Introduction
The clinical evaluation plan (CEP) is a crucial document in the assessment of a medical device's safety and performance. It serves as a guide for collecting and analyzing clinical data, ensuring compliance with regulatory standards. An effective CEP requires a meticulous approach to data analysis and presentation, as discrepancies can undermine the integrity of the evaluation.
Additionally, the integration of post-market surveillance data is often overlooked but plays a critical role in continuous assessment and improvement of medical devices. By incorporating expert experiences and aligning with recognized standards, the quality and effectiveness of the clinical evaluation can be enhanced, ultimately improving patient access to innovative and safe medical devices. With the increasing prevalence of chronic conditions, rigorous and transparent evaluations are more important than ever to enhance patient outcomes and public health.
Understanding the Purpose of a Clinical Evaluation Plan
The evaluation plan (CEP) is the cornerstone document which guides the evaluation of a medical product's safety and performance. This systematic and strategic document is crucial for demonstrating that the device achieves its intended benefits while adhering to the regulatory standards. Creating a successful CEO necessitates not only a thorough comprehension of its objective but also a meticulous method to the analysis and presentation of medical information.
A well-structured CEP ensures that the data narrative is supported by detailed analysis and coherent presentation, particularly in data tables and references. This cohesion is crucial to establish a clear connection between the data presented and the conclusions drawn. Inconsistencies in documentation, such as the assessment report (CER) and risk management files, can frequently suggest a lack of harmony in data gathering and analysis approaches, which can compromise the integrity of the assessment.
Furthermore, the effective use of post-market surveillance data is often an overlooked component in the clinical evaluation process. This information plays a vital role in ongoing evaluation and enhancement of healthcare equipment, which is essential for the progress of MedTech and safeguarding patient safety.
As emphasized by Bijan Elahi, an experienced specialist in safety risk management for healthcare equipment, 'Safety Risk Management for Healthcare Equipment' unravels the intricacies of risk management, empowering professionals with clarity and assurance. This underscores the importance of standardization in risk management practices, aligning with the FDA's commitment to fostering innovation and ensuring health equity through diverse and reflective study populations.
Incorporating the knowledge of specialists and aligning the assessment process with acknowledged criteria can significantly improve the quality and effectiveness of the assessment, ultimately facilitating patient entry to innovative and safe healthcare apparatus. Given the high occurrence of long-term illnesses such as diabetes affecting a considerable segment of society, it is increasingly crucial to guarantee that healthcare equipment goes through thorough assessments that are open, uniform, and mirror practical application to enhance patient results and public well-being.
