Introduction
Navigating the regulatory landscape of medical device approval is crucial for ensuring patient safety and compliance with U.S. Food and Drug Administration (FDA) standards. The FDA's device classification system forms the backbone of this process, stratifying devices into Class I, II, and III based on the level of risk they present to patients. Innovative medical technologies have the potential to transform patient care, and the FDA plays a critical role in facilitating access to these advancements.
The Breakthrough Devices Program provides a streamlined pathway for devices that offer more effective treatments or diagnostics for life-threatening or irreversibly debilitating diseases. The FDA's dedication to public health is further supported by its rigorous standards for device safety and effectiveness. As the medical device sector continues to evolve, the FDA's updates and programs reflect a proactive and collaborative approach to regulation, aiming to deliver high-quality, safe, and effective medical devices to patients while promoting health equity and innovation.
Understanding FDA Device Classification
Navigating the regulatory landscape of medical device approval is a pivotal aspect of healthcare innovation. The FDA's device classification system forms the backbone of this process, stratifying devices into Class I, II, and III, based on the level of risk they present to patients. Class I devices pose the least risk and are subject to the least regulatory control, whereas Class III devices, which pose the greatest risk, require the most stringent regulatory scrutiny, including premarket approval.
Innovative medical technologies have the potential to transform patient care, and the FDA plays a critical role in facilitating access to these advancements. The Breakthrough Devices Program is a testament to this, providing a streamlined pathway for devices that offer more effective treatments or diagnostics for life-threatening or irreversibly debilitating diseases. To date, the program has granted Breakthrough Device designation to over 830 devices and has authorized marketing for 77, underscoring the agency's commitment to fostering medical innovation and public health.
The final guidance issued by the FDA for the Breakthrough Devices Program emphasizes the agency's holistic approach to device effectiveness, considering the totality of available information, the potential for clinically meaningful impact, and a comparison of risks and benefits against standard care. The guidance also highlights the importance of device accessibility and the FDA's transparency in disclosing Breakthrough status post-marketing authorization.
The FDA's dedication to public health is further supported by its rigorous standards for device safety and effectiveness. These standards are bolstered by the use of voluntary consensus standards developed by Standards Development Organizations (SDOs) that adhere to principles of transparency, stakeholder participation, and due process. The integration of these consensus standards into the regulatory framework enhances the quality and efficiency of the regulatory review process, ensuring that devices meet the high bar set for safety and effectiveness.
As the medical device sector continues to evolve, the FDA's updates and programs reflect a proactive and collaborative approach to regulation, aiming to deliver high-quality, safe, and effective medical devices to patients while promoting health equity and innovation.
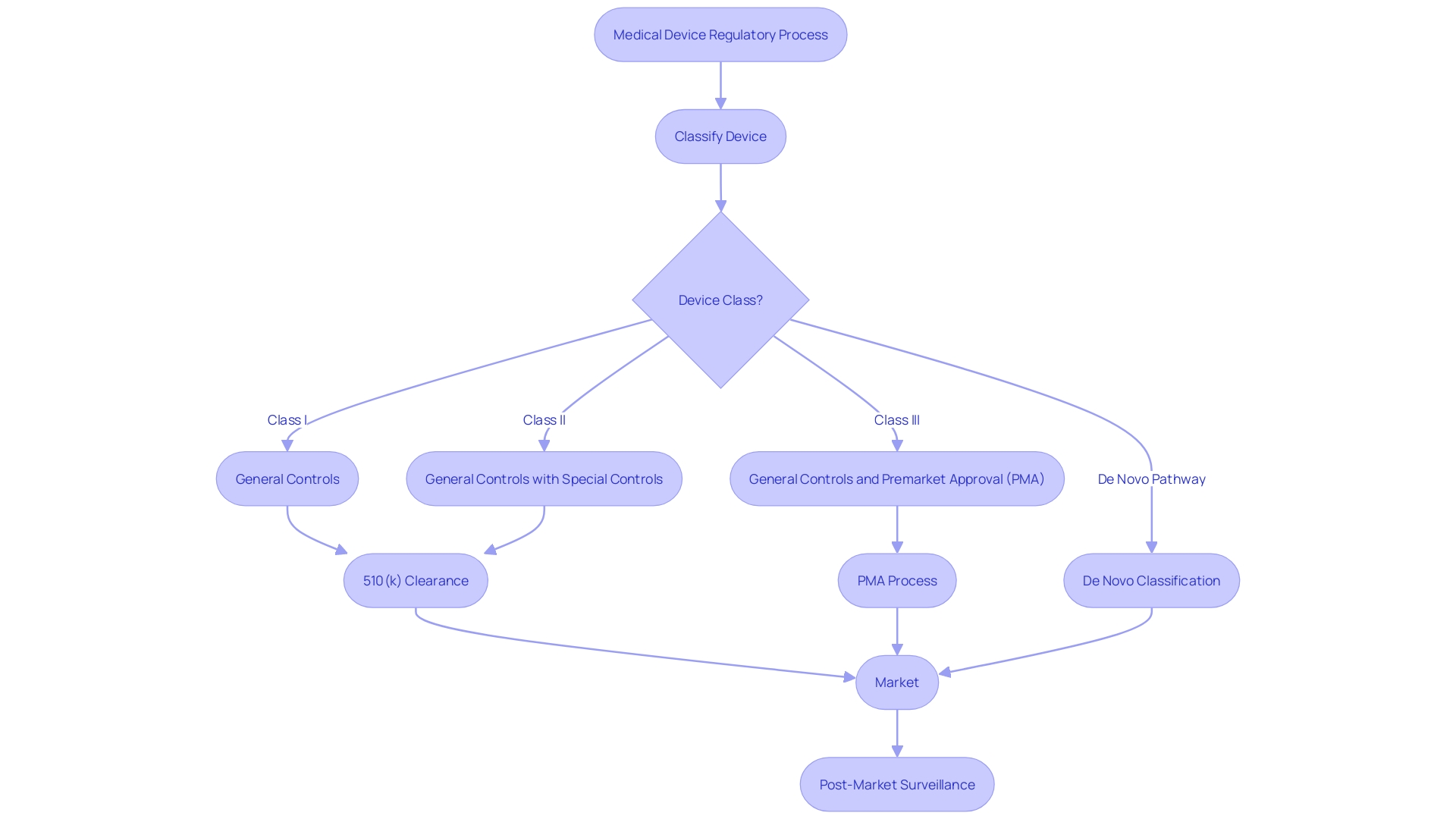
What is FDA Clearance?
