Introduction
Good Clinical Practice (GCP) serves as the gold standard for conducting ethical and scientifically sound clinical trials. These trials are essential for advancing medical knowledge and ensuring the safety and well-being of participants. The scope of GCP encompasses guidelines that guide researchers and clinicians in every stage of the trial, from its inception to the dissemination of findings.
At the heart of GCP is the principle of prioritizing participants' rights and safety, ensuring that trials are conducted with integrity. The impact of GCP extends beyond ethical considerations, as it directly influences clinical efficacy and patient care. Trials conducted under the GCP framework contribute to the development of safe and effective treatments, with a clear understanding of potential side effects.
Furthermore, GCP emphasizes transparency in clinical research, promoting the availability of trial protocols and the sharing of raw data, ultimately benefiting the wider medical community. As the clinical trial landscape evolves, GCP continues to adapt, incorporating new technologies and approaches to enhance efficiency and patient safety. The integration of artificial intelligence (AI) and real-world evidence (RWE) is revolutionizing data collection and analysis, while risk-based monitoring strategies are optimizing resource allocation.
These innovations pave the way for more patient-centered trials that accelerate therapeutic development and improve patient outcomes. In summary, GCP is critical for ensuring the ethical conduct of clinical trials, guaranteeing data integrity, and advancing medical knowledge for the benefit of patients worldwide.
Definition and Scope of Good Clinical Practice (GCP)
Good Clinical Practice (GCP) is the gold standard in the realm of clinical trials, encompassing a broad spectrum of guidelines that ensure trials are conducted ethically and with scientific integrity. These guidelines are pivotal in safeguarding the rights, safety, and well-being of trial participants and guaranteeing the authenticity and trustworthiness of clinical data. Spanning the entirety of clinical trials from inception through to their culmination and dissemination of findings, GCP is the foundation that supports the construction of credible medical evidence.
At the core of GCP is the principle of putting participants first, a notion echoed by recent restructuring of medical standards that emphasize the application of professional judgment tailored to individual circumstances. While GCP guidelines are not prescriptive rules, they function as a beacon, guiding clinicians and researchers towards the ethical high ground in trial conduct.
The significance of GCP extends beyond ethical considerations, directly impacting clinical efficacy and patient care. Trials conducted under the GCP framework contribute to establishing treatments that are not only safe but also optimized for efficacy, with a clear understanding of potential side effects. This is crucial, as the treatments and interventions tested in trials often become the standard of care upon successful completion and approval.
In the context of GCP, the moving narrative of patients participating in trials, such as those with transthyretin-mediated amyloidosis, underlines the human element that these guidelines protect. These participants, often facing severe health challenges, contribute to a legacy that could lead to breakthroughs for future generations.
The commitment to GCP is also seen in the push for transparency within clinical research. The availability of trial protocols, statistical analysis plans, and the eventual sharing of raw data are steps toward making research more transparent, reproducible, and beneficial to the wider community. In doing so, GCP ensures that the advancements made in the lab can be effectively translated into real-world outcomes, ultimately improving patient care and advancing medical knowledge.
