Overview
Creating a comprehensive clinical trial plan is essential for researchers, particularly in the context of accelerated medical device studies in Latin America. This article outlines critical components such as:
- Study objectives
- Design
- Stakeholder engagement
- Budgeting strategies
A well-structured plan significantly enhances the likelihood of successful outcomes and regulatory compliance in a competitive research landscape. By addressing these elements, researchers can navigate the complexities of clinical trials more effectively and drive innovation in medical technology.
Introduction
In the dynamic landscape of clinical trials, particularly within the realm of accelerated medical device studies in Latin America, meticulous planning is not merely beneficial—it is essential for success. Researchers must adeptly navigate a myriad of components, ranging from the precise definition of study objectives to the establishment of timelines and the engagement of key stakeholders.
With the increasing complexity of regulations and the pressing need for efficient data management, the stakes have never been higher. As the industry continues to evolve, a comprehensive understanding of the intricacies involved in clinical trial planning becomes paramount.
This article delves into the essential elements that contribute to effective clinical trial execution, highlighting strategies and insights that can pave the way for innovative medical advancements and improved patient outcomes.
Essential Components of a Clinical Trial Plan
To develop a comprehensive clinical trial plan, researchers must incorporate several essential components crucial for effective study execution, particularly in the context of accelerated medical device clinical studies in Latin America, where bioaccess® excels.
- Study Objectives: Clearly articulate both primary and secondary aims of the study, as these guide the overall direction and focus of the research.
- Study Design: Specify the type of study—such as randomized controlled trials or observational studies—and detail the methodology that will be employed to ensure robust results. Bioaccess® specializes in various research designs, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), which are critical for innovative medical devices.
- Study Population: Define the inclusion and exclusion criteria for participant selection, essential for ensuring the relevance and applicability of the findings.
- Intervention Details: Provide a thorough description of the intervention(s) being tested, including specifics on dosage and administration methods, to facilitate reproducibility and clarity.
- Outcome Measures: Identify both primary and secondary endpoints that will be measured to evaluate the effectiveness of the intervention, ensuring that these measures align with the research objectives.
- Statistical Analysis Plan: Outline the statistical methods that will be utilized to analyze the data gathered during the experiment, essential for validating the findings.
- Timeline: Present a detailed timeline for the study, highlighting key milestones and deadlines to keep the project on track. Bioaccess® emphasizes prompt execution, which is essential in the rapid-paced research setting.
- Budget: Include a comprehensive budget that outlines the financial resources required for the study, reflecting the increasing demand for transparency and accountability in research.
In 2025, the significance of these components is underscored by the fact that 45% of Alcon's information is recorded on the same day as the visit date, stressing the necessity for real-time information management in research trials. This shift towards real-time data entry aligns with the growing demand from sponsors for technology that allows direct access to live data, as highlighted by industry trends. Furthermore, as the medical market becomes increasingly crowded, optimizing development journeys is essential not only for regulatory approval but also for achieving commercial success.
Max Baumann from Treehill Partners warns of fundamental business model challenges facing biotech, further emphasizing the need for effective research planning. Creating a clinical trial plan by addressing these components enables researchers to establish a well-organized research framework that lays the groundwork for successful execution and ultimately contributes to advancing medical technology. Moreover, the case analysis titled "Commercial Focus in Drug Development" illustrates the challenges biotech companies encounter and the emphasis on optimizing development journeys, reinforcing the article's focus on effective planning.