Navigating the FDA's regulatory landscape is critical for medical device manufacturers, and understanding the nuances between clearance, approval, and compliance is foundational for market success. The FDA categorizes medical devices into three classes based on potential risks, with Class I representing low-risk devices and Class III encompassing high-risk devices, such as life-sustaining implants. Devices falling into Class I and II typically undergo a less complex regulatory process, with many requiring a 510(k) premarket notification.
This process is designed to establish that a new device is substantially equivalent to an existing device that has already been legally marketed and is not subject to premarket approval.
The 510(k) clearance pathway allows for a faster route to market for devices that can be compared to a predicate device. However, this process has come under scrutiny, as highlighted in the 2018 documentary 'The Bleeding Edge', which shed light on instances where devices fast-tracked through the 510(k) process resulted in patient harm due to the lack of required clinical trials for clearance.
Despite these concerns, the 510(k) process remains a pivotal part of the FDA's regulatory framework. As the medical device market continues to grow, with projections estimating a reach of $60.2 billion by 2030, understanding the distinction between FDA terms like 'Cleared', 'Approved', and 'Granted' is not only beneficial but essential for regulatory professionals. These terms reflect different levels of scrutiny and approval processes, with 'Cleared' typically associated with Class I and II devices through the 510(k) process, 'Approved' for Class III devices requiring a more stringent Pre-Market Approval (PMA), and 'Granted' for those that pass through the De Novo process.
Given the critical role that regulatory pathways play in market access and patient safety, it is of utmost importance for the medical device industry to remain vigilant and informed about FDA regulatory requirements. The success of a device hinges not only on its innovation and efficacy but also on the careful navigation of the regulatory process that ensures devices are safe and effective for public use.
What is FDA Approval?
For high-risk medical devices, the FDA requires a thorough review process before granting marketing approval. This involves a Premarket Approval (PMA) application, which must include substantial scientific evidence that demonstrates the device's safety and efficacy. For instance, the Impella Connect System, which assists in critical care by providing temporary ventricular support, went through such scrutiny.
Its web-based user portal and remote link module are designed to work with the Automated Impella Controller, enabling remote monitoring of the pump's performance and providing vital, patient-specific notifications in real-time. These features, essential for detecting life-threatening conditions and issuing timely alarms, are considered device functions under section 201(h) of the Act and therefore require premarket authorization. This is just one example of the FDA's commitment to safeguarding public health by ensuring the safety, effectiveness, and security of medical devices, as well as other products under its jurisdiction.
Recent advancements in healthcare technology, such as those from Medtronic, showcase the industry's effort to address challenging health problems. With a global presence and an array of devices and therapies, companies like Medtronic are at the forefront of innovation, delivering technologies that transform lives every day. In light of these developments, regulatory bodies continuously adapt, aiming for more efficient approval processes, especially for devices that meet urgent medical needs.
The FDA's classification system places devices into three categories based on risk, with class three including critical devices like pacemakers. These devices undergo more rigorous processes due to their high-risk nature and crucial role in patient care, representing a smaller percentage of FDA-regulated devices but requiring longer approval times. The drive for faster regulatory pathways is also influenced by factors such as the complexity of the device and the nature of the medical condition it addresses, underscoring the importance of safety and efficacy in medical device development.
