Introduction
The term Investigational New Drug (IND) holds significant importance in the field of medical and biotechnological research. An IND refers to a pharmaceutical compound or biological substance that is undergoing rigorous examination for safety and effectiveness. Before a potential treatment can be tested in clinical trials on humans, an IND application must be meticulously prepared and submitted to regulatory bodies such as the U.S. Food and Drug Administration (FDA).
The IND application plays a crucial role in paving the way for clinical studies and is an essential component in the intricate drug development journey. In this article, we will explore the purpose of an IND application, the different types of INDs, the key components of an IND application, and the process of preparing and submitting an IND application. We will also delve into the phases of clinical trials, the role of the regulatory authority in IND review, and the importance of ensuring the safety and rights of clinical trial participants.
Additionally, we will discuss the evaluation of scientific quality in IND applications. Join us as we delve into the world of INDs and gain a deeper understanding of their significance in the realm of medical research and development.
What is an IND?
The term Investigational New Drug (IND) plays a pivotal role in the field of medical and biotechnological research. It pertains to a pharmaceutical compound or biological substance that is under rigorous examination for its safety and effectiveness. Prior to a potential treatment being given to humans in trials, an IND application must be carefully prepared and submitted to a regulatory body such as the U.S. Food and Drug Administration (FDA). The importance of the INDies not only in its capability to pave the way for clinical studies but also as a strategic component in the intricate pharmaceutical development journey.
Contemporary medication exploration is a complex procedure in which encouraging medication applicants are recognized and enhanced to interact with particular biological targets, aiming to achieve a therapeutic effect. A case in point involves the cutting-edge application of machine learning techniques, like image segmentation, to swiftly and accurately analyze data from experiments. For instance, Vertex Pharmaceuticals employed machine learning to improve the analysis of microscopic images, thereby expediting the evaluation of how medication candidates affect biological materials.
Another example is Cardinal Health, which successfully navigated the complexities of IND applications by developing comprehensive regulatory strategies. This approach facilitated timely and well-rounded submissions, demonstrating the critical nature of strategic planning in the regulatory approval process.
The biopharmaceutical industry frequently distinguishes between scientific findings and the subsequent creation of novel pharmaceuticals. This distinction highlights the fact that discoveries such as penicillin, which took more than 15 years to progress from a laboratory curiosity to a commercially produced medication, necessitate substantial investment and progress efforts to become practical treatments.
Thorough examination and future-oriented declarations, like those from Intellia Therapeutics regarding their investigational gene editing therapies, emphasize the ever-changing environment of treatment creation and the uncertainties involved in bringing new therapies from idea to market. With trials extending from an average of 41 months to 44 months for Phase 3, and 80% of these studies failing to meet their timelines, the pressure to accelerate development is immense. This is particularly urgent given the competitive nature of the market and the pressing need for treatments for conditions with high unmet medical needs.
In the quest to innovate, companies must navigate a myriad of challenges, from safeguarding intellectual property to ensuring regulatory compliance and managing collaborations. As the sector continues to develop, motivated by advancements and external influences, such as legislative modifications impacting medication pricing, the significance of strategic, well-informed IND applications cannot be emphasized enough, as they are the gateways to medical progression and ultimately, patient care.