Historical Background and Development of GCP
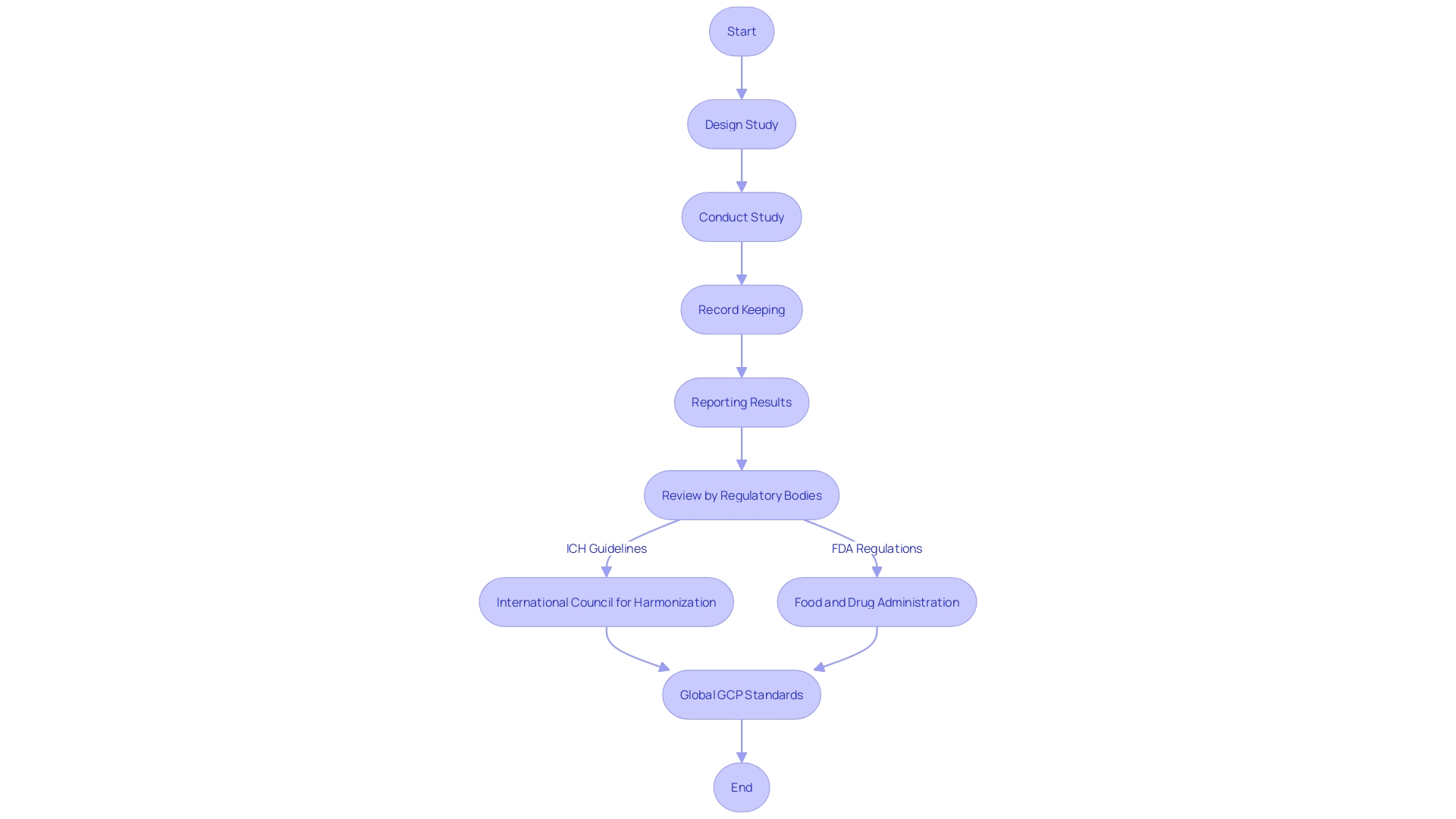
Good Clinical Practice (GCP) and Good Laboratory Practice (GLP) are cornerstones of clinical research and pharmaceutical development, ensuring the ethical treatment of trial participants and the reliability of laboratory results. The inception of GCP can be traced back to the Declaration of Helsinki in 1964, which set forth ethical guidelines for medical research involving human subjects. This milestone document emphasized the necessity of informed consent and the primacy of the subject's welfare. GCP has since evolved, with international bodies like the International Council for Harmonization (ICH) elaborating on these principles to harmonize regulations globally. This ensures that no matter where research is conducted, the rights, safety, and wellbeing of trial subjects are paramount, and the data generated is credible and accurate. GLP, on the other hand, focuses on the integrity of non-clinical laboratory studies, which are critical in the early phases of drug development. Together, GCP and GLP form a robust framework that guides researchers and ensures that clinical trials and laboratory studies are conducted to the highest standards of quality and ethics.
