Introduction
Navigating the regulatory landscape for medical devices in Argentina involves understanding the intricate frameworks set by the National Administration of Medicines, Food and Medical Technology (ANMAT) and the Mercosur trade bloc. These regulatory bodies ensure that medical devices meet high standards of safety and efficacy before entering the market. The collaborative efforts within Mercosur aim to harmonize regulatory guidelines across member countries, simplifying compliance and enhancing market access.
This article delves into the classification and registration processes for medical devices in Argentina, highlighting the essential requirements for compliance, the emerging trends in clinical trials, and the challenges and opportunities in this dynamic market. By examining these facets, manufacturers and researchers can better navigate the complexities of the Argentine medical device sector, ensuring successful market entry and ongoing compliance.
Regulatory Framework: ANMAT and Mercosur
In Argentina, the regulatory framework for healthcare instruments is primarily managed by the National Administration of Medicines, Food and Medical Technology (ANMAT). This agency guarantees that health instruments fulfill strict safety and effectiveness criteria prior to being sold. Additionally, Argentina is part of the Mercosur trade bloc, which includes Brazil, Paraguay, and Uruguay. Mercosur has established common regulatory guidelines to harmonize the approval process for health devices across member countries, aiming to facilitate trade and streamline the regulatory pathway for manufacturers. This collaboration not only simplifies regulatory compliance but also improves accessibility, benefiting both manufacturers and healthcare providers. Recent partnerships, such as the one between TTG and AccesoFarm in Mexico, demonstrate the growing emphasis on international collaboration to bring advanced healthcare technologies to new markets, thereby improving health outcomes.
Medical Device Classification and Registration Process