Identifying Key Stakeholders in Clinical Trials
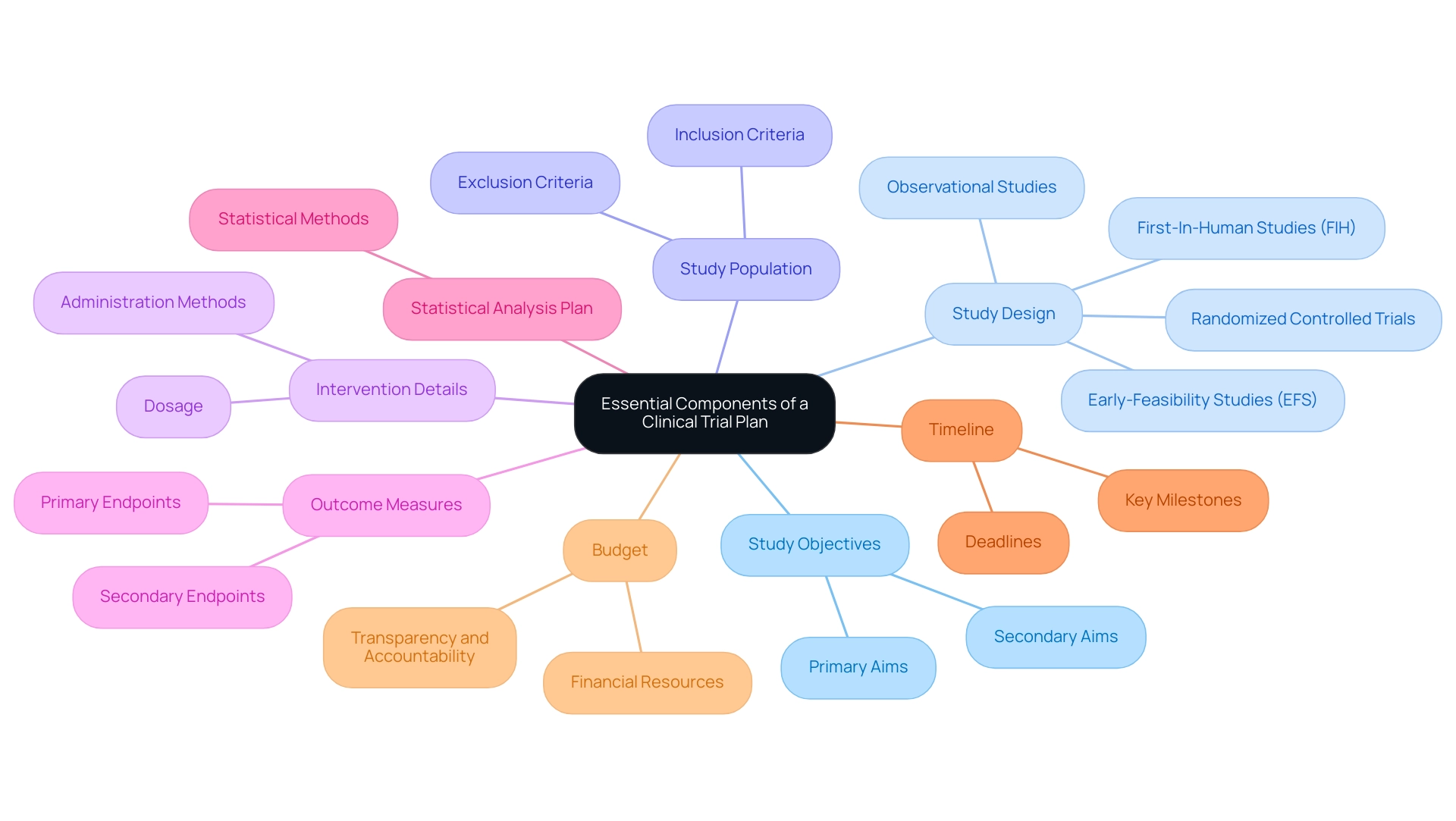
Identifying key stakeholders is crucial for the success of a clinical study. The following primary stakeholders should be considered:
-
Patients: As the primary participants, their health outcomes are the focal point of the research. Engaging patients early can lead to better recruitment and retention rates, especially in a competitive landscape like Colombia, where bioaccess® has demonstrated success in enhancing patient recruitment through its established networks.
-
Investigators: These researchers carry out the study and ensure participant safety, making their expertise vital for the study's integrity. Bioaccess® emphasizes the importance of selecting qualified principal investigators (PIs) to ensure compliance with regulatory standards and ethical guidelines.
-
Sponsors: Organizations or individuals financing the study, including pharmaceutical companies and contract research organizations (CROs), play a significant role in resource allocation and strategic direction. Collaborations, like those between bioaccess® and Caribbean Health Group, demonstrate how strategic partnerships can improve the clinical research landscape in Latin America by offering access to local expertise and resources.
-
Regulatory Authorities: Entities such as the FDA or EMA are essential for overseeing compliance with legal and ethical standards, ensuring that the study adheres to necessary regulations. Colombia's regulatory framework, backed by INVIMA, provides a streamlined process for medical device evaluations, making it an appealing location for first-in-human studies. Bioaccess® assists in navigating these regulatory requirements, ensuring that all necessary approvals and compliance reviews are conducted efficiently.
-
Ethics Committees/IRBs: These groups review the study protocol to ensure that ethical standards are met, safeguarding participant rights and welfare. Their involvement is critical in maintaining the integrity of the proceedings.
-
Information Management Personnel: Responsible for collecting and managing trial information, their role is increasingly important as sponsors shift towards in-house information management to enhance quality and operational efficiency. As mentioned by the Head of Clinical Information Engineering, 'Traditionally, management was outsourced to our CRO vendor partners.' Part of the initiative is to bring all our research in-house so that our internal teams can start working on it. They can be more hands-on, and we implement research in-house and we are able to take control of our data, and we deliver for our patients with high quality.
