Introduction
In the ever-evolving landscape of medical device development, the formulation of a comprehensive clinical development plan (CDP) stands as a cornerstone. This detailed roadmap not only guides the progression from concept to market launch but also ensures regulatory compliance, patient safety, and market competitiveness. The CDP integrates key components including clearly defined objectives, meticulous study design, a robust regulatory strategy, and precise budget and resource allocation.
Each element is meticulously crafted to align with the overarching mission of improving patient outcomes, addressing specific populations, and navigating the complex legal landscape. By incorporating these critical elements, a well-structured CDP facilitates a seamless and effective transition through the various stages of medical device development, ultimately ensuring that the final product is both safe and efficacious.
Key Components of a Comprehensive Clinical Development Plan
A thorough development plan (CDP) is essential for the successful advancement of a medical device. It encompasses various elements that collectively ensure a seamless progression from concept to market launch.
-
Objectives: Clearly defined goals for clinical trials that align with the overall mission of improving patient outcomes. For instance, consider how HSPC transplant initially targeted terminally ill patients, setting clear, life-saving objectives from the outset.
-
Study Design: A detailed description of the study methodology, including trial phases, endpoints, and participant demographics. This includes addressing specific populations in drug development, ensuring that the study is inclusive and comprehensive.
-
Oversight Strategy: An outline of the compliance pathway, including FDA interactions and approvals. Navigating the complex legal and compliance landscape is essential, particularly as obtaining market approval for new products is a top priority for 47% of medical equipment leaders, despite the rising legislative challenges.
-
Budget and Resources: Estimation of financial resources and staffing needs throughout the development process. This encompasses pre-market research, development, and clinical evidence, which are crucial for the post-market safety, efficacy, and performance of medical products.
By combining these elements, a CDP not only fulfills compliance requirements but also responds to market dynamics, ensuring that the medical instrument is both safe and effective. Moreover, the CDP should be adaptable to changes, such as updates in regulatory submissions due to design modifications, ensuring continuous improvement and compliance.
Defining the Target Product Profile (TPP)
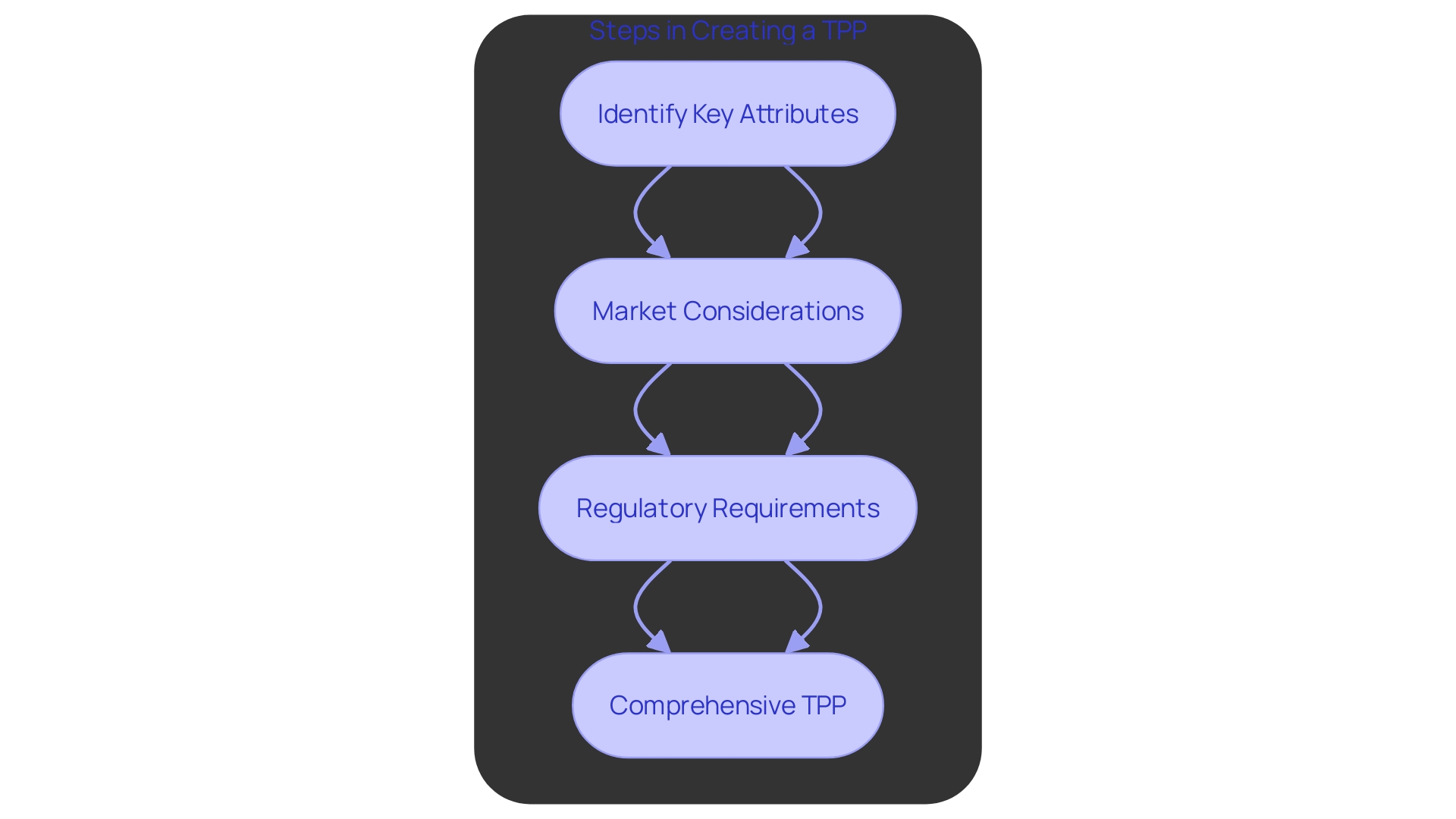
The Target Product Profile (TPP) is a crucial strategic document that details the key characteristics and specifications needed for a medical instrument to fulfill its intended use and market needs. Developing a comprehensive TPP involves several crucial steps:
-