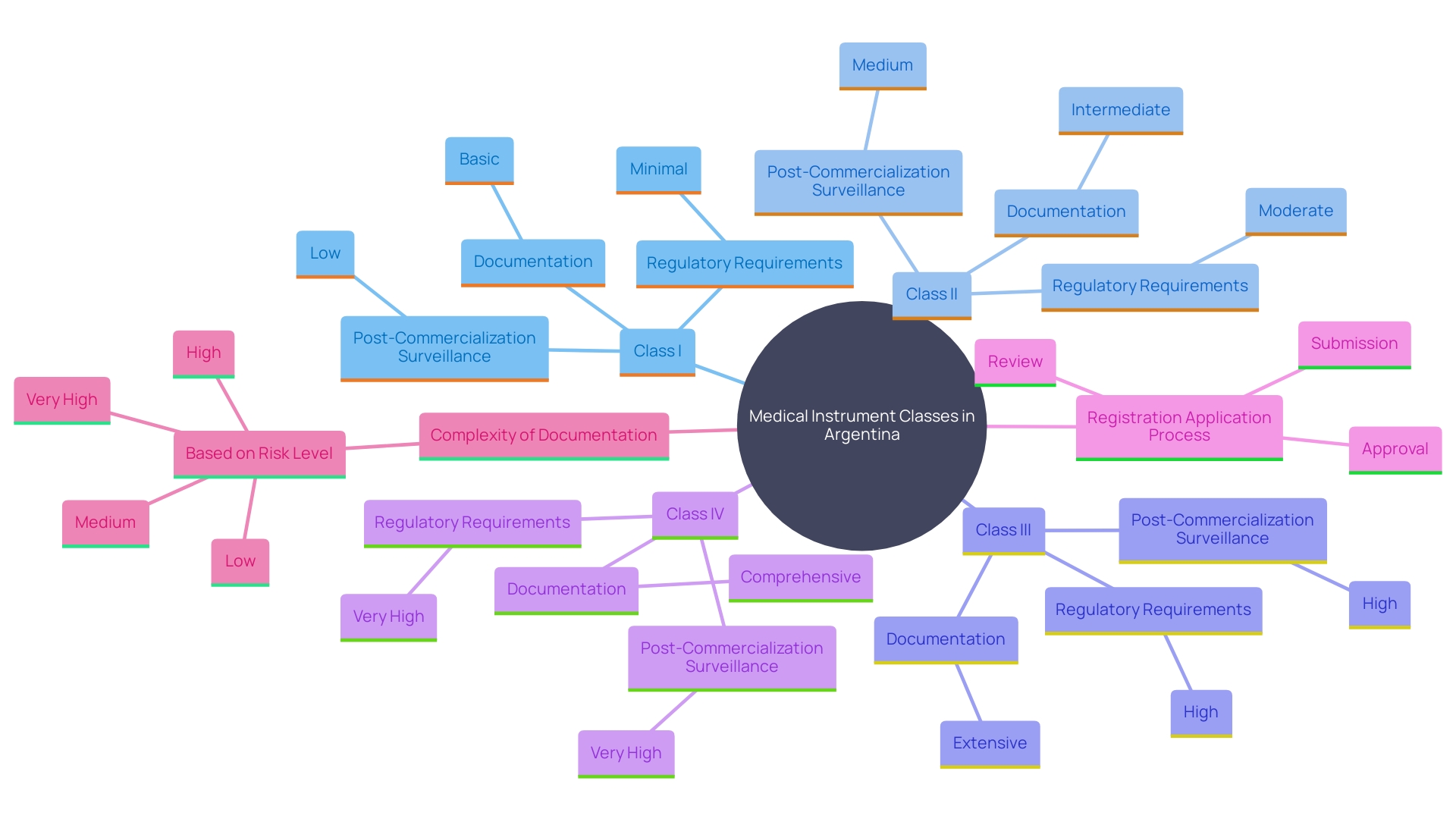
In Argentina, medical instruments are categorized into four classes based on their risk levels: Class I (low risk), Class II (medium risk), Class III (high risk), and Class IV (highest risk). This classification framework determines the regulatory requirements for registration and the necessary compliance pathways. Producers are required to provide a thorough registration application to the National Administration of Drugs, Foods, and Medical Devices (ANMAT), including detailed documentation such as product specifications, research data, and quality management system certifications.
The complexity of the registration process varies with the risk classification. Higher-risk instruments, such as Class III and IV, require more stringent research evidence and comprehensive documentation to ensure safety and efficacy. 'Based on a recent study, among the 710,800 registered clinical trials, 2,669 were focused on AI/ML-enabled healthcare tools, highlighting the significance of advanced technologies in healthcare product development.'. These trials were predominantly carried out in China, the United States, Japan, India, and Korea, emphasizing a global effort in health research.
'Registration also involves post-commercialization surveillance activities, including post-approval studies (PAS) and 522 studies, to continually assess the product's performance and safety once it is available. The European Databank on Medical Devices (Eudamed) system combines various electronic systems to gather and manage information about healthcare products, which manufacturers must comply with, ensuring transparency and ongoing oversight.
The evolving technological landscape and stringent regulatory requirements underscore the need for manufacturers to stay abreast of the latest developments to ensure compliance and successful market entry. 'With the appropriate strategic approach, companies can navigate the regulatory environment efficiently while contributing to advancements in healthcare innovations.'.