Core Principles of ICH GCP
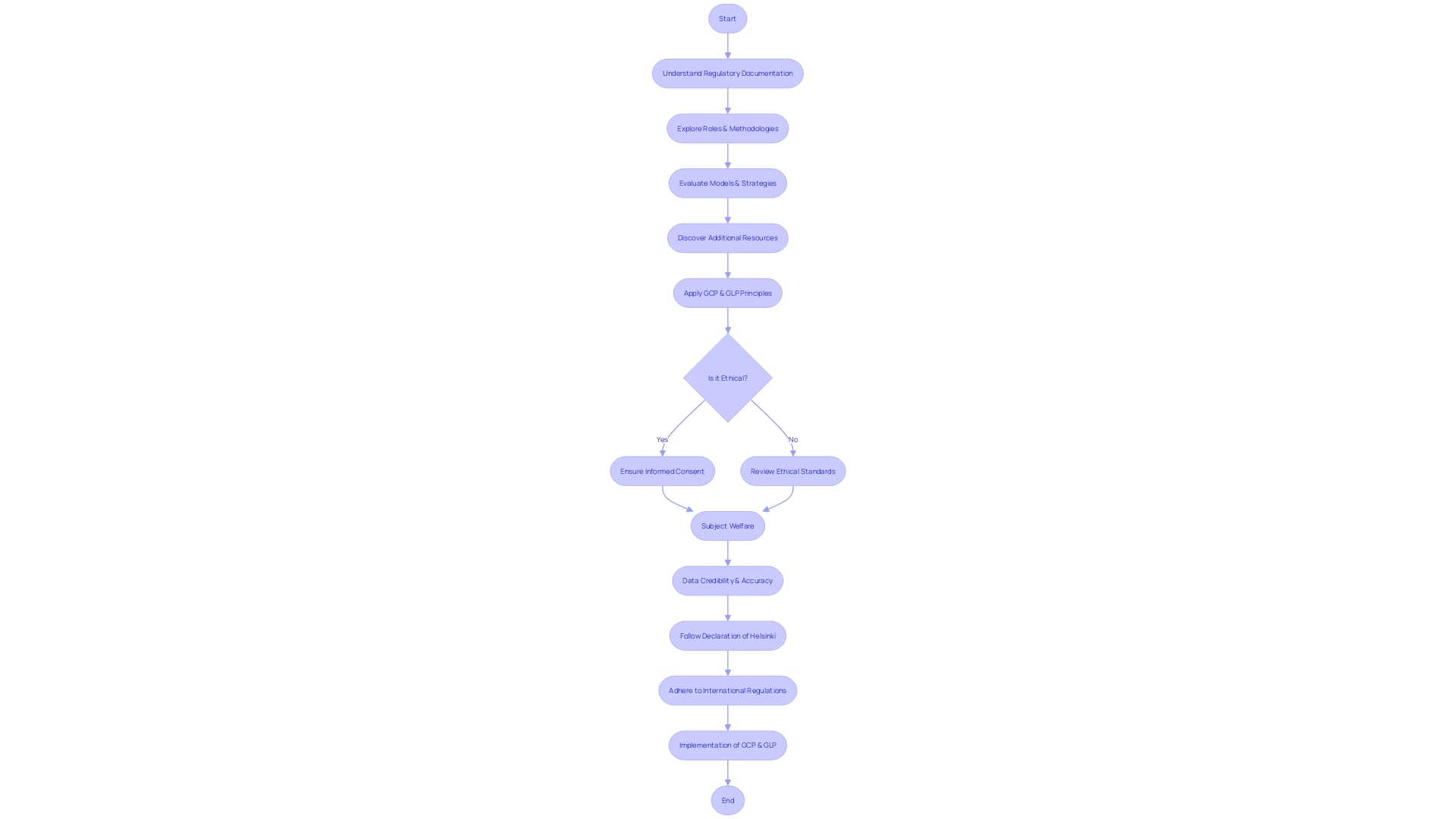
The foundation of clinical trials lies in a set of core principles that ensure ethical and reliable research practices, as delineated by the International Council for Harmonization (ICH) Good Clinical Practice (GCP) guidelines. These principles are critical for safeguarding participant welfare and ensuring the integrity of trial data. Key among these is the ethical conduct of trials, which mandates that participant welfare and rights are paramount, aligning with ethical standards. Adherence to a scientifically robust and thoroughly documented protocol is another cornerstone, ensuring the trial's methodological soundness.