Identify Key Attributes: Determining the essential performance and safety parameters is paramount. This encompasses outlining the shape, size, and purpose of the instrument, which are essential for guaranteeing that it fulfills its intended role efficiently and securely.
-
Market Considerations: Assessing the competitive landscape and identifying unmet needs are essential for positioning the product in the market. This involves understanding the diverse nature of medical tools, which range from simple items like bandages to complex machinery like MRI equipment. By recognizing these market dynamics, developers can better tailor their products to address specific gaps and demands.
-
Regulatory Requirements: Ensuring the TPP aligns with FDA standards and guidelines is crucial for compliance. The oversight environment for medical instruments is strict, aiming to ensure patient safety and product effectiveness. Good Manufacturing Practice (GMP) frameworks, such as ISO 13485 and FDA regulations, provide the necessary guidelines to maintain consistent quality and compliance. However, navigating these compliance waters can be challenging due to the complexity and detailed nature of the requirements.
By meticulously addressing these steps, the TPP serves as a blueprint that guides the development process, ensuring that the final product is safe, effective, and competitive in the market.
Regulatory Considerations and Strategy
Grasping regulatory requirements is essential to creating a successful strategy for medical devices. Key strategies for ensuring compliance and effectiveness include:
-
Pre-Submission Meetings: Engage with the FDA early to discuss your plans and gather feedback. Initial engagement can pave the way for success by specifying the necessary information and aligning expectations. According to Michelle Tarver, acting director at CDRH, having an Investigational Device Exemption (IDE) before a de novo submission is a significant indicator of success, as it demonstrates thorough consideration of clinical data requirements.
-
Compliance with Guidelines: Ensure all aspects of the study meet FDA guidelines. Regulatory documentation, defined as documents submitted to health authorities to support all phases of product development and marketing, must comply with the laws and regulations governing medicinal products. Creating a shared vocabulary among team members at the project's outset is critical to maintaining compliance and ensuring understanding across various roles.
-
Documentation: Maintain thorough records of all compliance communications and submissions. Proper documentation supports research, development, marketing applications, and post-marketing activities. As highlighted by Owen Faris from CDRH’s Office of Product Evaluation and Quality, comprehending the advantages, dangers, and verification of the product, along with an acceptable degree of uncertainty, is essential for decision-making in governance. Comprehensive documentation guarantees that all these factors are clearly conveyed and comprehended by both the oversight authorities and the project team.