Key Requirements for Medical Device Registration
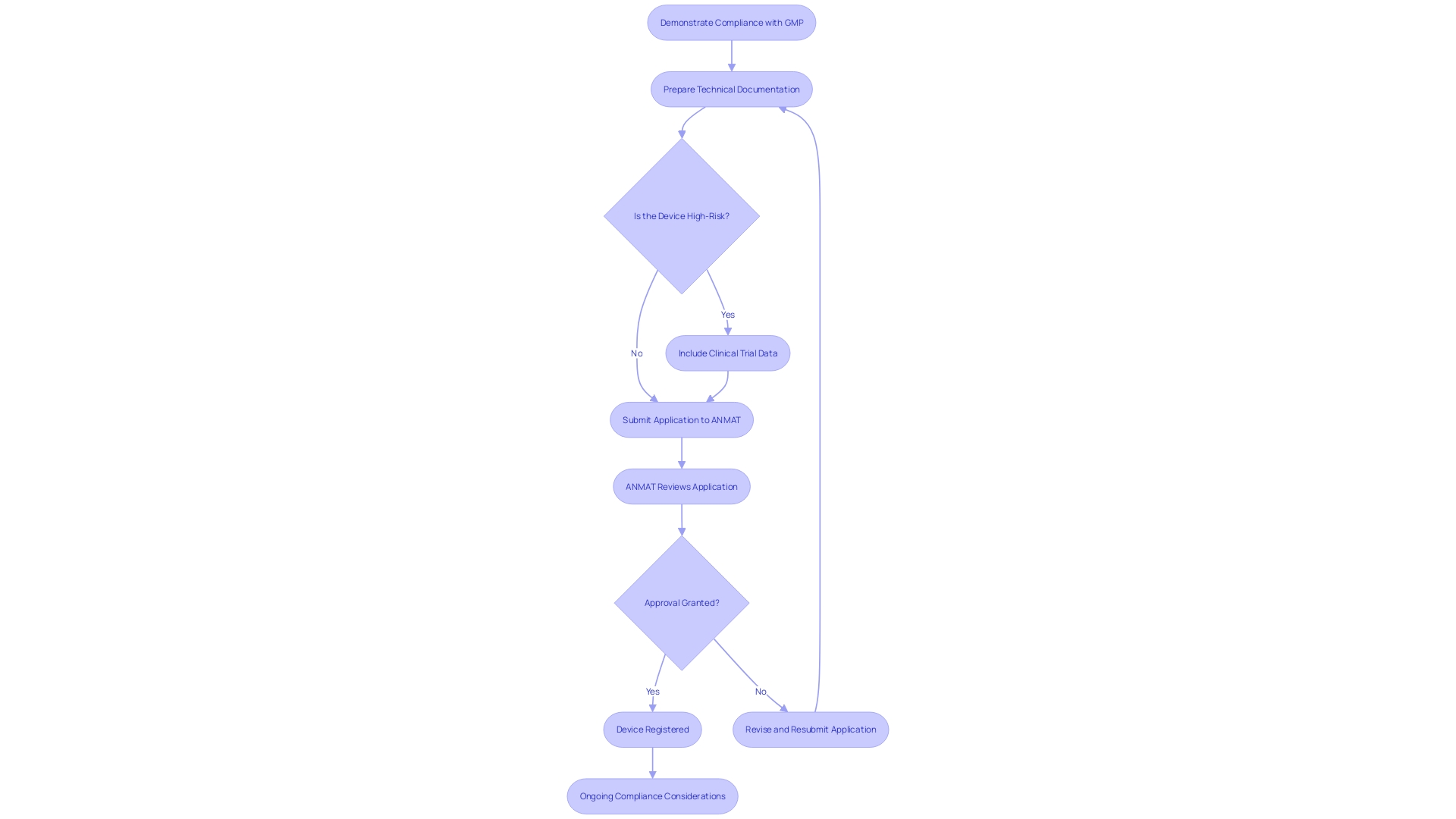
To register a medical instrument in Argentina, manufacturers must adhere to several crucial requirements. Firstly, they need to demonstrate compliance with Good Manufacturing Practices (GMP). This includes meeting the quality system requirements as specified, without delegating the responsibility for compliance, even if the actual work is outsourced. A thorough technical document is also crucial, outlining the product's design, intended application, manufacturing procedure, and medical assessments. The technical documentation must be clear, organized, and meet the Essential Principles of Safety and Performance.
For higher-risk items, clinical trial data is often mandatory to establish safety and efficacy. The Argentine regulatory authority, ANMAT, processes initial applications for Phase III and IV low-risk trials within 14 days. Once ANMAT reviews and approves the submission, the product is granted a registration certificate, permitting its marketing in Argentina.
The regulatory environment for healthcare instruments is changing, with recent modifications highlighting human factors and post-market monitoring. Understanding these requirements is vital for successful market entry and ensuring ongoing compliance.
