Introduction
The Pre-Market Approval (PMA) process is an essential regulatory pathway for medical devices, ensuring that they meet the highest standards of safety and efficacy before entering the market. Administered by the U.S. Food and Drug Administration (FDA), PMA involves a comprehensive evaluation of devices to assess their quality and conformity to regulations. This article explores the challenges of PMA, the importance of comprehensive assessments, key components of a PMA submission, the rigorous review process, navigating the submission process, choosing the right pathway between PMA and 510(k), and the role of expert consultants in PMA submissions.
By understanding and navigating the PMA process, businesses and healthcare providers can bring innovative medical solutions to the market efficiently and responsibly.
What is PMA?
Pre-Market Approval (PMA) is not just a stringent regulatory process by the U.S. Food and Drug Administration (FDA) but also a crucial stage in the lifecycle management of healthcare instruments. It evaluates the safety and effectiveness of healthcare products to make sure they meet high quality criteria prior to being introduced in the U.S. market. In contrast to other practices in the industry, achieving success in this field remains uncertain until the product is being used, which requires a concentration on crucial characteristics for the initial release without burdening with production intricacies. A substantial amount of time is dedicated to developing and perfecting a manufacturing system that is strong and capable of producing a product that is both secure and efficient.
The challenges of PMA extend beyond U.S. borders; companies must navigate international regulations, which, despite alliances like the EU, can vary significantly. Last-minute changes in packaging, labeling, and localization can introduce delays. Understanding the logistical intricacies of shipping, such as size and weight restrictions, can have a profound impact on costs and efficiency. Moreover, post-launch production adjustments may be necessary to cater to market demands or to address manufacturing issues identified after product release. This fact can be unsettling for manufacturing personnel who prefer a consistent procedure, yet it is crucial for the medical equipment industry's advancement.
For example, Medtronic's Coronary and Renal Denervation business recently achieved approval for its Symplicity blood pressure procedure in over 70 countries, demonstrating the worldwide influence and magnitude of successful PMA procedures. This global reach underlines the importance of a scalable supply chain, particularly post-Phase Two, as large acquirers often prize this capability.
The FDA's CDER underscores the importance of comprehensive assessments in the development of new drugs and therapeutic biological products. This is done through clear guidance to developers about study design and data requirements. CDER's approval of new molecular entities and biological products each year is a testament to the FDA's commitment to public health. Furthermore, in the context of servicing and remanufacturing of equipment, the FDA offers vital guidance to guarantee ongoing adherence to safety and performance specifications.
Ultimately, the PMA procedure serves as a cornerstone for regulating healthcare equipment, guaranteeing that only products that adhere to the highest standards of quality and effectiveness reach patients. For businesses and healthcare providers, comprehending and maneuvering through this system is vital to introducing innovative healthcare solutions to the market efficiently and responsibly.
When is PMA Required?
The PMA (Pre-Market Approval) process is a rigorous regulatory pathway for medical instruments that are high-risk or represent breakthrough technologies, such as instruments intended for life-sustaining or life-support functions, or those that have a significant impact on body structures or functions. Notably, items that have not previously undergone the FDA's 510(k) clearance require PMA. This includes innovative offerings like cardiac equipment, surgical robots, and insulin pumps, which are integral to companies like Medtronic, a leader in healthcare technology. Medtronic's commitment to alleviating pain, restoring health, and extending life is evident in their expansive portfolio, which addresses 70 health conditions and benefits two individuals every second of every day.
The significance of PMA extends to alterations of existing equipment as well. The recent guidance clarifying 'servicing' versus 'remanufacturing' activities underscores this, as changes that significantly affect a product's performance or safety can necessitate a new PMA submission. This guidance is essential for preserving the quality, safety, and effectiveness of objects throughout their lifecycle, including those that necessitate preventive maintenance or repair.
In the dynamic field of healthcare technology, regulatory oversight evolves with experience and emerging challenges. Recent revisions in regulations reflect this, providing procedural guidance for drug-device combinations and setting the stage for managing the lifecycle of these complex products. In an era where cybersecurity poses significant risks, the FDA's focus on considering these threats at every stage of a product's life—from design to maintenance—is a testament to the proactive approach required to ensure patient safety.
Bijan Elahi, a well-known specialist in safety risk management for devices, highlights the significance of risk management in his book, in which he provides practitioners with the resources and understanding to establish acceptance criteria for risk and establish the boundaries of risk reduction. His insights serve as proof of the crucial role that risk management plays in the creation and upkeep of healthcare equipment, in accordance with industry standards and international norms such as ISO 14971.