By adhering to these strategies, research teams can maneuver through the intricate compliance environment more efficiently, ultimately resulting in more successful product approvals and market entries.
Risk Management and Contingency Planning
Efficient risk management is essential for addressing obstacles in medical development. This encompasses:
-
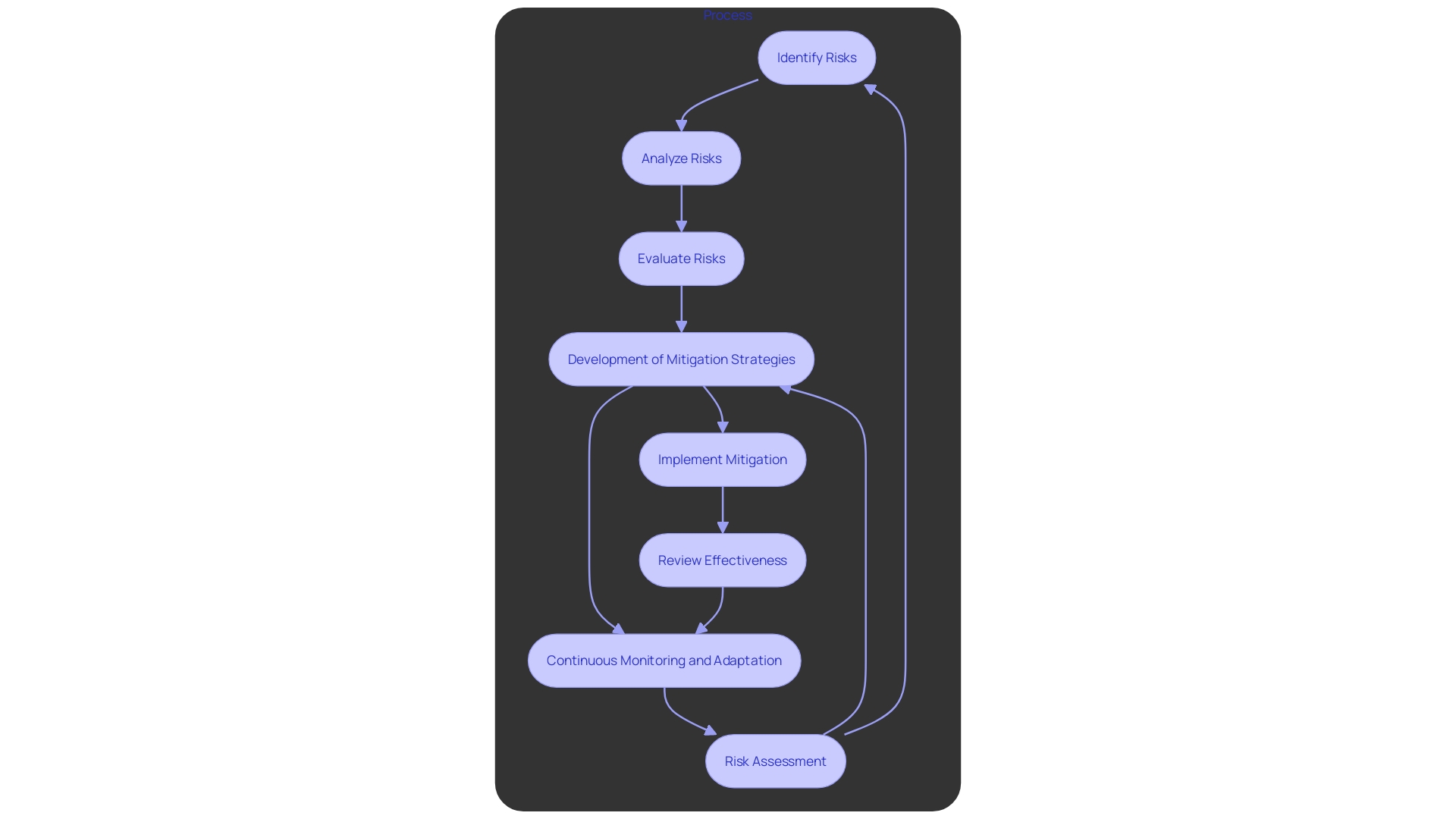
Risk Assessment: Identifying potential risks, including safety, efficacy, and regulatory issues, is paramount. With the rapid expansion of digital health solutions and AI-driven tools, understanding and categorizing risks becomes crucial. Regulatory bodies such as the FDA and EMA emphasize a “risk-based approach” to ensure transparency and safety.