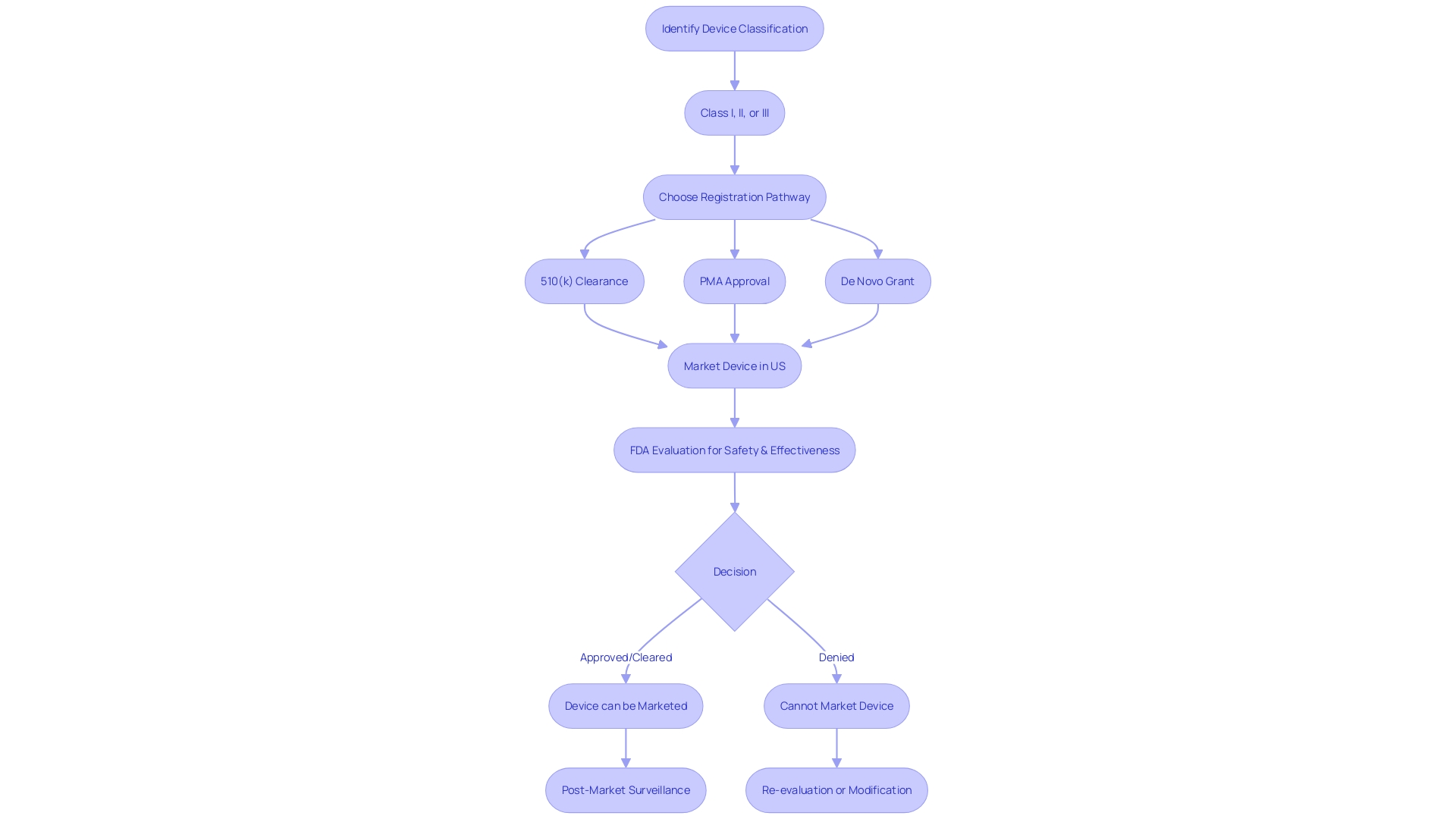
Key Differences Between FDA Cleared and FDA Approved Devices
Navigating the regulatory landscape of medical device approval is crucial for ensuring patient safety and compliance with U.S. Food and Drug Administration (FDA) standards. Medical devices are classified by the FDA into three categories based on the level of risk they pose to patients. Class I devices are deemed low risk, Class II devices are considered moderate risk, and Class III devices are categorized as high risk.
Understanding the differences between FDA clearance, approval, and granting authority is essential for regulatory professionals.
Class II devices typically undergo the FDA's 510(k) Premarket Notification process, where a manufacturer must demonstrate that a new device is substantially equivalent to a predicate device already on the market. This process is less rigorous than Pre-Market Approval (PMA), which is required for most Class III devices. PMA demands extensive scientific evidence proving the safety and effectiveness of a device, often including clinical trial data.
The De Novo process, alternatively, is used when a novel device doesn't have a comparable predicate but is of low to moderate risk and thus does not warrant the stringent PMA process.
The FDA's mission to protect public health encompasses rigorous evaluation of medical devices, ensuring that they meet safety and effectiveness standards. For instance, the FDA recently published rules to ensure clarity and neutrality in direct-to-consumer prescription drug advertisements. This reflects the agency's commitment to transparent communication that is understandable to consumers.
Moreover, the FDA's vaccine assessment covers the entire lifecycle of development and use, highlighting the thoroughness of FDA's regulatory oversight. It's important to recognize that not all products are subject to FDA approval. For example, dietary supplements do not require FDA approval and cannot claim to treat or prevent diseases, while generic medicines must meet the same standards as their brand-name counterparts.
In summary, the pathway to market for a medical device is contingent upon its classification and associated risk. The 510(k) clearance process allows for moderate risk devices to be fast-tracked based on equivalence to existing devices, whereas PMA is reserved for high-risk devices that necessitate a more comprehensive review of safety and efficacy. Understanding the nuances of these regulatory terms and pathways is fundamental to maintaining compliance and ensuring the safety of medical devices offered to consumers.