-
Healthcare Providers: Professionals who may refer patients to the study or provide care during the research, their involvement can facilitate patient recruitment and support.
Creating a clinical trial plan allows for the early identification of these stakeholders, enabling researchers to establish effective communication and collaboration throughout the study. In 2025, the significance of stakeholder involvement is emphasized by the fact that 45% of medical data is entered on the same day as the visit date, highlighting the need for timely and precise data management. Furthermore, as the trial landscape becomes more crowded, understanding the dynamics of stakeholder relationships will be essential for navigating challenges and ensuring successful outcomes.
Max Baumann from Treehill Partners cautions about essential business model difficulties confronting biotech as market spaces grow congested, highlighting the necessity for effective stakeholder engagement. Case analyses show that sponsors who actively engage key stakeholders from the beginning can obtain higher quality data delivery and ensure greater transparency and ownership of their research data. For example, bioaccess® has played a crucial role in enhancing Medtech research, offering affordable, high-quality research services that help expedite trials, connecting innovative Medtech firms with the opportunities of performing research in Latin America.
Moreover, bioaccess® provides extensive services such as feasibility studies, setup of experiments, project management, and compliance evaluations, guaranteeing that all elements of the research process are efficiently overseen.
Budgeting Strategies for Clinical Trials
Effective budgeting is crucial for the success of medical studies, especially in the evolving landscape of 2025. To navigate this complexity, researchers should consider several strategies:
-
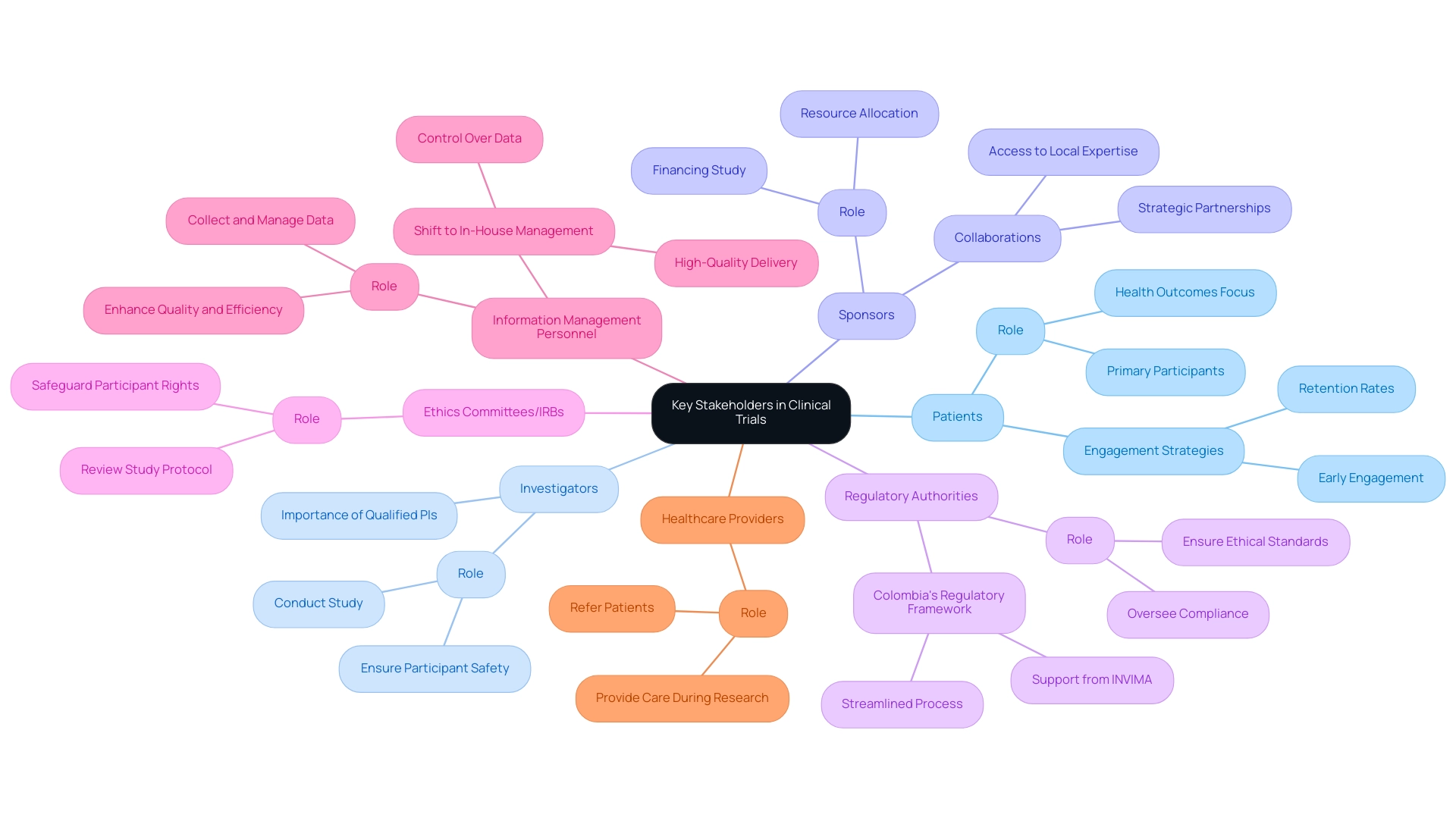
Conduct a Coverage Analysis: Assess which costs will be covered by the sponsor and which will fall to the site or participants. This analysis is essential for understanding financial responsibilities and ensuring all necessary expenses are accounted for.
