innovative approaches will be crucial for advancing medical technologies and enhancing patient care. By prioritizing flexibility, collaboration, and patient engagement, the future of clinical research in Argentina appears promising, paving the way for breakthroughs that can transform healthcare delivery. The momentum gained from these methodologies will undoubtedly play a pivotal role in shaping the next generation of clinical trials, ensuring that new therapies reach patients more swiftly and effectively.
Frequently Asked Questions
What are adaptive study designs in clinical research?
Adaptive study designs are flexible methodologies that allow researchers to make real-time adjustments to study protocols based on emerging data, such as modifying sample sizes, treatment regimens, or endpoints as the study progresses.
How do adaptive study designs improve clinical research outcomes?
They enhance testing efficiency and reduce costs by reallocating participants to more effective treatment groups, minimizing unnecessary exposure to less effective therapies.
What are the cost benefits of adaptive study designs in Argentina?
Implementing cost-efficient trial designs can lead to a 20-30% reduction in overall research costs, helping researchers comply with local regulations while maintaining high study standards.
How do platform studies function in clinical research?
Platform studies allow for the simultaneous evaluation of multiple interventions under a single master protocol, making them particularly useful in oncology where several therapies may target the same indication.
What advantages do platform studies offer in Argentina?
They enhance collaboration among researchers and sponsors, streamline patient recruitment, and accelerate data collection, resulting in significant cost reductions for Medtech firms.
Can you provide examples of successful platform studies?
Successful platform studies include ENSEMBLE, RECOVERY, and ACTIV, which have effectively addressed public health challenges, such as the rapid evaluation of therapies during the COVID-19 pandemic.
What are basket studies in clinical research?
Basket studies evaluate a single investigational drug across various disease types that share a common genetic mutation or biomarker, particularly beneficial in oncology for targeted therapies.
How are basket studies advancing personalized medicine in Argentina?
They optimize resource distribution and manage expenses effectively, aligning with the region's strategic initiatives to accelerate personalized treatment options.
What is the significance of the Basket of Baskets (BoB) study?
The BoB study has successfully treated over 170 individuals by utilizing a multidisciplinary approach to offer personalized therapy options, demonstrating the effectiveness of basket studies in enhancing patient care.
What do experts say about the effectiveness of basket studies?
Experts suggest that the choice of study method should be based on priorities and the expected distribution of treatment effects, underscoring the importance of tailored approaches in clinical research.