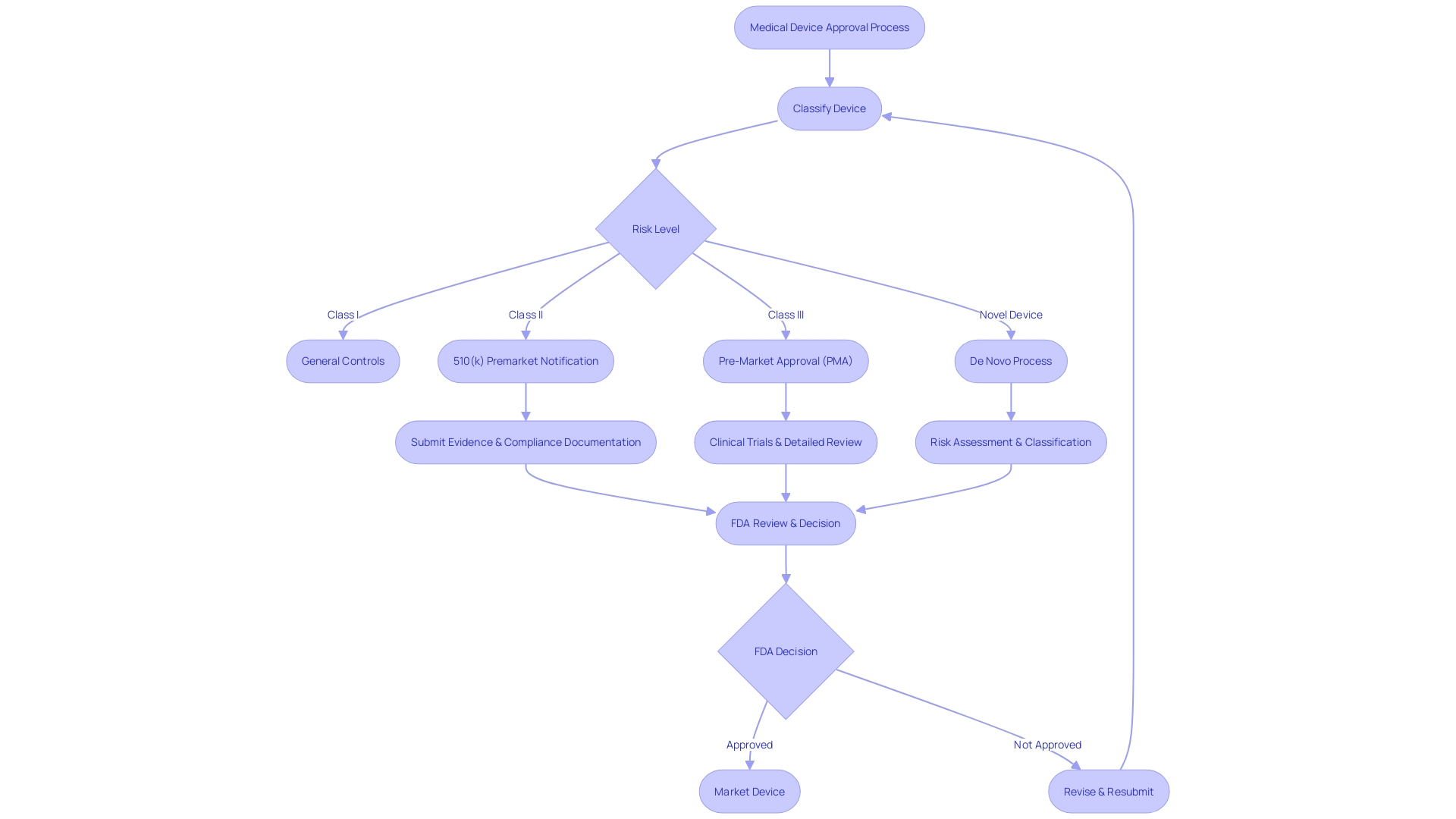
The 510(k) Submission Process for FDA Clearance
Navigating the complexities of the FDA's 510(k) submission process is a critical step for medical device manufacturers aiming to achieve market clearance in the United States. This process requires manufacturers to meticulously demonstrate that their new medical device is substantially equivalent to an existing device, referred to as a predicate, that has already been legally marketed. The goal is to ensure that the new device is as safe and effective as its predicate.
To substantiate this claim of substantial equivalence, manufacturers must provide detailed data including performance testing results and, in some cases, clinical data. Understanding the device's intended use, users, and safety instructions, along with analyzing the competitive landscape, is fundamental. A comparative analysis of the new device against potential predicate devices based on technological characteristics and intended use is constructed, often aided by a comparative table.
This thorough evaluation, which includes reviewing Summaries of Safety and Effectiveness from the FDA's 510(k) database, is pivotal in discerning similarities and differences that impact the submission. The FDA classifies medical devices into three categories based on patient risk, and each classification has a corresponding regulatory pathway: 510(k) for devices of low to moderate risk, Pre-Market Approval (PMA) for high-risk devices, and the De Novo process for novel devices of low to moderate risk that lack a comparable predicate. To legally distribute a device in the U.S., it must be either FDA Cleared, Approved, or Granted Authorization through the De Novo process.
The distinctions among these terms denote varying levels of regulatory scrutiny and are crucial for regulatory professionals to comprehend. The FDA's role in safeguarding public health extends to ensuring that medical devices meet stringent standards of safety and effectiveness before reaching consumers, a responsibility highlighted by critiques such as the 2018 documentary 'The Bleeding Edge', which revealed potential risks associated with the fast-tracked clearance of certain medical devices. As the medical device landscape continues to evolve, with technological advancements such as 3D printing forecasted to generate significant market growth, the importance of rigorous regulatory processes becomes ever more apparent.