-
Itemize Costs: Break down the budget into detailed categories, including personnel, equipment, participant compensation, and other operational costs. This granularity aids in identifying specific areas where savings can be made.
-
Negotiate with Vendors: Actively seek competitive bids from vendors to secure the best rates for services and supplies. Effective negotiation can significantly reduce costs and enhance overall budget efficiency.
-
Utilize Historical Information: Leverage insights from prior experiments to accurately estimate expenses and identify potential budget pitfalls. As Nitya Maddodi, Business Operations Services Manager, states, "Historical information is very helpful for precisely forecasting expenses in medical studies and can assist in avoiding overspending." An analysis of historical data has proven essential in forecasting expenses and avoiding overspending, enabling more effective optimization of clinical studies budgets and resource distribution. For instance, the case study titled "Leveraging Data" emphasizes how historical data can be used to forecast expenses and avoid overspending, offering insights into various cost factors related to experiments.
-
Monitor Expenses: Implement a robust system for tracking expenses throughout the testing period. Regular monitoring helps to avoid budget overruns and ensures that spending aligns with the planned budget.
-
Plan for Contingencies: Allocate a portion of the budget for unexpected costs that may arise during the experiment. This proactive approach can mitigate financial risks and ensure that the experiment remains on track.
-
Conduct a Cost-Benefit Analysis: Ensure that each budget item adds value to the project and helps maximize ROI. This analysis is crucial for the target audience of Directors of Clinical Research.
By employing these strategies, researchers can create a practical budget that not only facilitates the successful implementation of their study but also maximizes return on investment (ROI). The significance of a thorough coverage analysis and the strategic use of historical data cannot be overstated, as they are key components in crafting a budget that is both effective and sustainable. With over 20 years of experience in the Medtech sector, bioaccess® understands the nuances of budgeting for studies and the critical role it plays in the success of medical technology advancements, particularly through their comprehensive management services, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies.
Establishing Timelines for Clinical Trial Activities