Purpose of an IND Application
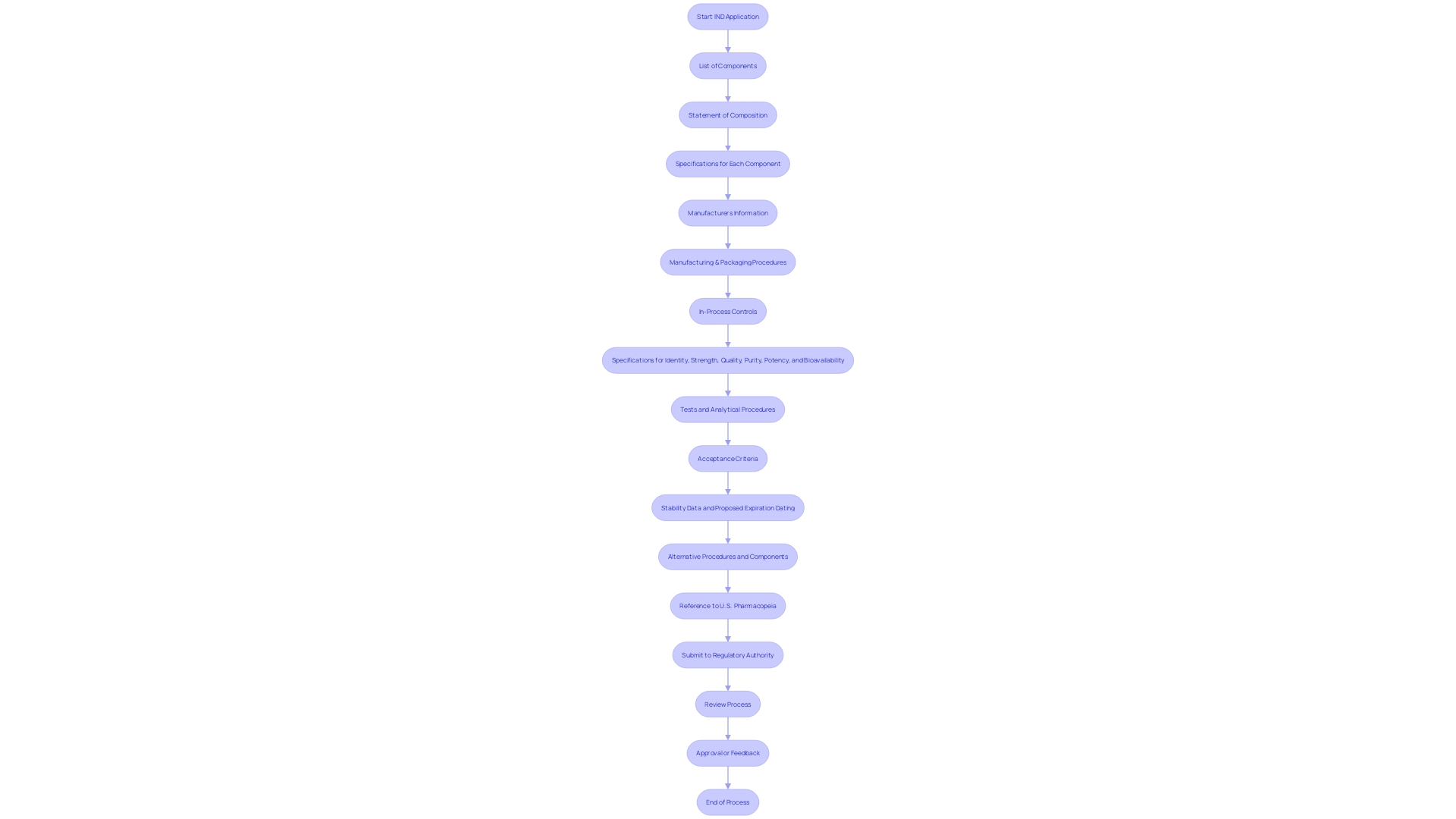
The Investigational New Drug (IND) application is a pivotal step in the journey from laboratory research to the introduction of a novel drug or biological product to the market. It encapsulates a wealth of crucial details, including the product's composition, the intricacies of its manufacturing process, the results of preclinical studies, and the methodologies proposed for clinical examination execution. The IND not only presents a logical argument for the initiation of human trials but also emphasizes the investigational product's safety profile and its potential therapeutic benefits. Thorough by its essence, it must encompass an extensive record of all components employed in the manufacture of the medication, specifications for each, and information on the manufacturing entities. This exhaustive documentation ensures a clear understanding of the product's identity, strength, quality, purity, potency, and bioavailability — critical factors backed by tests, analytical procedures, and stability data.