Key Components of a PMA Submission
The Premarket Approval (PMA) pathway for medical instruments is a demanding and intricate regulatory procedure, ensuring that instruments are safe and effective for their intended uses. As a component of this procedure, producers are required to furnish an exhaustive dossier that covers the complete range of usage of the apparatus, which incorporates clinical data, production specifics, and labeling, along with proof that validates the security of the apparatus through rigorous clinical investigations and scientific data. The PMA submission must comprehensively address any potential risks, articulating how the product's benefits surpass these concerns.
Recent revisions to this process reflect the learning curve since the implementation of new regulations, with regulatory and procedural guidance now covering integral drug-technology combinations and their lifecycle management, co-packaged healthcare products, and the consultation procedures for technologies with an ancillary medicinal substance and companion diagnostics. These modifications arise from real cases and experiences, determining how such products should be labeled and regulated—whether within the pharmaceutical or equipment framework.
Furthermore, the Food and Drug Administration (FDA), the guardian of public health for drugs and healthcare equipment, underscores the significance of transparent communication in drug advertisements, asserting that the display of significant adverse effects and contraindications must be evident, prominent, and unbiased. This standard aims to ensure information is consumer-friendly and easily understandable, which is also a cornerstone of the PMA process.
FDA's expectations are particularly stringent when it comes to In Vitro Diagnostic Devices (IVDs), with a focus on the risk of false results and the interpretation of these results, highlighting the critical nature of accurate and reliable clinical data in regulatory submissions. Crucially, Bijan Elahi, a prominent authority in safety risk management for healthcare equipment, has highlighted the importance of clarity and assurance in risk management procedures. His work has been crucial in training professionals to establish risk acceptance criteria and determine when risk reduction efforts can be stopped, ensuring that equipment meets the high standards set by regulatory authorities.
In today's digital era, patient communication is also undergoing a transformation, with pharmaceutical and biotech industries leveraging digital channels to improve patient engagement and medication adherence. This development in patient engagement is proof of the industry's continuous dedication to enhancing health results, which is inherently connected to the thorough assessment of healthcare instruments via the PMA evaluation.
Rigorous Review Process
The Premarket Approval (PMA) process, overseen by the FDA, is a crucial step in guaranteeing that medical instruments are secure and efficient before they become accessible to the public. This thorough evaluation examines every aspect of the product, from its performance and manufacturing processes to its labeling and clinical trial data. Crucial to this evaluation is a group of diverse professionals who thoroughly authenticate the scientific reliability of the submitted information, ultimately relying on a thorough risk-benefit analysis of the apparatus.
The PMA process is not static; it evolves with the progress in the field and actual case studies. Recent updates have focused on the lifecycle management of integral drug-instrument combinations, such as pre-filled syringes, and the specific labeling requirements for healthcare products co-packaged with instruments. The FDA also addresses the consultation procedures for instruments containing ancillary medicinal substances and companion diagnostics, ensuring a holistic regulatory approach.
In the midst of the global panorama, the MHRA has set forth a policy intention to acknowledge approvals of healthcare gadgets from different worldwide regulators, simplifying the approval procedure and encouraging creativity. This policy underscores the commitment to patient safety while reducing redundant assessments, signaling a shift towards more efficient regulatory practices.
The medical equipment industry must navigate through different FDA classifications, each associated with specific patient risk levels. Determining the accurate categorization is vital as it determines the suitable regulatory pathway, whether it's a 510(k) notification, PMA, or De Novo procedure. Understanding these terms is crucial for regulatory professionals, as each represents a separate phase of approval and is vital to the market journey of the product.
When grasping the importance of PMA's significance, it becomes evident that it is not just a regulatory obstacle but a comprehensive safeguard for societal health, reflecting the FDA's unwavering commitment to maintaining the highest standards of safety and efficacy in the field of healthcare technology.