Investigators bear the weighty responsibility of ensuring the trial is conducted as per the protocol, with participant safety at the forefront. Comprehensive documentation of all trial-related procedures is vital for maintaining consistency and accountability. The accuracy, completeness, and verifiability of trial data are central to maintaining data integrity, a fundamental aspect of clinical research. Prompt reporting and management of adverse events are essential for participant safety and the integrity of the trial outcomes.
Quality control and assurance systems are in place to uphold the standard of trial conduct and data quality. Meticulous recordkeeping enables the preservation of all trial documents for future inspection and review. Finally, regulatory compliance is non-negotiable, as trials must adhere to the relevant regulatory requirements and guidelines.
These principles are not just theoretical but are applied in real-world contexts as evidenced by the ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial, which highlighted the nuanced differences in clinical outcomes based on gender, emphasizing the need for equitable risk factor control. Furthermore, the recent update to the European GCP guidance, which introduces a new section focused on data governance, underscores the evolving landscape of clinical trial management, highlighting the importance of patient safety, efficient trial design, and risk mitigation strategies—all reflective of the principles espoused by ICH GCP.
In the realm of non-interventional studies, a robust causal framework is necessary to avoid bias and ensure decision-grade evidence. Tools like the ROBINS-I and GRACE checklist help evaluate bias, while RECORD-PE and Start-RWE promote transparency in reporting. The harmonized protocol template HARPER, supported by regulators, exemplifies efforts to communicate key study parameters effectively.
Ethical Considerations in GCP
Good Clinical Practice (GCP) is an international ethical and scientific quality standard that ensures the rights, safety, and well-being of trial subjects are protected, while also guaranteeing that clinical trial data are reliable and credible. It embodies principles of respect for persons through informed consent, beneficence by maximizing benefits and minimizing harms, and justice in the equitable distribution of research burdens and benefits. Regulatory authorities and ethical review boards meticulously assess trial protocols to verify adherence to these principles. The recent introduction of a data governance section in European GCP guidelines highlights the importance of protecting participant data, managing computer systems, and supporting critical decision-making processes such as data finalization and trial design changes. Compliance with GCP is vital, as trials must be carefully designed to safeguard participant well-being and ensure the integrity of the results, as emphasized at the Outsourcing in Clinical Trials (OCT) Europe 2024 conference.
Roles and Responsibilities in GCP
Good Clinical Practice (GCP) is a framework that ensures clinical trials are conducted ethically, and with the highest standards of safety for participants, while Good Laboratory Practice (GLP) ensures the generation of high-quality and reliable non-clinical (laboratory) data related to the safety of chemicals and pharmaceuticals. Within this framework, multiple stakeholders play pivotal roles:
- Sponsors manage the clinical trial's initiation, monetary aspects, and regulatory compliance, ensuring the study adheres to GCP protocols.
- Investigators execute the trial according to the protocol, maintaining participant safety and rights.
- Ethics committees critically review the trial's protocol for ethical soundness and participant protection.
- Regulatory authorities grant approval for trial conduct, verifying adherence to GCP and pertinent regulations.
- Monitors verify ongoing trial adherence to the protocol, safeguarding participant well-being and data accuracy.
- Data management and analysis specialists handle the intricate tasks of data collection, management, and analysis, ensuring data integrity and validity.
- Trial participants commit to the study protocol and contribute essential data through their involvement.
A recently updated section in the European GCP guidance, set to be implemented in late 2024, underscores the significance of data governance, particularly the design and execution of clinical trials that prioritize patient safety and result reliability.
The evolving clinical trial landscape now emphasizes the need for Diversity, Equity, and Inclusion (DEI) to ensure more personalized medicine approaches for varied patient populations. The traditional reliance on a homogenous study group is being challenged to achieve more inclusive and effective care.
Moreover, the advent of digital technologies and AI-driven methodologies in clinical trials necessitates a strategic data approach. This includes a comprehensive plan that considers the collection of diverse data from traditional and digital sources, ensuring that data insights are utilized effectively for informed drug development decisions.
As the industry moves forward, it is crucial to acknowledge the clinical research professionals who are vital yet often overlooked in the ecosystem. Their role and professional identity need to be clearly defined to address the workforce crisis and improve clinical research outcomes.