-
Mitigation Strategies: Developing comprehensive plans to address identified risks ensures that contingencies are in place. The FDA's Software as a Medical Device (SaMD) regulations highlight the importance of good practice standards in design, development, and validation. This is essential for mitigating biases and maintaining the effectiveness of AI models used in clinical trials.
-
Monitoring and Adaptation: Continuous monitoring and adaptation of strategies are necessary to manage risks effectively. 'The changing legal framework for medical devices, particularly for SaMD, demands that professionals stay informed of global updates.'. AI-driven regulatory tools can streamline this process by promptly alerting professionals to changes, thus reducing risk and expediting market entry.
'Ensuring adherence to these strict guidelines not only safeguards patient safety but also boosts the trustworthiness and dependability of trial information.'. The FDA's dedication to varied and inclusive trials further emphasizes the significance of strong risk management in attaining generalizable and dependable results.
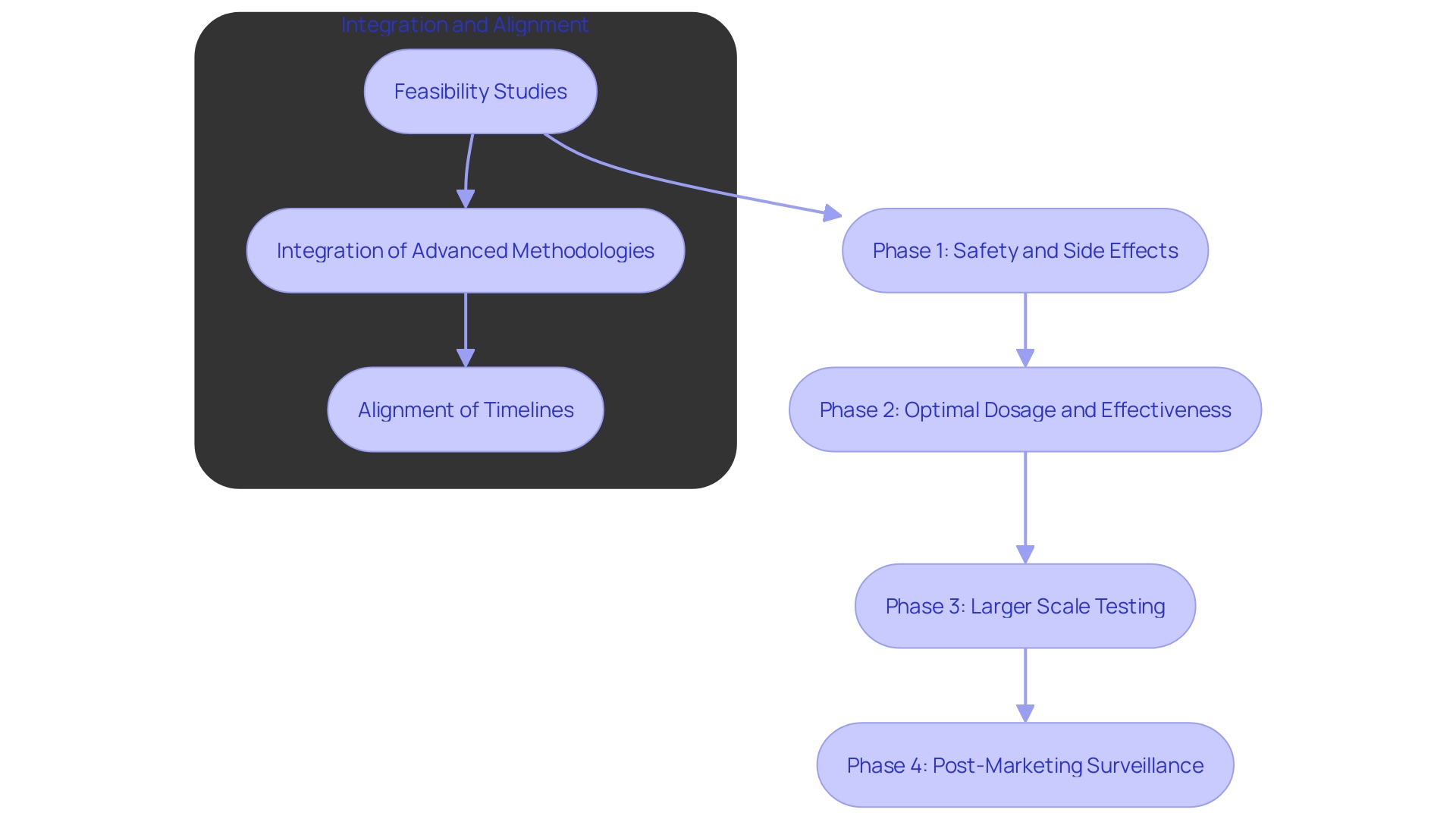
Creating a Logical Sequence of Clinical Trials
Creating a series of clinical trials is crucial for effectively collecting essential information, ensuring adherence to regulations, and obtaining significant results.