Emerging Trends in Medical Device Clinical Trials in Argentina
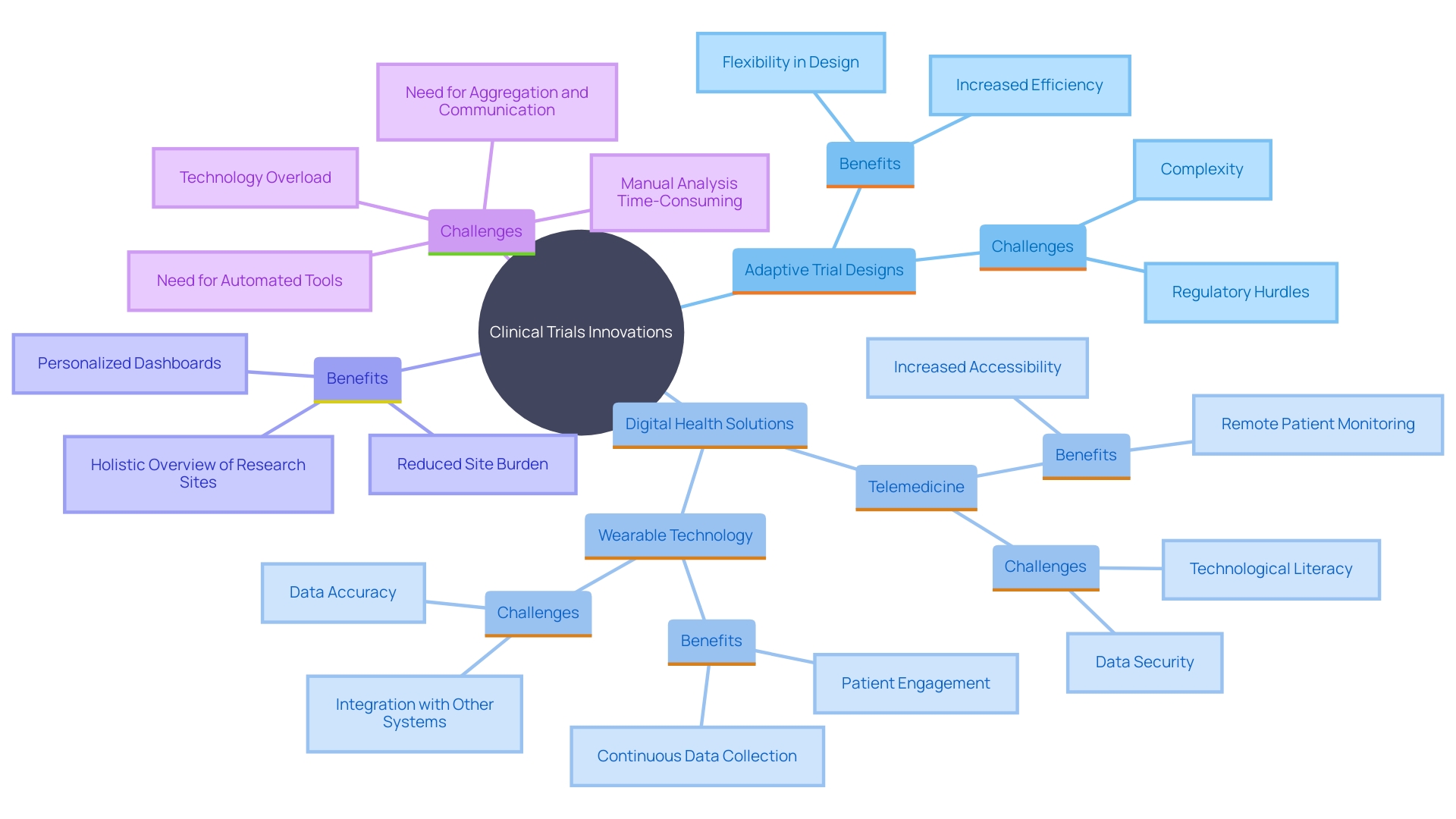
Current patterns in medical equipment research studies in Argentina indicate an increasing focus on innovation and digital health technologies. Adaptive trial designs, which allow for modifications to the trial protocols based on interim results, are increasingly being utilized, enhancing both efficiency and flexibility. This approach aligns with the global trend of incorporating advanced methodologies to streamline research processes in the health field. Furthermore, the incorporation of digital health solutions, such as telemedicine and wearable technology, is becoming more common in clinical trials. These technologies not only improve patient engagement but also facilitate data collection and monitoring, leading to more robust trial outcomes. According to the National Institutes of Health, the use of tech equipment like digital patient engagement tools, wearable devices, and sensors enables faster outcome assessments, improves patient compliance, reduces data entry errors, and provides real-time data updates. This technological integration is further supported by the Health Economics and Outcomes Research (HEOR) service launched by Curavit Clinical Research, which focuses on assessing the value of digital therapeutics. This service aims to quantify the financial impact of digital therapeutics, providing medicine manufacturers with insights into product value and commercial potential. As emphasized by Melissa Easy, VP & General Manager of Clinical Technologies at IQVIA, technological advancements have greatly enhanced the trial landscape by expanding remote monitoring capabilities, improving patient recruitment strategies, and fostering the decentralization of trials. However, it is essential to address challenges such as data security, privacy issues, and technological literacy to ensure the successful implementation of these innovations.