Creating a clinical trial plan with a clear timeline for clinical trial activities is crucial for successful execution, particularly in the context of accelerated medical device clinical services offered by bioaccess, which boasts over 20 years of experience in Medtech. The following essential steps will guide you in creating an effective timeline:
-
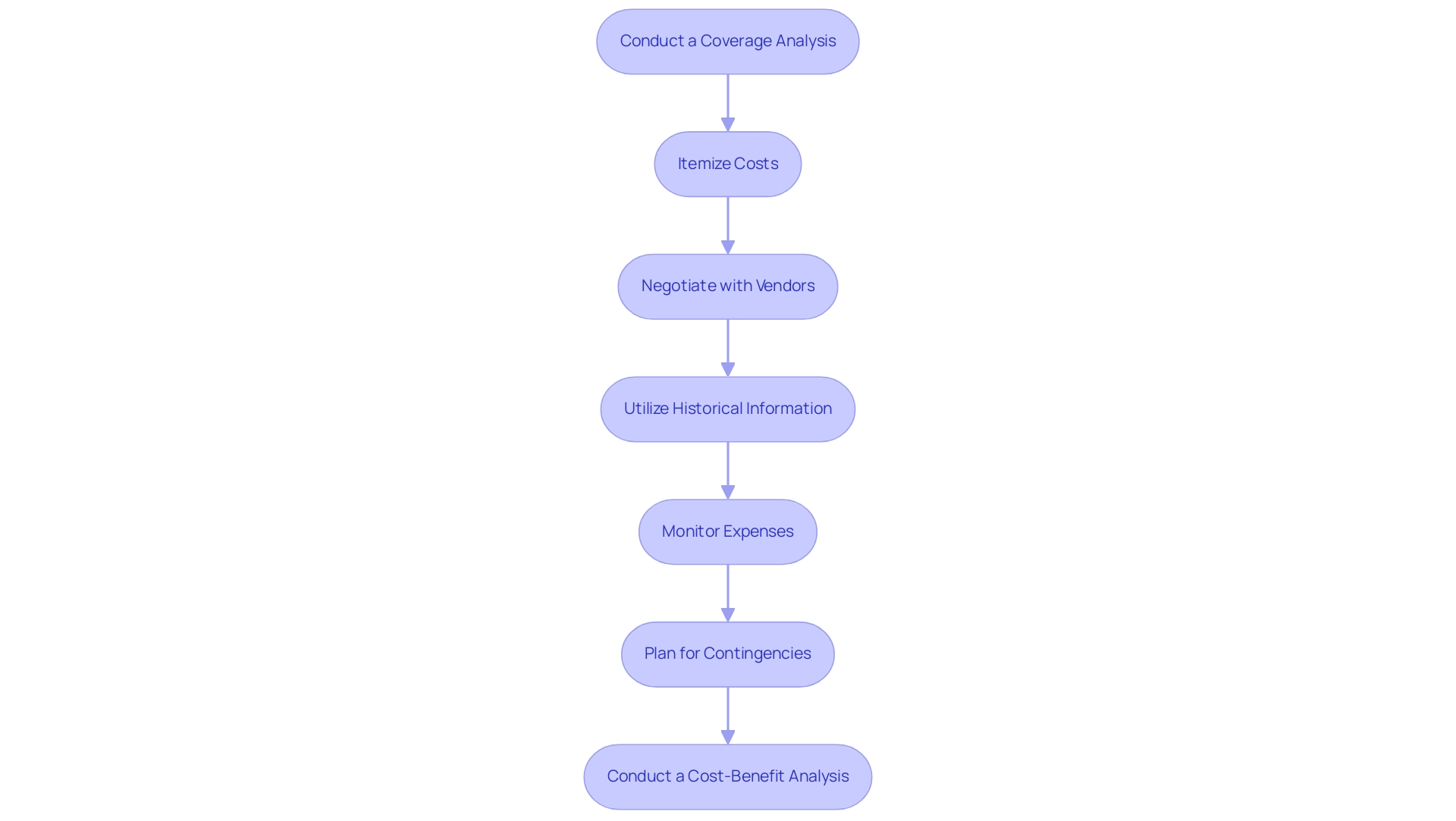
Define Key Milestones: Identify critical milestones such as protocol approval, participant recruitment, and information collection phases. Recognizing these milestones early on is vital for tracking progress and ensuring accountability, especially in studies like Early-Feasibility and First-In-Human, where timely execution is paramount.
-
Estimate Duration for Each Activity: Assess the duration of each activity based on historical information and expert insights. For instance, recent statistics indicate that 45% of medical information is recorded on the same day as the visit date, underscoring the significance of effective information management in timeline estimation and the necessity for prompt information entry to sustain the study's momentum.
-
Create a Gantt Chart: Utilize a Gantt chart to visualize the timeline, illustrating the sequence of activities and their interdependencies. This visual representation aids in understanding the flow of the process and facilitates communication among team members. Integrating AI-driven analysis and interactive dashboards can further enhance information visualization and clarity in research design, which is essential for overseeing intricate projects like pivotal and post-market follow-up assessments.
-
Incorporate Buffer Time: Allow for buffer time between activities to accommodate potential delays. This proactive strategy is crucial, especially considering the trend where sponsors increasingly manage information internally to improve control and quality in research. This shift highlights the importance of having a flexible schedule that can adapt to changes in data management practices, particularly regarding bioaccess's customized strategy for managing studies in Latin America.
-
Regularly Review and Adjust: Continuously monitor progress against the timeline and make necessary adjustments to stay on track. This iterative process is vital for creating a clinical trial plan, maintaining momentum, and ensuring that key milestones are met. As highlighted by Peng Lu, standardizing the use of specific results and result measures for medical research will aid in the creation of practice guidelines and future indirect comparisons among interventions, which can also impact timeline modifications.