- Phased Approach: A stepwise approach should be adopted, starting with feasibility studies and advancing to pivotal trials. 'This methodical progression not only validates the initial hypothesis but also allows for adjustments based on early information, minimizing risks and maximizing resource utilization.'. According to recent research, seamless designs and master protocols can significantly streamline this process, reducing the time and cost associated with traditional trial phases.
'- Information Integration: Effective information collection and analysis are critical at each trial phase.'. Trials typically gather information in closed systems, creating a burden on both clinicians and patients. Combining electronic health records (EHR) and utilizing real-world information sources can improve information quality and decrease redundancy. 'A comprehensive information strategy should be established before protocol design, incorporating advanced AI-driven methodologies to manage and analyze large volumes efficiently.'. 'This approach not only supports information flow but also enriches insights through patient-reported information and connected devices, as noted by experts in the field.'.
- Timeline Alignment: Ensuring that all trials are logically scheduled is essential for smooth transitions and ongoing information flow. The use of technology and multiple systems has been identified as a significant challenge, often leading to extended timelines and decreased efficiency. Aligning trial schedules with a robust data strategy can mitigate these issues, facilitating seamless transitions between phases and improving overall trial management. A recent study emphasized that despite heightened investments in technology, many research operations still encounter longer study timelines due to increased complexity. Streamlining these processes is crucial for timely and effective trial completion.
Executing the Clinical Strategy and Monitoring Progress
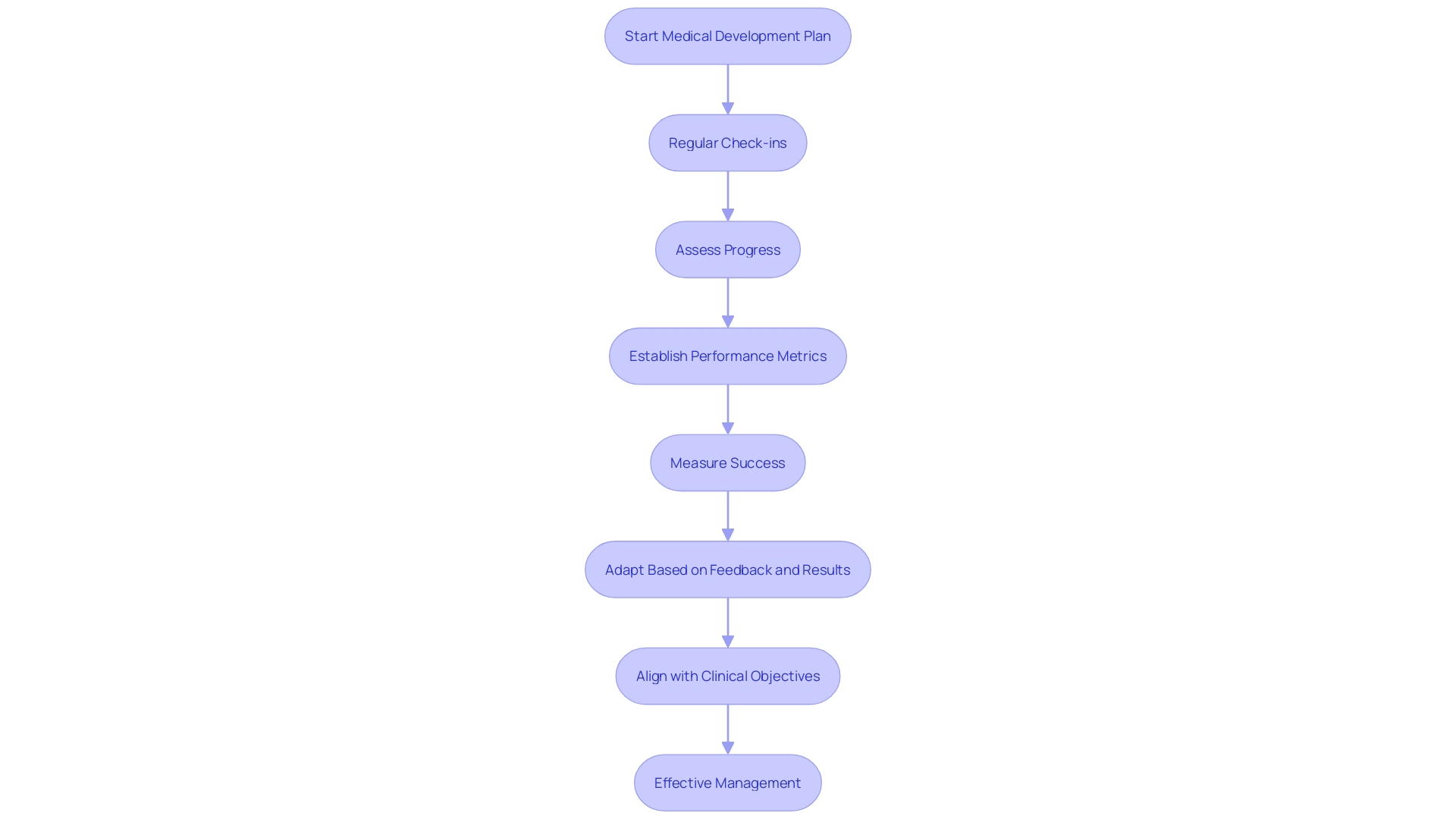
Implementing a successful medical development plan requires strict supervision and ongoing monitoring. Key practices to ensure effective management include:
-
Regular Check-Ins: Frequent meetings are crucial to evaluate progress against established milestones. This approach mirrors the iterative development used in creating innovative tools, such as the sepsis AI tool, where ongoing assessments ensure alignment with clinical objectives.
-