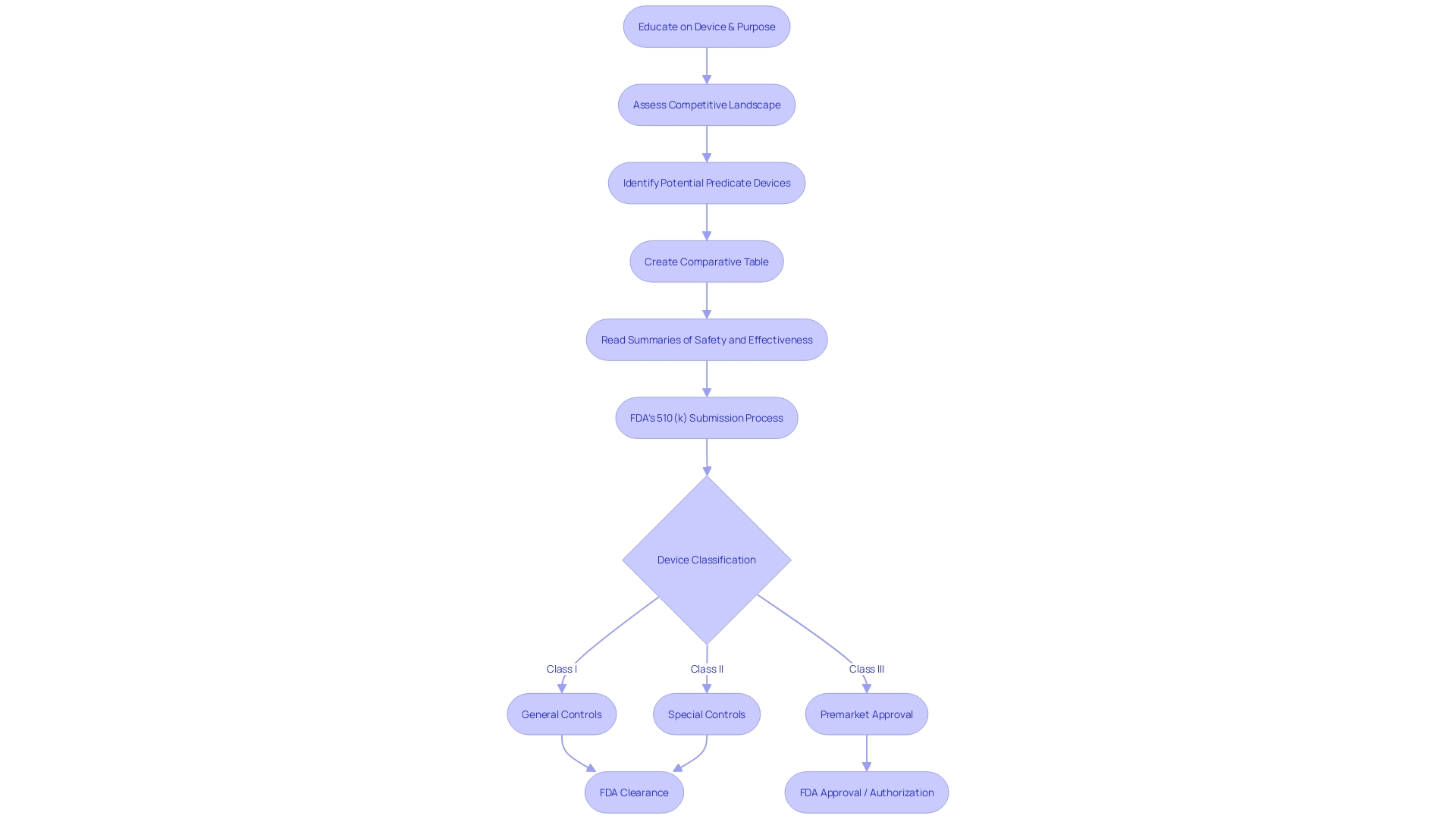
The Premarket Approval (PMA) Process for FDA Approval
Navigating the complex landscape of FDA device classifications and approval pathways is essential for ensuring that high-risk medical devices meet the rigorous safety and efficacy standards set by the agency. Class III devices, which include life-sustaining or implantable devices, such as pacemakers, are subject to the most stringent scrutiny and require Pre-Market Approval (PMA). This approval process mandates the submission of a comprehensive application that presents robust scientific evidence, verifying both the safety and the effectiveness of the device.
The determination of a device's classification hinges on its associated patient risk, and it's imperative to choose the appropriate regulatory route—be it a 510(k) Premarket Notification, a PMA, or the De Novo process. The PMA pathway, utilized by roughly 10% of medical devices, is reserved for those that carry the highest risk. Unlike the 510(k) clearance, which allows for market entry by demonstrating equivalence to an existing device, the PMA process involves an extensive review by the FDA, where clinical trial data often plays a critical role.
This rigorous evaluation includes not only clinical data but also laboratory and manufacturing information to ensure the highest level of patient safety. The approval timeline can be lengthy, reflecting the thorough examination required for these complex devices. Recent industry initiatives and the exigencies of the COVID-19 pandemic have spurred efforts to streamline these regulatory pathways, enhancing collaboration between the FDA, industry stakeholders, and other regulatory bodies to quicken the approval process for innovative devices that address significant medical needs.