Key Requirements for a Clinical Evaluation Plan
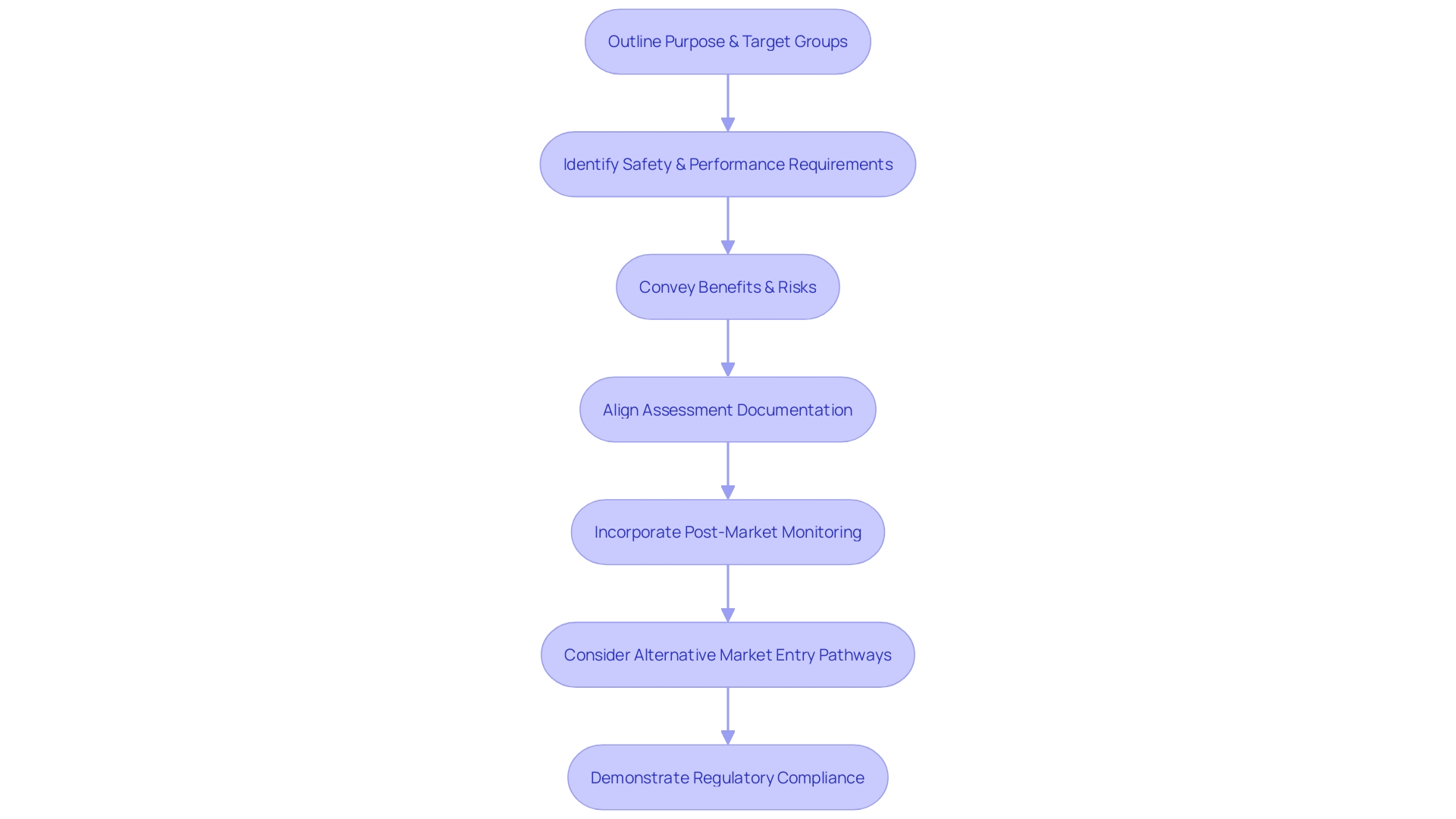
A successful evaluation plan is a foundation of safety and performance for an instrument, as it outlines the pathway for collecting and analyzing data, which is crucial in demonstrating compliance with regulatory requirements. The plan must outline the intended purpose and target groups of the instrument, guaranteeing that the general safety and performance requirements (GSPRs) are clearly identified and met. Importantly, the plan should also convey the benefits and remaining risks associated with the utilization of the apparatus.
The significance of this paper is emphasized by its role in assessments, which are vital appraisals of a healthcare instrument's safety and performance based on data from medical trials. This procedure is not only about fulfilling regulatory standards but also about guaranteeing openness and comprehensibility to a range of stakeholders, including patients, clinicians, and hospital staff, who all interact with the apparatus in various manners.
Recent policy developments, such as the MHRA’s statement of policy intent for international recognition of healthcare tools, highlight the evolving landscape of healthcare tool regulation and the importance of aligning assessment documentation. This includes the Clinical Evaluation Report (CER), a critical component of the technical documentation required for CE marking in the EU, which must be meticulously analyzed and coherently presented.
A typical challenge is the alignment and consistency of medical information across different documents, including assessment plans, CERs, and risk management files. The incorporation of post-market monitoring information is also a crucial component that is often underestimated, yet it offers priceless insights into the real-world performance of healthcare instruments.
Moreover, the assessment plan should take into account the potential qualification for alternative pathways to enter the market, as suggested by recent developments in the industry, like Cardiawave's readiness for a Series B funding round to back marketing and medical research in the U.S. prior to submitting an FDA pre-market approval application.
To reduce risks and improve patient results, it is essential for manufacturers of healthcare equipment to prioritize clear communication about their tools, including their intended use, development, performance, and when possible, the underlying logic of their operation. With more than 1.7 million injuries and 83,000 deaths potentially associated with defective healthcare instruments in a recent 10-year span in the U.S., the importance of comprehensive assessment strategies has never been greater. The ultimate goal is to ensure that medical devices are safe, effective, and beneficial for all users, from patients to the healthcare professionals who serve them.