Performance Metrics: Establishing key performance indicators (KPIs) is essential for measuring success. For instance, in drug development, leveraging data from connected devices and AI-driven methodologies can exponentially increase the breadth of data, allowing for more precise performance tracking and smarter decision-making.
-
Adaptability: Flexibility is vital in adjusting strategies based on interim results and external feedback. The evolving landscape of healthcare, influenced by remote and digital approaches, underscores the importance of incorporating feedback to refine and optimize strategies continuously. This adaptability is also reflected in the FDA's commitment to fostering innovation and advancing public health through updated regulatory processes.
Conclusion
A comprehensive clinical development plan (CDP) is crucial for the successful advancement of medical devices from concept to market. This strategic framework integrates clearly defined objectives, meticulous study designs, robust regulatory strategies, and precise budget allocations. By doing so, the CDP ensures regulatory compliance, prioritizes patient safety, and addresses market dynamics, ultimately leading to enhanced patient outcomes.
The Target Product Profile (TPP) acts as a vital blueprint, detailing the essential characteristics needed to meet market demands. By focusing on key attributes and aligning with regulatory requirements, the TPP guides the development process, ensuring the final product is both safe and competitive.
Navigating the complex regulatory landscape is essential for success. Engaging in pre-submission meetings, adhering to guidelines, and maintaining thorough documentation are critical strategies that facilitate the approval process and enhance the credibility of clinical trial data.
Effective risk management and contingency planning are key to overcoming challenges in clinical development. Identifying potential risks, developing mitigation strategies, and continuously monitoring the regulatory environment help safeguard patient safety and ensure reliable outcomes.
Finally, executing the clinical strategy with rigorous oversight and adaptability is vital for successful trial management. Regular evaluations and a commitment to refining strategies based on feedback are essential for achieving meaningful results. By embracing these practices, organizations can navigate the complexities of medical device development, delivering innovative solutions that satisfy both regulatory standards and patient needs.