By following these steps, researchers can ensure that their medical research activities are well-structured and punctual, ultimately enabling a smoother implementation process. The emphasis on milestone identification and creating a clinical trial plan is particularly pertinent in 2025, as the landscape of medical studies evolves with new challenges and opportunities. Moreover, as emphasized in the case analysis on 'Commercial Viability Challenges in Biotech,' optimizing development processes is essential for securing not only regulatory approval but also commercial success, closely linking the necessity for efficient timeline management in research phases.
Crafting the Clinical Trial Protocol: Writing Tips
Creating a clear and thorough clinical trial plan is vital for the success of any investigation. To achieve this, consider the following effective writing strategies:
-
Use Clear Language: Employ straightforward language to ensure that the protocol is accessible to all stakeholders, including regulatory bodies and medical personnel. Clarity in communication is essential, as research indicates that protocols composed in clear language significantly minimize misunderstandings and errors.
-
Follow a Standard Template: Utilizing established templates, such as those developed by the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) for phase 2 and 3 clinical trials, ensures that all necessary sections are included. This approach streamlines the writing process and enhances compliance with regulatory standards.
-
Be Specific: Clearly define research objectives, methodologies, and outcome measures. Specificity removes ambiguity, preserving the integrity of the research and ensuring that all team members are aligned with the research objectives.
-
Incorporate Feedback: Actively seek input from colleagues and stakeholders during the drafting process. Engaging diverse perspectives enhances the protocol's clarity and completeness, ultimately leading to a more robust research design.
-
Review Regulatory Requirements: Ensure that the protocol adheres to all relevant regulatory guidelines and standards. Given the increasing complexity of regulations, including data privacy requirements, staying informed about current guidelines is crucial for successful execution. Regulatory preparedness is becoming increasingly important due to these complexities. Bioaccess provides extensive services that include compliance evaluations to ensure that documents meet country requirements, significantly assisting in this process. Additionally, obtaining approvals from the ethics committee and health ministry is critical for trial setup, ensuring that all ethical considerations are addressed.
-
Include a Schedule of Activities: Provide a detailed schedule outlining all learning activities and timelines. A well-structured timeline aids in project management, helps anticipate potential delays, and ensures that all milestones are met. Bioaccess's project management services can assist in monitoring these timelines effectively. Furthermore, Bioaccess's expertise in feasibility assessments and site selection ensures that researchers can develop protocols that are not only well-organized but also based on practical realities.
By applying these strategies, researchers can create a protocol that is thorough and aids in the successful execution of the study. Case analyses have demonstrated that well-designed protocols play a crucial role in advancing medical knowledge, shaping the comprehension of new therapies, and directing medical choices. For instance, the case analysis titled 'Conclusion on Trial Statistics' emphasizes the significance of acknowledging trial statistics and utilizing suitable methods.
Thus, creating a clinical trial plan is a critical step that involves investing time in protocol development during the research process. Moreover, as noted by Meri Beckwith, researchers often conduct power analysis to determine the minimum sample size needed to detect an effect if one exists, further emphasizing the importance of thorough protocol planning. With Bioaccess's expertise in feasibility studies and site selection, researchers can ensure that their protocols are not only well-structured but also grounded in practical realities.
Navigating the Review Process for Clinical Trial Plans
Navigating the review process for research study plans in Colombia requires a systematic approach to ensure timely approval and adherence to ethical standards. Here are the essential steps to follow:
-
Submit to Ethics Committees/IRBs: Start by carefully preparing and submitting the research protocol to the appropriate ethics committee or institutional review board (IRB). This submission is crucial as it initiates the review process and sets the stage for subsequent evaluations.
-
Address Feedback Promptly: After submission, be ready to respond swiftly to any feedback or requests for clarification from the review board. Research indicates that timely responses can significantly enhance approval rates, as delays in addressing concerns can prolong the review process.
-
Ensure Compliance with Regulations: Prior to submission, it is vital to verify that the protocol adheres to all relevant regulatory requirements, including obtaining study approval from Colombia's regulatory agency (INVIMA) and the Ministry of Health (MoH). This encompasses comprehending local and global regulations that oversee research studies, which can differ greatly across areas. Notably, many researchers have withdrawn their applications due to difficulties associated with legislation, impacting the approval process.
-
Engage Stakeholders: Involving key stakeholders—such as research investigators, Regulatory Affairs specialists, and patient representatives—throughout the review process ensures that diverse perspectives are considered. This collaborative approach can lead to more robust protocols and smoother approvals.
-
Document Changes: Maintain comprehensive records of any modifications made to the protocol during the review process. This documentation is crucial for openness and can aid future submissions or modifications, as it offers a clear history of the protocol's development.