Importance of GCP in Protecting Trial Subjects
Good Clinical Practice (GCP) is not merely a set of guidelines but the foundation upon which clinical trials are built to safeguard participants' rights, safety, and welfare. By enforcing GCP, researchers and sponsors are committing to an ethical framework that prioritizes informed consent, conveying all aspects of the study alongside its potential risks and benefits. The proactive monitoring of trial participants is a critical component of GCP, allowing for the immediate identification and management of any adverse events, thereby maintaining the integrity of the research and the trust of the community.
In a practical context, the adoption of GCP principles is exemplified by Unity Health Toronto's collaboration with Google Cloud in developing a generative AI tool to streamline the review of intricate research contracts. This innovation is set to expedite the approval process for clinical trials, enabling quicker access to novel health interventions. The legally binding clinical trial agreements, which detail each party's responsibilities, underscore the importance of meticulous adherence to GCP to ensure ethical conduct and participant protection.
The ethical considerations of compensating clinical trial participants have evolved, recognizing the fairness of reimbursement for their contributions to public health advancements. This shift in perspective is a testament to the evolving ethical landscape and the recognition of participants' sacrifices, akin to those made by public servants like firefighters who are fairly compensated.
Furthermore, the implementation of new guidelines from the European Medicines Agency (EMA) has highlighted the complexity of aligning technology and systems with regulatory expectations. The experience shared by ICON demonstrates the rigorous processes involved in achieving compliance, from initial risk assessment to the implementation of mitigations.
As we consider the ethical dimensions of clinical trials, it is crucial to reflect on the personal experiences of participants, as shared by researchers like Gamertsfelder and Osipenko. Participants often bear a significant personal burden, enduring invasive tests and managing additional health risks, motivated by the hope that their involvement may benefit future generations.
The Declaration of Helsinki, a pivotal document in medical research ethics, is undergoing revisions to address long-standing ethical considerations. This presents an opportunity to align research conduct with the current understanding of ethical principles involving human participants.
In the broader scope of research integrity, studies such as those conducted by CLI, which shed light on issues like the impact of chemical abortions, demonstrate the profound responsibility researchers bear in conducting and publishing meticulous work. Such research not only contributes to public discourse but also underscores the necessity for GCP and GLP to maintain the highest standards of research quality and ethics.
GCP and Data Quality
Good Clinical Practice (GCP) guidelines serve as the cornerstone of clinical research to ensure the integrity of data collected. These guidelines mandate that data must be accurate, complete, and reliable, facilitating a comprehensive evaluation of investigational products. Harmonizing the data collection process through standardization, from the point of inception all the way through to analysis, is critical. This means implementing thorough documentation practices and robust validation processes. Such rigor in data handling is imperative not only for the credibility of the trial results but also for maintaining the trust of stakeholders and regulatory bodies. In essence, GCP's stringent data quality standards are analogous to the service level indicators used in the software industry to measure application reliability. Just as service level objectives anchor the performance thresholds in technology, GCP establishes clear benchmarks for clinical data quality, ensuring that it is fit for purpose and can stand up to rigorous scrutiny.