Importance of Correct Terminology in Medical Device Marketing
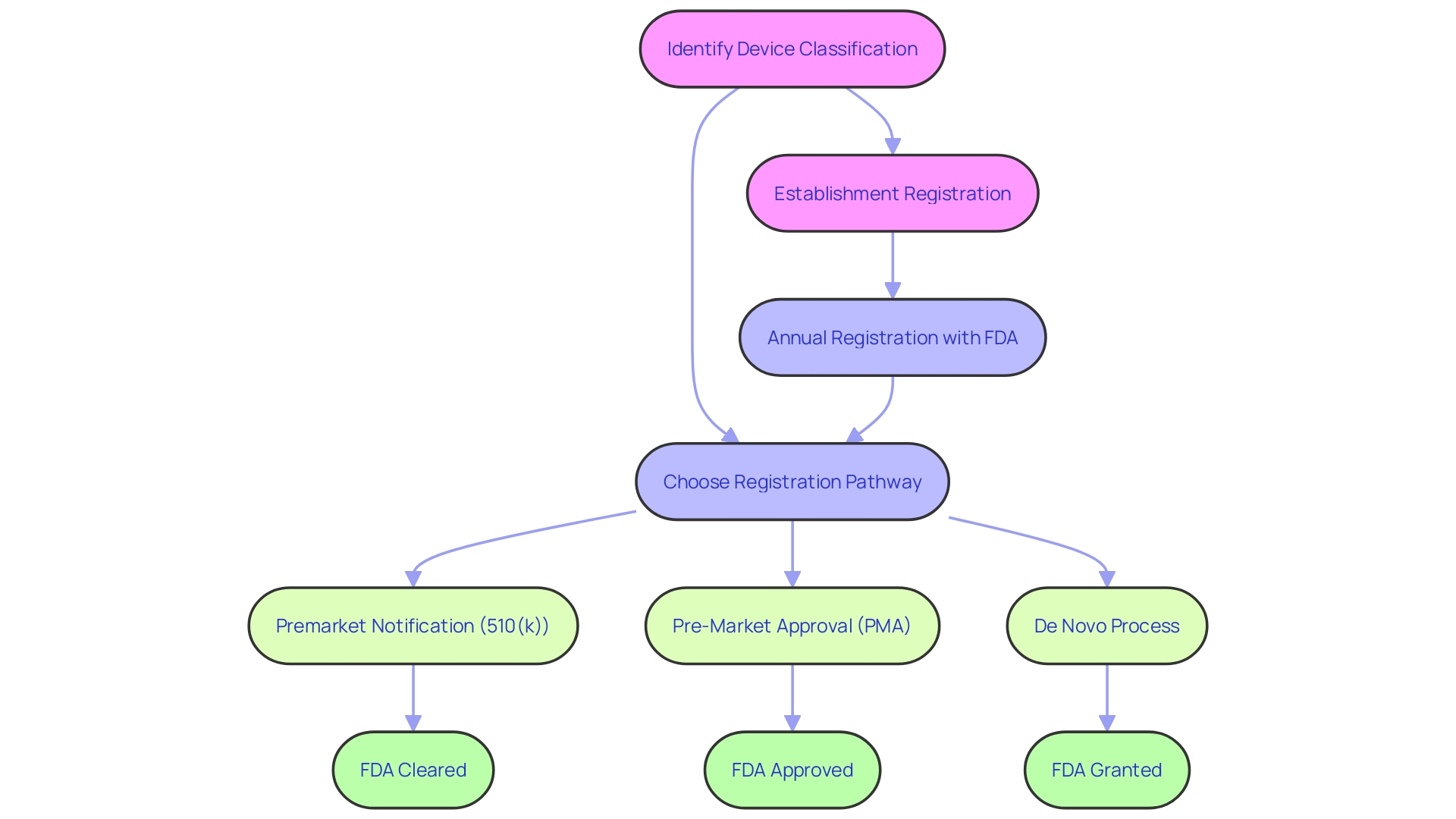
Clear communication of regulatory terms is vital in medical device marketing to ensure that stakeholders understand the level of regulatory compliance. Medical devices in the United States undergo rigorous evaluations by the FDA, which classifies them into three risk-based categories. Depending on their classification, devices must navigate through one of the registration pathways—Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo process—to be legally marketed.
Each term—Registered, Cleared, Approved, and Granted—reflects a specific regulatory status and implies a certain level of FDA evaluation. For instance, 510(k) clearance often involves demonstrating that a new device is substantially equivalent to an existing legally marketed device. In contrast, PMA is a more stringent process requiring scientific evidence to assure safety and effectiveness.
The De Novo process provides a route for novel low-to-moderate risk devices without existing predicates. It is imperative to use these terms correctly to avoid misunderstandings among healthcare providers, patients, and other parties involved in the use of medical devices. Furthermore, it is not just the FDA's role to ensure the safety and effectiveness of medical devices; after FDA approval or clearance, coverage and reimbursement decisions by payors and healthcare providers ultimately influence the accessibility of these devices to patients.