Challenges and Opportunities in Argentina's Medical Device Market
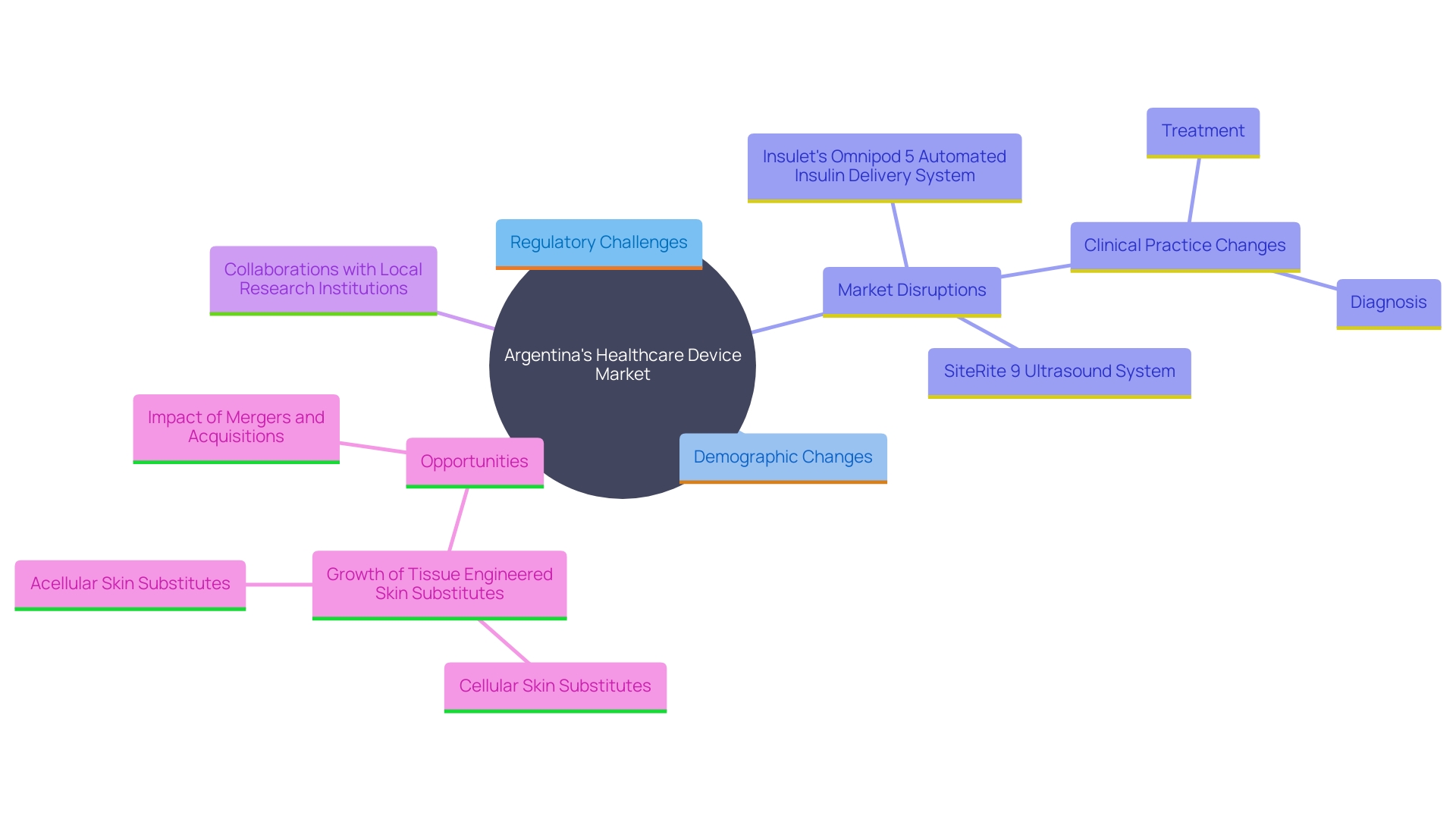
Argentina's healthcare device market presents a complex landscape filled with both challenges and opportunities for manufacturers and researchers. Navigating regulatory hurdles is a significant obstacle, as approval processes are often lengthy and stringent. A quote from Cochereau highlights that some countries require clinical trial data from local populations, which can increase development costs. Nevertheless, the growing demand for innovative medical technologies, spurred by an aging population and rising chronic disease prevalence, opens up substantial growth avenues.
The tissue engineered skin substitutes sector in Argentina can grow due to changes in population demographics and disease incidence. However, macroeconomic issues and geopolitical considerations can also drive market disruption. Disruptions could arise from sudden changes in medical practices aimed at enhancing healthcare overall.
Collaborations with local research institutions and investments in clinical research capabilities are crucial strategies to tackle these challenges. For example, Insulet’s OmniPod 5 Automated Insulin Delivery System and SiteRite 9 Ultrasound System are innovations that have shown potential in other markets like the USA. Such partnerships can promote the creation of new health tools, ensuring they comply with local regulatory requirements while addressing the unique needs of the community.
In terms of deal volume, the device sector has seen significant activity, with 938 M&A deals recorded in 2023, including 27 mega-deals valued over $1 billion. These mergers and acquisitions indicate a dynamic business environment where companies are striving to stay ahead by aligning with major industry themes.
The medical technology sector in Argentina, similar to that in the United States, gains from heightened funding in research and development. 'This investment drives rapid growth and positions the sector for future advancements.'. By leveraging local and international expertise, the Argentine market can overcome regulatory challenges and harness opportunities for innovation and growth.