Challenges and Evolutions in GCP
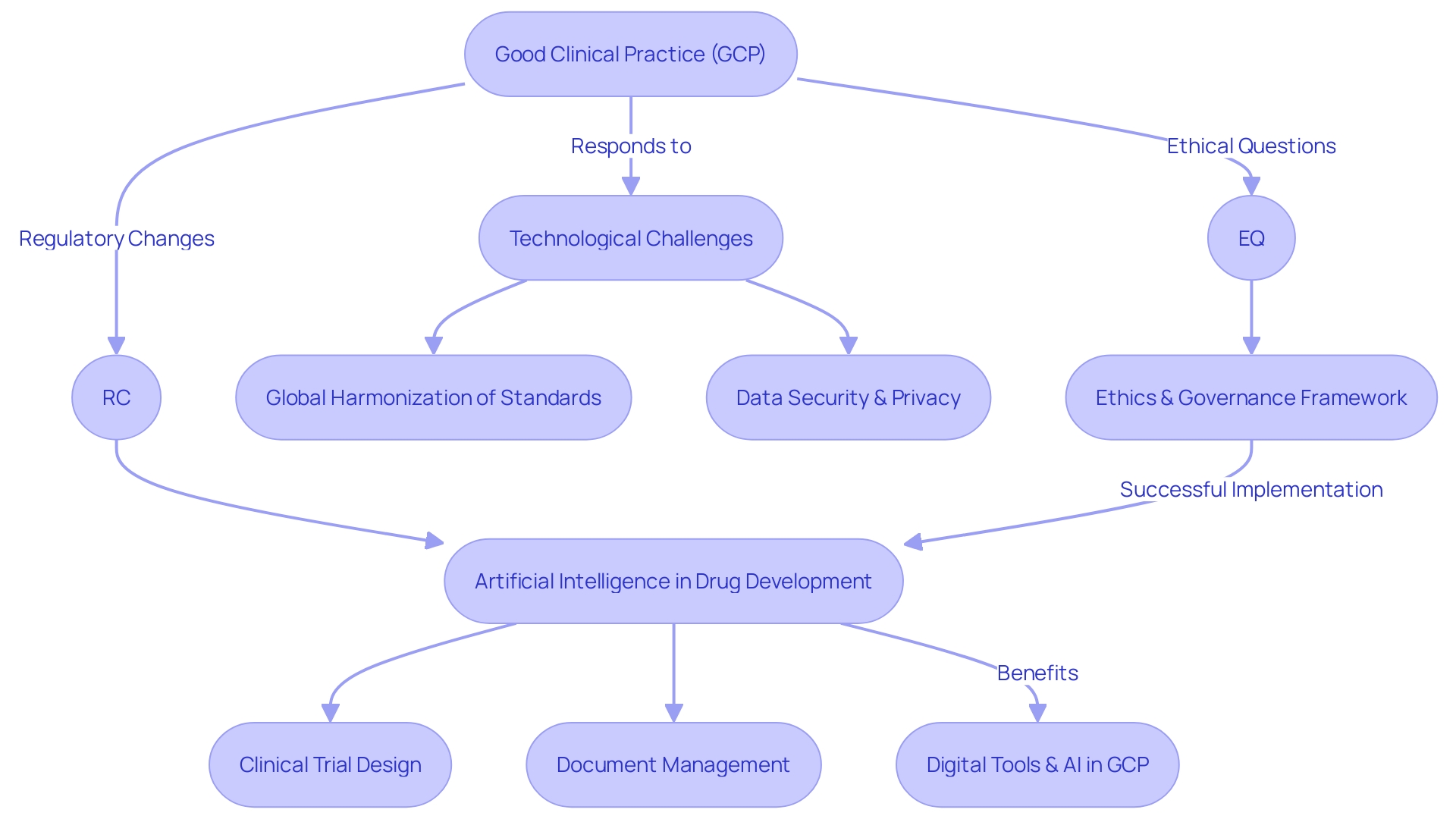
As the landscape of clinical research evolves, Good Clinical Practice (GCP) is adapting to meet new technological challenges, regulatory changes, and ethical questions. Global harmonization of standards is vital in creating consistent methodologies across borders. Meanwhile, the digital transformation necessitates robust strategies for data security and privacy to protect sensitive patient information. In response to the increasing intricacies of clinical trials, there's a drive to innovate with new technologies and approaches aimed at bolstering efficiency, simplifying processes, and enhancing patient safety. An integral part of this innovation is the application of artificial intelligence (AI) to various facets of drug development, including clinical trial design and document management. Ai's growing role in core systems necessitates a solid governance framework to ensure success. Embracing AI and digital tools can help address the challenges of GCP implementation by improving data literacy, fostering trust among stakeholders, and streamlining collaboration within the life sciences sector.