By following these steps, researchers can effectively navigate the intricacies of the review process in Colombia, thereby enhancing the chances of prompt approvals and successful execution when creating a clinical trial plan. A recent analysis of 150 site activations highlighted the importance of these strategies, revealing that well-prepared submissions significantly reduce site initiation times. Furthermore, understanding the common challenges faced during the ethics committee review process, such as regulatory hurdles and competition, can help researchers anticipate potential hurdles and streamline their submissions.
As Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, emphasizes, diligence and preparation are key to overcoming these challenges. Additionally, researchers should consider the financial implications of their submissions; for instance, the optional introduction on June 10, 2025, costs 155 EUR for Academic/Not for Profit and 245 EUR for Pharma/For Profit, which underscores the need for careful planning and resource allocation. Bioaccess provides extensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, to aid researchers in navigating these complexities effectively.
Understanding Regulatory Compliance in Clinical Trials
Grasping regulatory compliance is essential for the successful implementation of research studies. Understanding the guidelines established by regulatory bodies such as the FDA and EMA is crucial. Staying updated on the latest regulations for 2025 ensures that your experiment aligns with current standards. Notably, the FDA is urging sponsors to develop Diversity Action Plans to improve diversity in research studies, addressing disparities in participation and promoting representative study outcomes.
Obtaining informed consent from all participants is a fundamental requirement. Evolving statistics indicate that informed consent processes are focusing on clarity and transparency to enhance participant understanding and engagement.
Adherence to privacy regulations is crucial to protect participant information. This involves complying with the General Data Protection Regulation (GDPR) and other pertinent laws, which are increasingly highlighted in research protocols. A recent statistic indicates that 45% of Alcon's data is recorded on the same day as the visit date, emphasizing the significance of timely data management in clinical studies.
Be vigilant about the reporting requirements for adverse events and study results. Regulatory bodies expect timely and accurate reporting, which is critical for maintaining participant safety and trial integrity. As Leianne Ebert, Head of Clinical Data Operations, stated, "This is something we monitor regularly, and last week, our records indicated that 45% of our information is entered on the same day as the visit date."
Prepare for regular audits by regulatory authorities to ensure adherence to all compliance standards. Institutions may need to adjust their standard operating procedures and allocate resources effectively to meet these requirements. There is a growing trend among sponsors to insource information management processes, moving away from reliance on CROs. This shift aims to enhance control over data quality and operational efficiency, reflecting a significant change in the research paradigm.
Incorporating extensive research management services provided by bioaccess, such as feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, is essential for navigating these complexities. Experts like Ana Criado, Director of Regulatory Affairs at Mahu Pharma, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, can provide invaluable insights into these processes.
By prioritizing regulatory adherence, researchers enhance the credibility of their studies and contribute to the overall success and integrity of the research process. This commitment to compliance is increasingly recognized as a best practice in the industry, reflecting a shift towards more rigorous data management and operational efficiency.
Common Challenges in Clinical Trial Planning and Solutions
Common challenges in clinical trial planning in 2025 include:
-
Recruitment Issues: Difficulty in recruiting eligible participants remains a significant hurdle, often leading to delays in study timelines. In fact, studies suggest that nearly 30% of medical studies fail to meet their recruitment goals. Solution: To combat this, researchers should develop targeted recruitment strategies, leveraging digital platforms and engaging with patient advocacy groups to reach potential participants effectively. Significantly, the partnership between bioaccess™ and Caribbean Health Group seeks to improve recruitment efforts in Barranquilla, establishing the city as a premier location for clinical studies in Latin America.
-
Budget Constraints: Limited funding can severely restrict trial activities, impacting everything from participant recruitment to data management. Creating a clinical trial plan that details all anticipated costs is essential. Additionally, exploring alternative funding sources, such as grants or partnerships, can provide the necessary financial support.
-
Regulatory Delays: Navigating the complex landscape of regulatory approvals can be time-consuming and fraught with challenges. Solution: Initiating the regulatory process early and maintaining open lines of communication with regulatory bodies can help streamline approvals. Recent innovations, such as the FDA's introduction of single IRB (sIRB) reviews, aim to reduce duplicative assessments and expedite project initiation, enhancing overall efficiency. As highlighted in a case study on regulatory innovation, these changes are crucial for streamlining processes while protecting participants' rights. The support from Colombia's Minister of Health for initiatives like those from bioaccess™ further underscores the importance of regulatory collaboration.
-
Information Management: Ensuring precise information collection and management is crucial yet difficult. Solution: Implementing robust information management systems and providing comprehensive training for staff can mitigate errors and enhance information integrity. The incorporation of artificial intelligence and machine learning in data management processes has been shown to lower expenses by up to 20%, further aiding effective execution.