Defining the Scope of the Clinical Evaluation
Developing a successful assessment in the medical field necessitates a thorough method for outlining its extent. It starts with establishing specific goals, which are the foundation of a targeted evaluation plan. It's essential to identify the suitable data sources and establish criteria for study inclusion. This precision in scope definition is crucial to a comprehensive assessment of medical devices, ensuring patient safety and device performance.
For example, think about the case study of a patient with chest pain, where a differential diagnosis was guided by a structured healthcare assessment, ultimately leading to an precise diagnosis. This underscores the importance of a robust clinical evaluation framework.
In the realm of medical instruments, the need for transparency is paramount. As demonstrated by the rise of Molds (Machine Learning Medical Devices), information about an apparatus's intended use, development, performance, and logic must be conveyed to relevant audiences. This covers the explainability of a gadget—the extent to which its logic can be comprehended by humans.
Bijan Elahi, an experienced specialist in safety risk management for healthcare devices, highlights the significance of a comprehensive examination and logical demonstration of data. This corresponds to data suggesting that over a period of ten years, there were more than 1.7 million harm instances and 83,000 fatalities that might have been associated with defective healthcare apparatus, emphasizing the crucial significance of thorough assessments.
In summary, a clearly outlined assessment plan is a fundamental aspect of the introduction of any novel healthcare product to the market, guaranteeing both innovation and patient safety and efficacy.
Developing a Clinical Development Plan
Creating a development plan for medical trials is a meticulous undertaking that shapes the foundation of any evaluation plan. A well-structured plan is designed to outline the roadmap for gathering the evidence that is pivotal for the appraisal of a medical device. It includes a range of essential elements such as the study's configuration, the demographics of the patient population, the endpoints, the size of the sample group, and the methodology for statistical analysis.
For example, improving eligibility criteria for trials is an iterative process that requires a delicate balance. If the criteria are too narrow, it risks insufficient participant enrollment, while overly broad criteria can introduce increased variability and amplify management costs. The precision in defining these criteria is essential to the integrity and efficiency of the study.
Moreover, the collaboration between the Sponsor and Developer is crucial, as seen in the case of Sabin, which expects Developers to provide timely feedback on both draft and final protocols and amendments. This partnership ensures that the protocol is mutually agreed upon before submissions to Institutional Review Boards (IRBs) or regulatory authorities like the MHRA, which offers comprehensive guidance for MedTech companies.
In addition, recent advances in regulatory strategies, such as those employed by Cardinal Health, underscore the necessity for comprehensive planning. They demonstrate how a well-devised regulatory strategy can lead to successful and timely IND submissions, further highlighting the importance of a well-formulated clinical development plan.
The importance of such planning is also supported by the perspectives of experienced healthcare sector leaders who stress the significance of comprehending the end goal: who is being assisted, what impact the equipment will have, and the core values propelling the development process. These insights align with the principles of ensuring safety, quality, and efficacy in medical equipment, as overseen by agencies like the FDA, which provides detailed guidance on the design and analysis of trials conducted under a master protocol.
To summarize, the basis of a strong development plan for healthcare lies in a comprehensive understanding of the issue at hand, the requirements of the users, whether medical professionals or patients, and the capability to effectively navigate the regulatory landscape. This comprehensive approach ensures the collection of high-quality clinical data that is crucial for a sound assessment of a device's safety and performance.
Conclusion
In conclusion, the clinical evaluation plan (CEP) is a crucial document for assessing the safety and performance of medical devices. It guides the collection and analysis of clinical data, ensuring compliance with regulatory standards. By incorporating post-market surveillance data and aligning with recognized standards, the evaluation can be enhanced to improve patient access to safe and innovative medical devices.
An effective CEP outlines the pathway for collecting and analyzing clinical data, demonstrating compliance with regulatory requirements. It communicates the device's purpose, target groups, safety and performance requirements, and the associated benefits and risks. Transparent communication and the integration of MLMDs are important considerations in today's medical device landscape.
Defining the scope of the clinical evaluation is essential, requiring clear objectives, appropriate data sources, and study inclusion criteria. This ensures a comprehensive assessment of medical devices, prioritizing patient safety. Collaboration between the Sponsor and Developer, refining eligibility criteria, and comprehensive planning are key elements in developing a robust clinical development plan.
In summary, a well-defined clinical evaluation plan is crucial for assessing the safety and performance of medical devices. By following recognized standards, incorporating post-market surveillance data, and aligning with expert experiences, the evaluation can be enhanced to ensure patient access to safe and innovative medical devices.