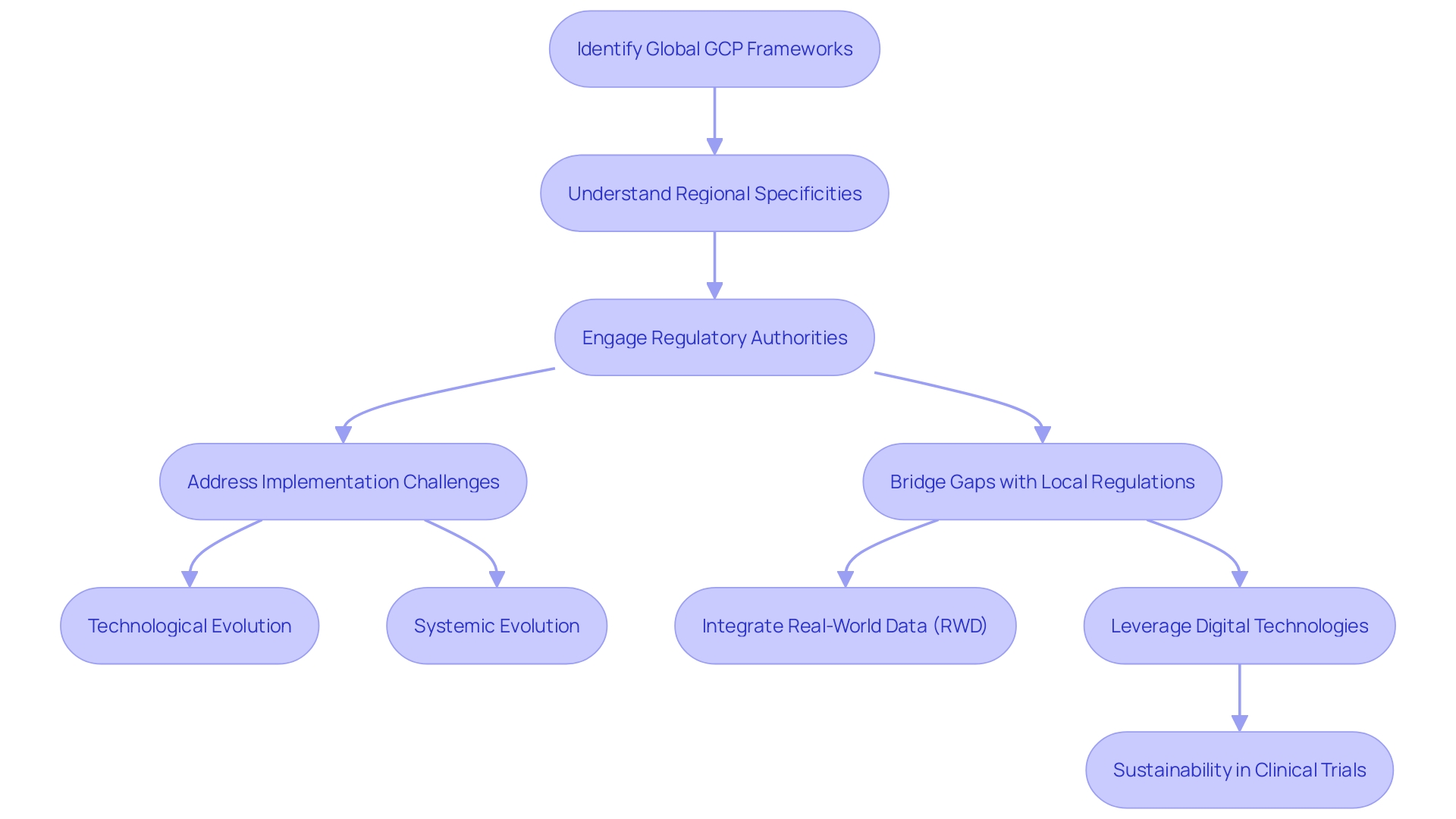
Integration of GCP with Local Regulations
Adherence to Good Clinical Practice (GCP) guidelines is vital for the ethical and scientific quality of clinical trials globally. However, the application of GCP is not a one-size-fits-all solution, as each country may have unique regulations and requirements that must be taken into account. To ensure comprehensive compliance, it is essential to harmonize GCP with both international standards and local laws. This is where regulatory authorities become instrumental, as they bridge the gap between global frameworks and regional specificities to oversee the successful execution of clinical trials.
The complexity of integrating GCP with local regulations is highlighted by the challenges faced during the implementation of the European Medicines Agency's (EMA) guidelines, which had a tight six-month timeframe from publication to the effective date. The multilayered nature of these guidelines and the required technological and systemic evolution to meet the expectations posed a significant challenge, especially with the variability of systems used in clinical trials. This includes ensuring adequate functionality, access control, direct access, and audit trails across different platforms, some of which may not be under the direct control of the trial's sponsor or clinical research organization (CRO).