-
Stakeholder Engagement: A lack of involvement from stakeholders can significantly hinder progress. Solution: Fostering strong relationships with all stakeholders, including sponsors, regulatory bodies, and patient groups, is vital. Consistent communication and updates can help sustain interest and support throughout the process. The partnership between bioaccess™ and GlobalCare Clinical Trials exemplifies effective stakeholder engagement, achieving over 50% reduction in recruitment time and 95% retention rates.
Furthermore, standardizing outcome measures across research will aid in the creation of guidelines and future comparisons among interventions. As Max Baumann, Head of Execution at Treehill Partners, states, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success."
By proactively tackling these challenges, researchers can significantly improve the chances of a successful medical study, paving the way for innovative healthcare advancements and contributing to local economic growth. The comprehensive services offered by bioaccess™, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, play a crucial role in overcoming these challenges and maximizing the impact of clinical trials on the local economy.
Conclusion
The successful execution of clinical trials, particularly in the rapidly evolving landscape of accelerated medical device studies in Latin America, hinges on meticulous planning and strategic execution. Key components—such as clearly defined study objectives, robust budgeting strategies, and effective stakeholder engagement—are essential to navigate the complexities inherent in clinical research. By addressing these elements, researchers can significantly enhance participant recruitment, streamline regulatory processes, and ensure timely data management—all critical for achieving favorable outcomes.
Moreover, the integration of innovative practices, such as real-time data entry and the use of historical data for budgeting, underscores the need for adaptability in clinical trial planning. As the industry faces challenges, including recruitment difficulties and regulatory delays, proactive measures and collaboration among stakeholders can lead to more efficient study execution and improved participant retention.
Ultimately, the commitment to thorough planning and compliance not only bolsters the credibility of clinical trials but also paves the way for groundbreaking medical advancements. As organizations like bioaccess continue to provide comprehensive support in trial management, the collective efforts of researchers, sponsors, and regulatory bodies will foster an environment conducive to innovation and enhanced patient outcomes. Emphasizing these strategies will not only optimize trial success but also contribute to the ongoing evolution of medical technology in the region.
Frequently Asked Questions
What are the essential components of a comprehensive clinical trial plan?
The essential components include study objectives, study design, study population, intervention details, outcome measures, statistical analysis plan, timeline, and budget.
Why are study objectives important in a clinical trial?
Study objectives clearly articulate both primary and secondary aims of the study, guiding the overall direction and focus of the research.
What types of study designs can be utilized in clinical trials?
Study designs can include randomized controlled trials, observational studies, Early-Feasibility Studies (EFS), and First-In-Human Studies (FIH).
How should researchers define the study population in a clinical trial?
Researchers should define inclusion and exclusion criteria for participant selection to ensure the relevance and applicability of the findings.
What should be included in the intervention details of a clinical trial?
A thorough description of the intervention(s) being tested, including specifics on dosage and administration methods, should be provided for reproducibility and clarity.
What are outcome measures and why are they important?
Outcome measures are the primary and secondary endpoints that will be measured to evaluate the effectiveness of the intervention, ensuring alignment with research objectives.
What is the purpose of a statistical analysis plan in a clinical trial?
The statistical analysis plan outlines the methods used to analyze the data gathered, which is essential for validating the findings.
Why is a timeline crucial in a clinical trial plan?
A detailed timeline highlights key milestones and deadlines, helping to keep the project on track, which is particularly emphasized by organizations like bioaccess®.
What should be included in the budget of a clinical trial?
The budget should outline the financial resources required for the study, reflecting the demand for transparency and accountability in research.
Who are the primary stakeholders in a clinical study?
Primary stakeholders include patients, investigators, sponsors, regulatory authorities, ethics committees/IRBs, information management personnel, and healthcare providers.
How can engaging patients early benefit clinical trials?
Engaging patients early can lead to better recruitment and retention rates, especially in competitive environments.
What role do investigators play in a clinical trial?
Investigators carry out the study and ensure participant safety, making their expertise vital for the study's integrity.
Why is it important to consider regulatory authorities in clinical trials?
Regulatory authorities oversee compliance with legal and ethical standards, ensuring that the study adheres to necessary regulations.
What is the significance of timely and precise data management in clinical trials?
Timely data management is crucial for ensuring accurate information collection and enhancing the quality of research outcomes.
How does bioaccess® contribute to the clinical research landscape in Latin America?
Bioaccess® enhances patient recruitment, navigates regulatory requirements, and provides extensive research services, ensuring efficient oversight of the research process.