Navigating the Submission Process
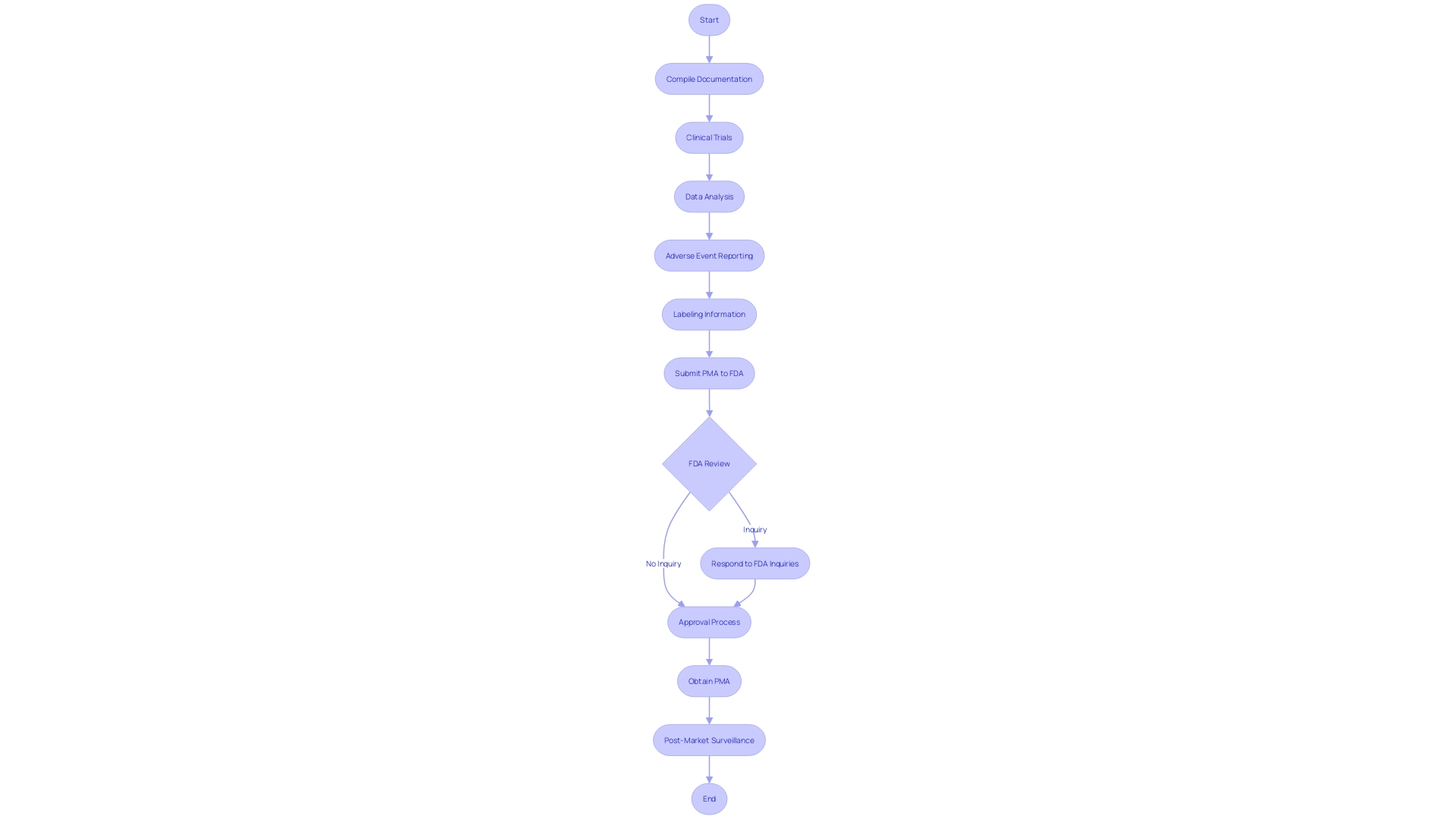
The Pre-Market Approval (PMA) pathway is a demanding journey that medical instrument manufacturers must navigate with accuracy and strategic planning. This involves a thorough compilation of various critical documents, including, but not limited to, detailed clinical trial protocols, comprehensive data analysis, reports of any adverse events, and precise labeling information. Manufacturers need to be prepared for a dynamic process that may include responding promptly to inquiries or additional information requests from the FDA.
In the design and development stages, manufacturers should embrace a structured approach, focusing on vital elements such as design planning and understanding the product's use environment. For example, it's crucial to have a thorough understanding of the equipment's users—clinicians, physicians, dentists, patients, etc.—and the instructions for use, which include warnings and cautions. This knowledge, alongside a familiarity with the competitive landscape derived from marketing insights and research literature, informs the creation of a comparative table that is critical for identifying potential substitute instruments with analogous intended uses and technological characteristics.