Conclusion
Understanding the regulatory landscape for medical devices in Argentina is crucial for manufacturers aiming to enter this dynamic market. The framework established by ANMAT, in conjunction with the Mercosur trade bloc, sets rigorous safety and efficacy standards that must be met before any medical device can be marketed. The harmonization of guidelines within Mercosur not only simplifies the compliance process but also enhances market accessibility, fostering an environment conducive to innovation and collaboration.
The classification and registration processes play a pivotal role in determining the path for market entry. By categorizing devices into four distinct classes based on their risk levels, ANMAT ensures that higher-risk devices undergo more stringent scrutiny. The requirement for comprehensive technical documentation, including adherence to Good Manufacturing Practices and clinical trial data for high-risk devices, underscores the importance of thorough preparation in the registration process.
Emerging trends in clinical trials highlight a shift towards adaptive designs and the integration of digital health technologies. These advancements not only improve the efficiency of trials but also enhance patient engagement and data collection. Nevertheless, challenges such as regulatory hurdles and the need for local clinical trial data remain significant concerns for manufacturers.
Addressing these obstacles through strategic collaborations and investments in research capabilities will be essential for leveraging the growing demand for innovative medical technologies in Argentina.
In conclusion, while the Argentine medical device market presents complexities, it also offers substantial opportunities for growth and innovation. By navigating the regulatory landscape effectively and embracing technological advancements, manufacturers can position themselves for success in this evolving sector.




