Introduction
The role of a Clinical Development Plan (CDP) in medical device regulation is crucial for ensuring the safety and efficacy of these devices. The CDP serves as a roadmap for conducting clinical trials and gathering the necessary data for regulatory submissions. However, there can be challenges in aligning data requirements between regulatory bodies and payors, which can impede patient access to new medical devices.
In addition, concerns have been raised about the FDA's 510(k) clearance process, which can expedite device availability but may compromise safety. To address these issues, regulatory agencies are developing frameworks that prioritize patient safety and international harmonization. Healthcare providers and payors need to understand the FDA's role in evaluating medical devices to make informed decisions on coverage and use.
A well-structured CDP that aligns with regulatory and payor requirements is essential to minimize obstacles in patient access to essential medical technologies.
Understanding the Role of CDP in Medical Device Regulation
A comprehensive Clinical Development Plan (CDP) is a foundational element in the lifecycle of medical equipment regulation. This strategic document delineates a clear roadmap for conducting clinical trials and assessing an equipment's safety and efficacy. With the aim of smoothing the pathway towards regulatory approval, the CDP is instrumental in ensuring a structured development process, establishing unequivocal study goals, and amassing the requisite data for regulatory submissions.
The CDP's importance is further highlighted by the reality that data submitted to the FDA for approval may not always align with the criteria used by payors to determine coverage. Therefore, despite FDA approval or clearance, there can be delays or denials in the coverage and usage of the instrument due to the additional data requirements from payors, such as CMS and private health plans. This misalignment can hinder individuals' ability to obtain new medical equipment.
Furthermore, the documentary 'The Bleeding Edge' emphasizes the potential risks linked to the FDA's 510(k) clearance process, which can enable products to bypass clinical trials by demonstrating similarity to existing items. This procedure can result in accelerated availability of equipment but also raises concerns about safety, as shown by examples of harm to individuals.
In reaction to these difficulties, organizations such as the MHRA in the UK are developing fresh regulatory structures aimed at improving patient safety, facilitating the availability of healthcare equipment, and attaining broader global harmonization. These frameworks prioritize patient-centric requirements and adapt to the rapid advancements in healthcare technology.
Grasping the FDA's function in evaluating the safety and efficacy of healthcare equipment is essential for healthcare providers and payors when making informed decisions on coverage and use. This emphasizes the significance of a well-organized CDP that conforms with both regulatory and payor prerequisites to reduce barriers in individuals' entry to necessary healthcare technologies.
Key Components of a Clinical Development Plan
Developing a clinical development plan for medical devices is a meticulous process that must incorporate several critical components to ensure the successful execution of clinical studies and the generation of reliable, meaningful results. The strategy should start with the identification of the target population, acknowledging the specific needs and characteristics of the group. This aspect is vital, as demonstrated by Teal's approach to cervical cancer screenings, which was designed with women's experiences in mind, significantly improving screening compliance and effectiveness.
Another crucial element is the selection of appropriate study endpoints, which are the specific events or outcomes that the study aims to observe. These endpoints must be measurable and meaningful to the population in question and regulatory bodies. Moreover, determining the optimal study design and sample size is paramount. This process is informed by previous studies, such as the MARRS study, which aims to enroll up to 220 patients to assess the efficacy of a new thrombectomy procedure.
Finally, a comprehensive data collection and analysis plan is essential. With the emergence of connected technology and advanced data analytics, it is feasible to collect and analyze substantial amounts of data from various sources. This approach was echoed in the insights provided by a National Institute on Aging Leadership Award-funded database, which offers real-time updates and annotations for Alzheimer's Disease clinical trials. The establishment of such a data strategy is a crucial step in ensuring the integrity and utility of the data collected throughout the study. All of these elements play a crucial part in the effort to bring secure, efficient, and patient-focused healthcare instruments to the market.
Strategic Planning for Medical Device Development
Developing a healthcare apparatus is a intricate journey that goes beyond mere technical design and addresses the ecosystem of its use, including individuals seeking medical care, healthcare professionals, and supporting staff. This holistic approach—frequently called service design—ensures that the equipment not only fulfills patient needs but also integrates seamlessly into the workflows of those who provide care. By embracing a user-centered design philosophy, which encompasses usability testing and iterative design processes, developers can create tools that enhance the individual experiences of all users, from clinicians to support staff.