Moreover, the Quality and Regulatory teams should be integral from the onset of product development. This proactive involvement allows for mapping out an efficient approval strategy and mitigates the need for retrospective changes that can be costly and time-consuming. As highlighted by industry experts, companies often realize the importance of these teams too late, when issues arise or during the final stages of development, where changes become more challenging to implement.
It is a common practice for companies to involve skilled consultants or firms who specialize in navigating the PMA procedure. These experts offer invaluable insights, ensuring that the information submitted to the notified bodies is adequate and meets the stringent requirements set forth. Attempting to gain certification without this expertise can lead to expensive and multiple submission rounds, which is not a preferred strategy.
In the end, the PMA submission is not only a regulatory challenge but a well-defined journey that demands a comprehensive understanding of both the product and the regulatory landscape, ensuring that devices entering the market are safe, effective, and of the highest quality.
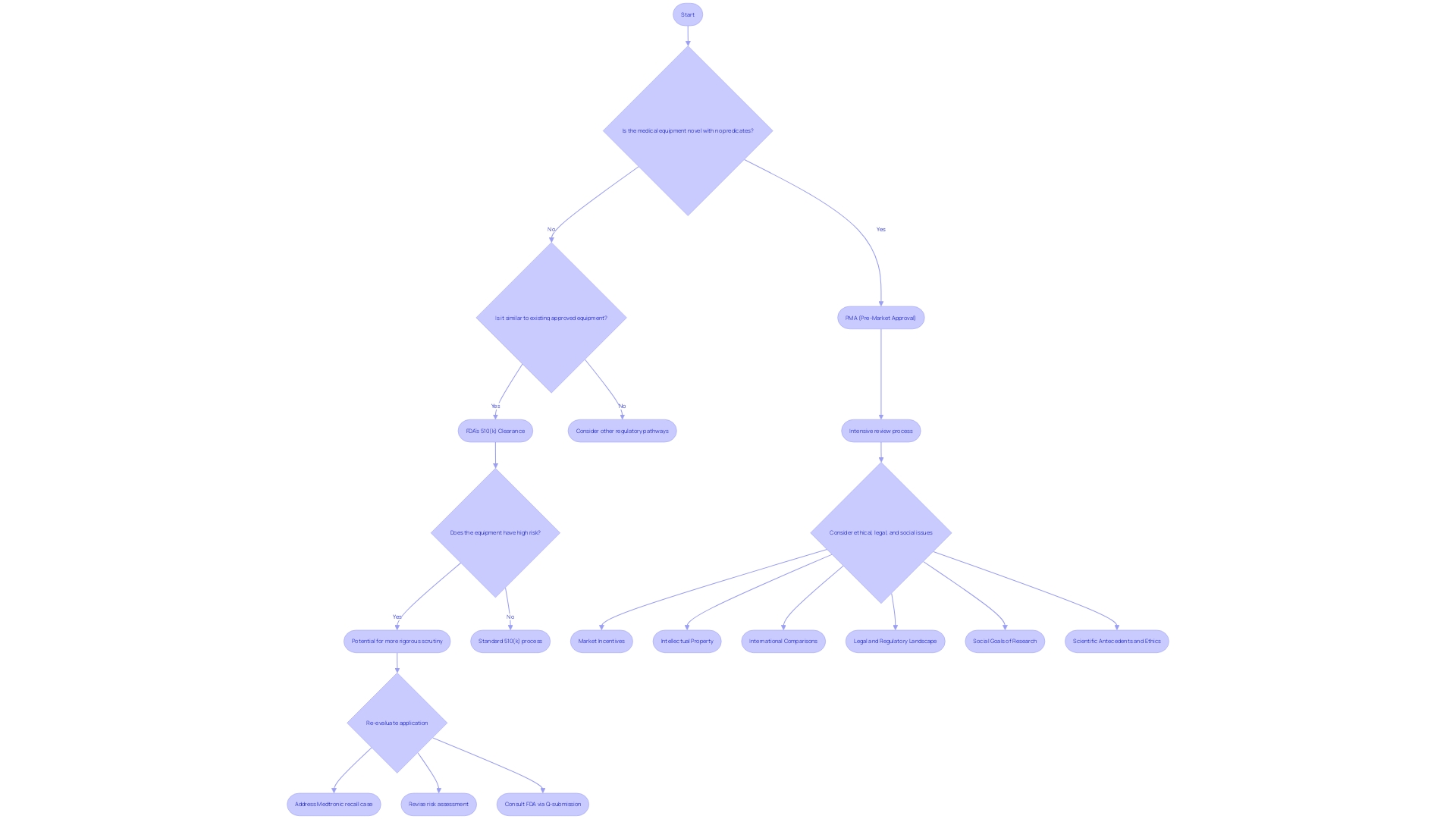
Choosing the Right Pathway: PMA vs. 510(k)
The route to market approval for medical equipment is a crucial point for manufacturers, involving a strategic choice between the Pre-Market Approval (PMA) process and the FDA's 510(k) clearance. While the 510(k) route offers a swifter approval for products that are substantially equivalent to those already on the market, PMA is indispensable for novel or higher-risk items that require rigorous review to ensure their safety and efficacy. This decision depends on various factors, including the intended application of the equipment, its technological complexity, and the potential risks it presents. For instance, a Michigan-based company specializing in instruments for orthopedic, dental, and cardiothoracic surgery would need to contemplate these options carefully, considering the critical nature of their products. Likewise, in the setting of medical conditions such as Autoimmune Pulmonary Alveolar Proteinosis (PAP), with its substantial prevalence and the requirement for dependable treatment apparatus, the choice between PMA and 510(k) becomes even more significant. The recent case of a Missouri man's unfortunate demise, allegedly connected to a delay in a Medtronic device recall, underscores the gravity of stringent regulatory scrutiny and the potential repercussions of the pathways chosen for device clearance.
Role of Expert Consultants in PMA Submissions
The procedure for Premarket Approval (PMA) is intricate and requires a team of expert consultants who bring a wealth of specialized knowledge to the table. These seasoned professionals are invaluable in guiding manufacturers through the labyrinth of regulatory requirements, ensuring that each step of the process is meticulously handled. Their expertise spans a broad spectrum, including clinical research know-how, regulatory frameworks, statistical analysis, and more, all of which contribute to a robust and successful PMA submission.