Legal and Regulatory Implications of Incorrect Terminology
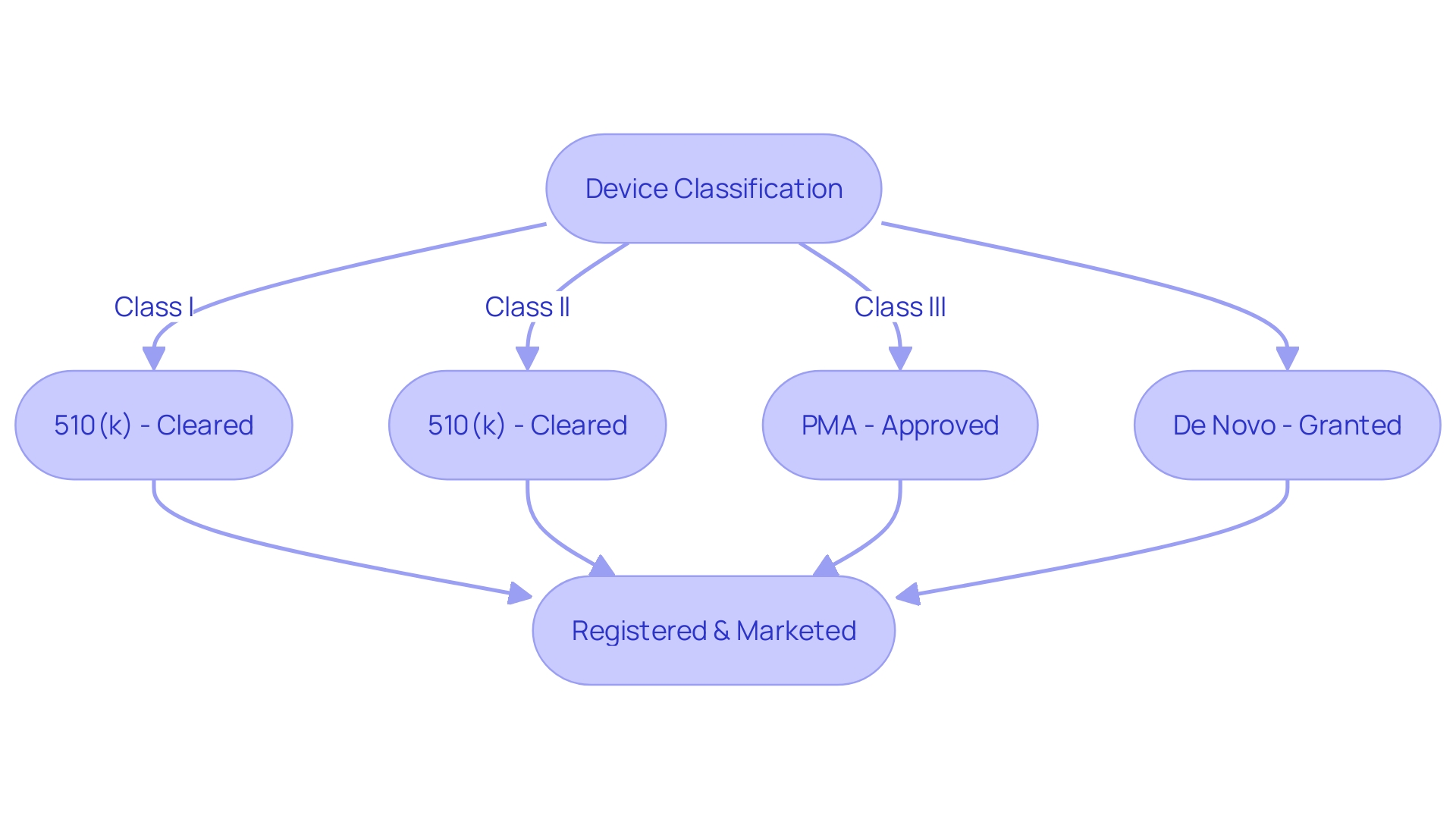
The nuances of FDA terminology are critical for medical device manufacturers to understand, as improper usage can lead to significant legal and regulatory repercussions. The terms 'Registered,' 'Cleared,' 'Approved,' and 'Granted' are commonly heard within the industry but are often misunderstood even by regulatory professionals. Each term relates to different levels of FDA classification and corresponding risk to patients, leading to specific registration pathways that must be navigated correctly.
For instance, a Premarket Notification, known as 510(k), is required for devices that are substantially equivalent to those already on the market, whereas Pre-Market Approval (PMA) is necessary for higher-risk devices. The De Novo process offers a route for low to moderate risk devices that lack a comparable predicate. Misrepresenting a device's FDA status not only muddies the clarity of its market position but can also attract enforcement actions, as seen during the Covid-19 pandemic when the FDA had to address widespread misinformation about various products.
The FDA's draft guidance on this topic suggests proactive steps for companies to correct misinformation, underscoring the importance of accurate communication. It is imperative for businesses to carefully determine the classification of their devices and choose the correct regulatory pathway, ensuring that marketing materials reflect this to prevent adverse outcomes, including potential patient harm. This approach is not only a regulatory mandate but a commitment to patient safety, as evidenced by the more than 1.7 million injuries and 83,000 deaths potentially linked to medical devices over a decade, as reported in a 2018 study of FDA data.