For medical startups, the path to success involves a clear vision of who the equipment will assist and the impact it will bring. This starts with a strategic plan that considers not only the end product but also the journey there. Key factors to take into account encompass the appearance of the apparatus, market size, distribution channels, and critical milestones. Moreover, having knowledge about the regulatory environment, like adhering to the guidelines of the Medicines & Healthcare products Regulatory Agency (MHRA) in the UK for products with a medical purpose, is crucial in shaping the roadmap for progress.
Recent industry advancements emphasize the significance of strategic planning. For instance, in the MARRS study for thrombectomy equipment, Perfuze plans to enroll patients across multiple sites, emphasizing the requirement for careful project management and adherence to timelines. The neurovascular thrombectomy market, led by companies like Medtronic, is burgeoning and projected to reach $1.9bn by 2033. Early detection and prevention, as shown by Everly Health's emphasis on kidney health, also have a crucial part to play in the advancement of technology.
A prosperous healthcare instrument venture typically ends in a tactical departure, which can be in the form of a purchase, IPO, licensing deal, or strategic partnership. These outcomes not only reflect the culmination of strategic planning but also underscore the qualities of perseverance and confidence that characterize the leaders of successful startups in the healthcare sector.
Integrating these diverse factors into the plan can ultimately aid in a smoother transition through regulatory pathways, maximize resources, and reduce risks—resulting in a successful introduction of the product into the market.
Regulatory Considerations and Compliance
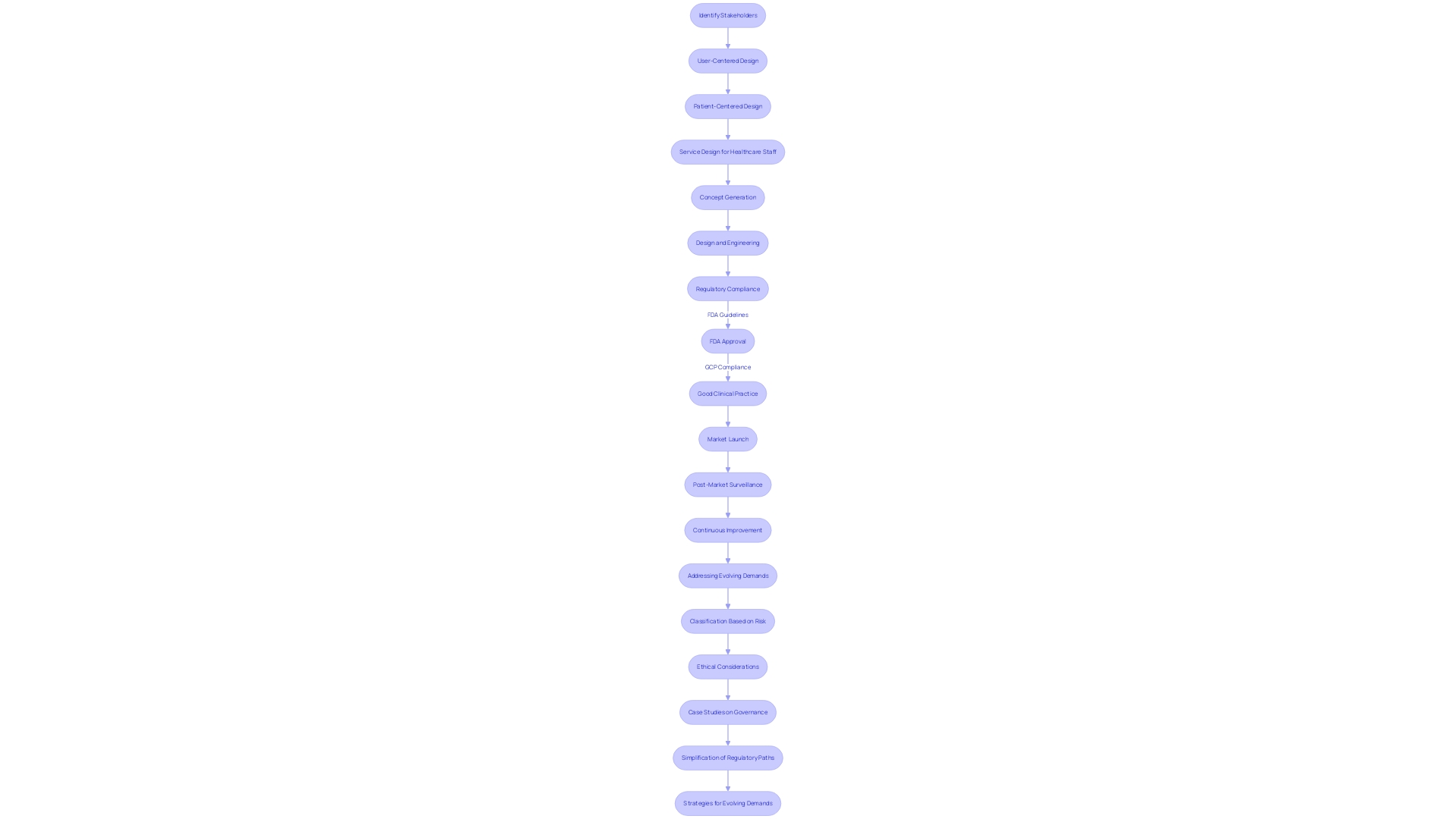
Creating a clinical development strategy for healthcare equipment necessitates a thorough comprehension of the regulatory environment, especially in line with the guidelines of the FDA. These guidelines are designed to ensure the safety, efficacy, and security of healthcare instruments, which requires compliance with Good Clinical Practice (GCP) and the acquisition of necessary approvals and clearances. The FDA classifies instruments into three categories based on risk, with each requiring a different level of regulatory scrutiny.