The significance of these consultants cannot be overstated, as they are not only adept at navigating current regulations but also possess the foresight to anticipate and adapt to evolving industry standards. For instance, the Program Management Improvement Accountability Act (PMIAA) underscores the necessity for key skills and competencies in managing complex projects, which parallels the demands of PMA submissions. Similarly, recent developments in healthcare regulation emphasize the importance of physician associates (Pas) and anesthesia associates (AAs), who are increasingly integral to the multi-disciplinary team, reflecting the collaborative nature of PMA efforts.
Furthermore, the expense and pricing approaches of healthcare equipment, as emphasized by firms such as Stryker, emphasize the significance of a carefully devised regulatory plan. This is where expert consultants shine, aligning PMA submissions with business goals and market realities to optimize the product's journey to market. Their role is a critical component in driving innovation and ensuring that new medical devices meet the highest standards of safety, efficacy, and quality before reaching patients.
Conclusion
In conclusion, the Pre-Market Approval (PMA) process is a crucial regulatory pathway for medical devices, ensuring they meet the highest standards of safety and efficacy before entering the market. It involves comprehensive assessments, a rigorous review process, and strategic navigation of the submission process.
Comprehensive assessments are vital in PMA submissions, requiring manufacturers to provide detailed dossiers that encompass the device's full use and substantiate its safety through robust clinical studies and scientific data. Recent revisions reflect the FDA's commitment to addressing integral drug-device combinations and consultation procedures for devices with ancillary medicinal substances and companion diagnostics.
The rigorous review process involves multidisciplinary experts who meticulously verify the scientific credibility of submitted information, ensuring a comprehensive risk-benefit analysis. This process evolves with the field's progress and actual case studies, reflecting the FDA's dedication to upholding high standards of device safety and efficacy.
Navigating the submission process demands precision and strategic planning, including compiling critical documents, embracing a structured approach in design and development, and involving quality and regulatory teams from the onset. Expert consultants provide invaluable guidance, ensuring a successful submission.
Choosing the right pathway between PMA and 510(k) clearance is a strategic decision based on factors such as intended application, technological sophistication, and potential risks. While the 510(k) route offers swifter approval for substantially equivalent devices, PMA is essential for novel or higher-risk devices requiring rigorous review.
Expert consultants play a vital role, bringing specialized knowledge to guide manufacturers through regulatory requirements and anticipate evolving industry standards. Their involvement ensures meticulous handling of each step, optimizing the product's journey to market.
By understanding and navigating the PMA process, businesses and healthcare providers can bring innovative medical solutions to market efficiently and responsibly. This upholds the FDA's dedication to public health and patient safety.