In the pursuit of optimizing clinical trials, innovative approaches such as utilizing real-world data (RWD) to enhance trial design and patient recruitment are gaining momentum. This aligns with health authorities' guidance, such as the FDA's encouragement to include RWD to reflect the real-world patient population that will use the drugs. The quality of RWD is of paramount importance, as noted by Flatiron's research, which emphasizes the need for data relevance and reliability to inform scientific inferences.
Furthermore, the healthcare sector, responsible for approximately 5% of global greenhouse gas (GHG) emissions, is recognizing the urgency to drive sustainability. Innovative digital technologies are being leveraged to reduce the environmental impact of clinical trials, as seen with the first pharmaceutical company to implement a Carbon Emission Index in trial design. This tool is crucial for calculating and minimizing GHG emissions, emphasizing the industry's responsibility to operate sustainably while advancing healthcare.
In conclusion, the harmonization of GCP with local regulations is a collaborative effort that requires the involvement of regulatory authorities, innovative technology, and a commitment to sustainability to ensure the ethical, scientific, and environmental integrity of clinical trials worldwide.
Future Directions and Innovations in GCP
Good Clinical Practice (GCP) is pivotal in ensuring that clinical trials are conducted ethically and efficiently, yielding reliable results for the advancement of medical science. With the integration of digital health technologies, GCP is poised to undergo transformative changes. The use of Artificial Intelligence (AI), for instance, has the potential to revolutionize the way data is collected and monitored, as evidenced by Google's commitment to leveraging AI for enhancing mental health resources. By training AI models on extensive mental health data, there is an opportunity to support tasks ranging from provider training to diagnosis and intervention implementation.
Moreover, the inclusion of real-world evidence (RWE) in clinical trials is gaining traction. RWE refers to healthcare information derived from multiple sources outside of traditional clinical research settings, which can include electronic health records, claims and billing activities, and product and disease registries. As real-world data accumulates, it offers a rich resource for generating evidence that complements information from randomized controlled trials (RCTs). This evidence is crucial in understanding how treatments perform in day-to-day medical practice, which can differ significantly from controlled trial environments. The U.S. FDA's initiative on RWE aims to align with patient-focused drug development, highlighting the importance of patient-centricity in advancing a health ecosystem that truly serves the needs of patients.
Additionally, risk-based approaches to trial monitoring are being implemented to enhance the quality of clinical trials. These approaches involve prioritizing monitoring efforts where it is most needed, based on the level of risk to patient safety and data integrity. Such strategies can lead to more efficient use of resources and potentially faster development of therapies.
The evolution of GCP is not without its challenges, as forward-looking statements in the pharmaceutical industry often involve significant risks and uncertainties. However, by harnessing the power of AI and RWE, while adopting risk-based monitoring strategies, the future of GCP holds the promise of more efficient and patient-centered clinical trials that can accelerate the development of new therapies and improve patient outcomes.
Conclusion
In conclusion, Good Clinical Practice (GCP) is the gold standard for conducting ethical and scientifically sound clinical trials. It prioritizes participants' rights and safety, ensuring trials are conducted with integrity. GCP contributes to the development of safe and effective treatments and emphasizes transparency in clinical research.
The integration of artificial intelligence (AI) and real-world evidence (RWE) is revolutionizing data collection and analysis in clinical trials. These innovations pave the way for patient-centered trials that accelerate therapeutic development and improve patient outcomes.
Moving forward, GCP faces challenges and opportunities. Harmonizing GCP with local regulations is essential for comprehensive compliance. The integration of digital health technologies, such as AI and RWE, holds promise for transformative changes in data collection and monitoring.
Risk-based monitoring strategies optimize resources and enhance the quality of clinical trials.
In summary, GCP is critical for ensuring ethical and reliable clinical trials and advancing medical science. By adhering to GCP guidelines, researchers and sponsors commit to an ethical framework that prioritizes participant welfare and data integrity. The future of GCP lies in embracing technological innovations and patient-centered approaches to accelerate therapeutic development and improve patient outcomes.