The importance of adhering to ethical standards cannot be overstated, as highlighted by case studies that explore the governance of technology within the healthcare sector. These studies uncover the ethical, legal, and social concerns that arise from the use of healthcare instruments and emphasize the necessity for a set of guiding queries to navigate these intricacies. Moreover, the case studies offer a historical background, which aids in comprehending the progression of technology and its current state, thereby ensuring that plans for growth are influenced by both previous encounters and current anticipations.
Simplifying regulatory paths has become a priority, as shown by recent FDA initiatives aimed at speeding up the approval process for healthcare apparatus, especially those that fulfill pressing health requirements. This includes the publication of standards that require drug advertisements to present major side effects and contraindications in a clear, conspicuous, and neutral manner, ensuring that the information is easily understandable to consumers.
Professionals in the industry are also adopting strategies to navigate the evolving regulatory demands, improving efficiency in document preparation to meet standards while minimizing delays. Having a thorough grasp of the market and regulations, along with the industry's readiness to fulfill these growing demands, is essential for adherence and effective maneuvering through the process of creating healthcare equipment.
Designing a Comprehensive Clinical Development Strategy
Developing a successful clinical development strategy for medical instruments involves more than just the technical specifications—it's a comprehensive process that understands the intricacy of healthcare settings and the multitude of stakeholders involved. It starts with a comprehensive comprehension of the purpose of the gadget and the individuals it serves, but it must also acknowledge the varied responsibilities of healthcare professionals, support staff, and even the logistics system that contribute to the care of an individual. A successful strategy considers the complete lifecycle of the product, from conception to implementation, aligning it with both regulatory frameworks and business objectives. Furthermore, it is crucial to incorporate user-focused design principles throughout the progression, guaranteeing that the apparatus fulfills the intricate requirements and encounters of all individuals, such as patients, caregivers, and healthcare practitioners. This comprehensive approach to design not only improves user satisfaction but also supports the safety and effectiveness of the product, ultimately enabling successful market entry and adoption. By embracing this strategic, user-centric mindset, companies can create a plan that is strong, scientifically validated, and customized to achieve significant impact in the healthcare landscape.
Creating a Successful Medical Device Development Plan
The process of creating healthcare tools is intricate and far-reaching, starting from the conception phase and extending to their implementation in healthcare settings. A key aspect often emphasized is user-centered design, prioritizing the distinct needs and experiences of end-users. While design focused on individuals requiring medical care is a subset, concentrating on solutions specifically for patients, an equally critical aspect is the consideration of clinical staff and other healthcare stakeholders in the design process. This comprehensive approach, referred to as service design, acknowledges that patient care is a collaborative endeavor involving different individuals including nurses, doctors, and support staff, all of whom interact with the healthcare equipment and impact patient outcomes.
Considering the wider influence of the healthcare apparatus, one must take into account the ultimate objectives: the advantage to the recipients, the societal values it maintains, and the vision it represents. It is imperative to visualize the final product, gauge the market size, identify potential buyers, and understand how the supply chain factors into overall success. Milestones must be well-defined and strategically placed throughout the timeline to track progress and facilitate goal attainment.