Consumer and Clinical Implications of FDA Clearance vs Approval
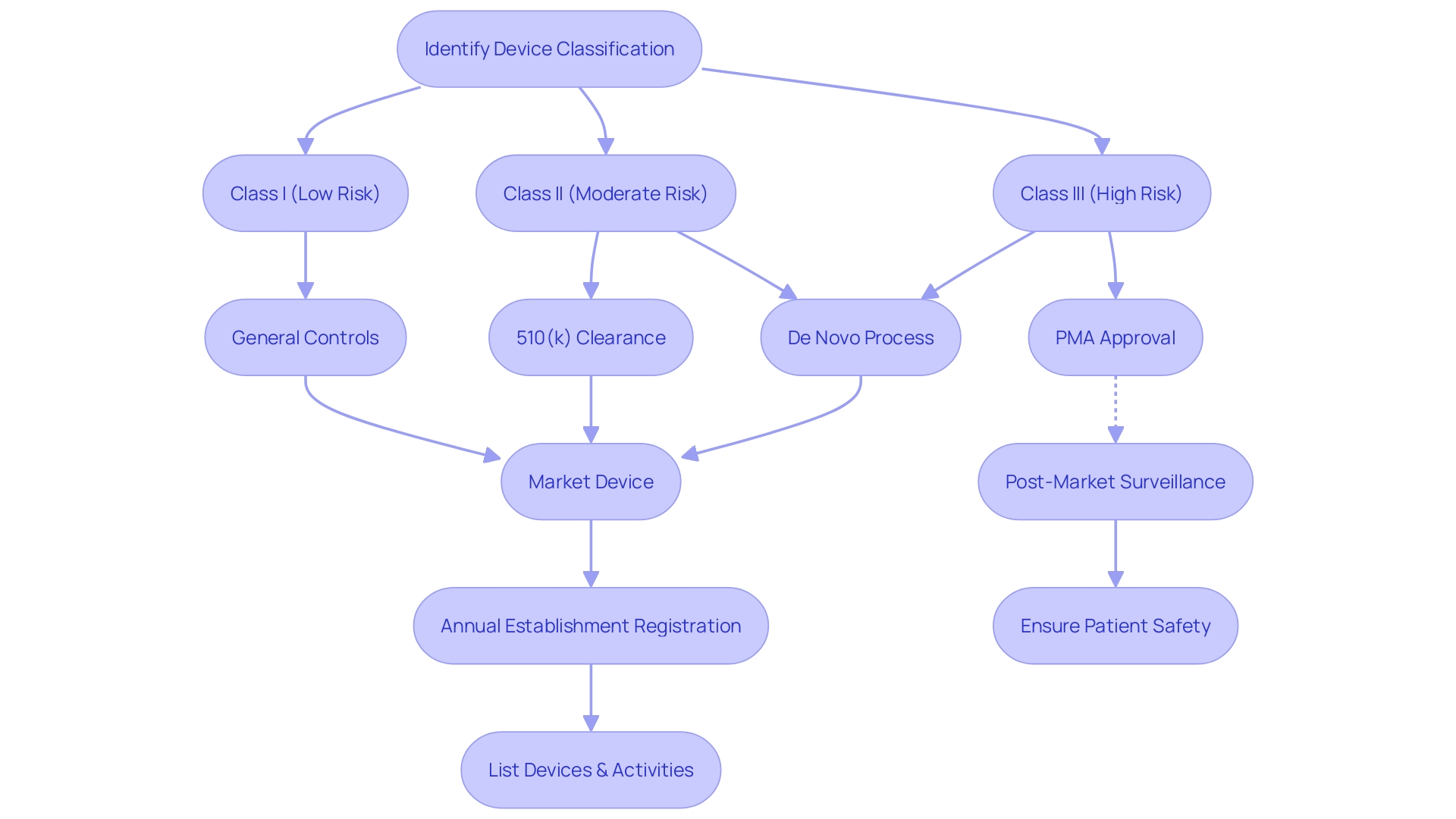
Grasping the nuances between FDA clearance, approval, and compliance is crucial for healthcare professionals. Devices given FDA clearance have undergone a review to confirm they are safe and effective for their intended use, typically through the 510(k) process. This process may involve demonstrating that the new device is substantially equivalent to a legally marketed device.
FDA approval, on the other hand, generally requires a Pre-Market Approval (PMA), which involves a more rigorous review and often must be supported by extensive clinical trial data to demonstrate safety and efficacy. Additionally, the De Novo pathway serves as an alternative for novel devices of low to moderate risk that lack a predicate device, leading to FDA granting authorization.
The FDA categorizes medical devices into three classes based on the level of risk they present to patients, with Class I posing the least risk and Class III the most. The classification determines the regulatory pathway a device must follow. For instance, Class I devices usually require less regulatory control, while Class II devices often go through the 510(k) process, and Class III devices typically require a PMA.
It's critical to recognize that the term 'FDA Registered' simply means the entity is registered with the FDA and doesn't reflect the approval status of a product. The differentiation between these terms can impact patient care, as seen in some cases where devices cleared through the 510(k) process without rigorous clinical testing led to adverse outcomes, highlighting the importance of understanding the level of evidence backing a device's market entry.
In essence, keeping abreast of FDA regulatory requirements and understanding the evidence supporting a device's clearance or approval are essential for making informed decisions about medical device usage and ensuring patient safety.
Conclusion
In conclusion, navigating the FDA's regulatory landscape for medical device approval is essential for patient safety and compliance. The FDA's classification system, Breakthrough Devices Program, and rigorous standards for safety and effectiveness demonstrate its commitment to public health.
Understanding the distinctions between FDA clearance, approval, and compliance is crucial for regulatory professionals. The 510(k) clearance process fast-tracks moderate-risk devices, while Pre-Market Approval (PMA) is required for high-risk devices. The De Novo process provides a pathway for novel devices of low to moderate risk.
Accurate communication of regulatory terms is vital in medical device marketing to ensure clarity. Misrepresenting a device's FDA status can have legal and regulatory consequences. Healthcare professionals must grasp the differences between FDA clearance and approval to make informed decisions and prioritize patient safety.
Overall, the FDA's regulatory framework promotes high-quality, safe, and effective medical devices. Its proactive and collaborative approach aims to foster innovation while ensuring health equity. By adhering to rigorous standards and providing streamlined pathways, the FDA strives to deliver innovative medical technologies that enhance patient care.