In the field of healthcare equipment development, user research and usability testing are crucial to creating tools that not only fulfill clinical requirements but also improve the interaction between the tool and all users, including caregivers and maintenance personnel. An iterative design process that incorporates feedback from these varied users ensures that the final product is not only effective for patients but also convenient and practical for all healthcare professionals who engage with it.
Furthermore, the commercial success of a healthcare instrument is not only reliant on its integration into the healthcare setting but also on the strategic business choices resulting in successful exits. These can manifest as acquisitions by larger companies, initial public offerings, lucrative licensing or partnerships, and forming strategic alliances, all of which are indicative of a successful venture and provide a substantial return on investment.
The importance of early detection and prevention of diseases like chronic kidney disease is highlighted by the initiatives of companies such as Everly Health, which, through advanced diagnostics and virtual care, strives to alter the trajectory of such illnesses. The inclusion of such healthcare tools into broader healthcare initiatives emphasizes the importance of companies specializing in the creation of health equipment in contributing to advancements in public health.
To sum up, the advancement of healthcare tools necessitates a synchronized approach that encompasses not only the technical and design aspects but also the commercial and healthcare implications, ensuring that the tool successfully transitions from concept to a valuable tool in the healthcare industry.
Case Study: Implementing a CDP for Medical Devices
Starting a clinical advancement strategy for a healthcare tool is a complex undertaking that necessitates a strong emphasis on the ultimate objective: the influence on individuals, healthcare principles, and the measurable results of the product. The journey involves an intricate process, beginning with a clear vision that guides the product from design to market-ready status. Key elements such as gap analysis, process and test strategy, supply chain management, and a well-defined regulatory pathway are pivotal. These steps are particularly valuable for startups aiming for funding milestones or established companies preparing for full-scale production. However, navigating this pathway to commercialization is complex, requiring adaptability to unforeseen deviations from the initial roadmap.
The strategies to streamline this process include a thorough review of product requirements, prioritizing those critical for performance, safety, and quality while deferring non-essential features. An approach that focuses on the needs of the user ensures that the equipment not only fulfills technical requirements but also delivers an instinctive and fulfilling experience for both healthcare professionals and individuals. The process is further bolstered by the recent policy initiatives by regulatory bodies like the MHRA, which aim to reduce assessment duplication and foster innovation by recognizing international regulatory approvals. This progressive strategy is intended to accelerate the distribution of secure and efficient healthcare technologies to the market, thereby improving care and healthcare distribution.
By understanding these critical aspects, companies can better position themselves for successful medical device launches, characterized by strategic partnerships, licensing agreements, or even IPOs. The ability to integrate software with hardware, for instance, provides a competitive edge in the digital health landscape. Ultimately, selecting a development partner with the requisite technical and regulatory expertise is essential for a seamless transition from concept to market, ensuring that the clinical development plan aligns with the overarching vision for patient care and healthcare innovation.
Conclusion
In conclusion, a well-structured Clinical Development Plan (CDP) is crucial for ensuring the safety and efficacy of medical devices. Challenges in aligning data requirements between regulatory bodies and payors can impede patient access to new devices. The FDA's 510(k) clearance process has raised concerns about expedited availability compromising safety.
Regulatory agencies are developing frameworks prioritizing patient safety and international harmonization.
Developing a comprehensive CDP involves identifying the target patient population, selecting study endpoints, determining study design and sample size, and establishing a data collection and analysis plan. These components bring safe, effective, and patient-centered devices to the market.
Strategic planning is essential, including a clear vision, understanding of the regulatory landscape, and consideration of market size and distribution channels. Successful ventures culminate in strategic exits like acquisitions, IPOs, licensing agreements, or strategic alliances.
Adhering to regulatory considerations and compliance is crucial. Streamlining regulatory pathways and improving document preparation efficiency are strategies embraced by professionals in the industry.
Designing a comprehensive clinical development strategy involves integrating user-centered design principles to enhance user satisfaction, safety, and efficacy. By adopting this strategic, user-focused mindset, companies can forge a development plan that is scientifically validated and tailored for meaningful impact in the healthcare landscape.
In conclusion, a synchronized strategy encompassing technical, design, commercial, and healthcare implications is necessary for successful medical device development. Navigating the pathway to commercialization requires adaptability and understanding of critical aspects like gap analysis, process and test strategy, supply chain management, and regulatory pathways. With the right approach and partnerships, companies can position themselves for successful medical device launches that contribute to patient care and healthcare innovation.