The application stands as evidence of the thoroughness of the drug creation process, addressing safety concerns for specific populations and preparing for investigations that can withstand examination from industry peers, academia, and regulatory bodies alike. As we have seen with Intellia's innovative gene editing therapies, such as NTLA-2001 for the treatment of transthyretin (ATTR) amyloidosis with cardiomyopathy, the IND is the backbone supporting the progression of programs. It is a statement that looks to the future, influenced by the ever-changing nature of the progress in medicine, where risks and uncertainties are plentiful.
The importance of the IND is further emphasized by recent McKinsey analysis, which highlights that the duration of trials has increased, with up to 80 percent not finishing on time. The focus on accelerated medical advancement is more noticeable than ever, with competitive pressures and legislative changes like the US Inflation Reduction Act reshaping priorities and underscoring the significance of being first to market. The IND thus becomes a vital instrument in navigating the intricate terrain of pharmaceutical advancement, where accuracy, adherence, and foresight are the trademarks of achievement.
Types of INDs
The regulatory environment for medication development is intricate and diverse, with different types of Investigational New Drug (IND) applications that serve various facets of research and patient care. These include commercial INDs, which are typically pursued by pharmaceutical companies aiming to bring new therapies to market. Research INDs are filed by researchers conducting studies without the intent of immediate commercialization. Emergency use INDs allow for the use of investigational medications in life-threatening situations when no suitable alternatives are available. Treatment INDs, also referred to as expanded access INDs, offer access to experimental medications for patients who do not meet the requirements for clinical trials.
Each IND type has unique requirements and considerations, essential for researchers and sponsors to comprehend to navigate the regulatory approval process effectively. For example, commercial INDs require a strong set of preclinical data to demonstrate safety, while treatment INDs concentrate on providing access to medications for patients with serious or immediately life-threatening conditions.
The aim is to ensure that new therapies are not only effective but also safe for patient use. Regulatory oversight at every stage is crucial to safeguard public health. For instance, the FDA's Center for Drug Evaluation and Research (CDER) examines innovative medications and biological products every year, many of which may provide new treatment choices or rival with existing products in the marketplace.
Comprehending these classifications and the linked data prerequisites is crucial for effective progression through the pharmaceutical advancement process, from preclinical investigations to trials and, ultimately, to market endorsement.
Commercial INDs
Commercial Investigational New Drug (IND) applications are crucial for pharmaceutical and biotech companies aiming to bring new therapeutic products to the market. These applications are based on strong preclinical data and are a necessary step in the process of obtaining regulatory approval for the marketing and sale of new pharmaceuticals or biological substances. According to a white paper created for the Netherlands government, biotechnology firms and small/medium-sized biopharmaceutical firms are primarily involved in the exploration of medicines, preclinical advancement, and initial-phase medical progress. This emphasizes the significance of a well-prepared commercial IND in the drug progress ecosystem.
The complexity and critical nature of the IND process are evident in the fact that the average duration of experiments has increased, as reported by McKinsey, with Phase 3 trials now averaging 44 months and Phase 2 trials 41 months. Furthermore, considering that nearly 80 percent of experiments do not conclude within the scheduled timeframe, the demand on organizations to hasten the progression of experiments is substantial. The importance of commercial INDs is further emphasized by the competitive landscape, where being first to market can lead to disproportionate success, especially in an era where new legislative measures like the US Inflation Reduction Act are reshaping the industry's approach to drug advancement and commercialization.
The commercial IND is a testament to a company's commitment to innovation and its ability to navigate the intricate patenting process, as governed by entities like the European Patent Office (EPO). With the high stakes involved in securing intellectual property rights and the increasing duration and scrutiny of clinical trials, companies must meticulously strategize their patent applications and clinical trial designs to ensure a timely and successful entry into the market.
Research INDs
Non-commercial research studies, often spearheaded by academic institutions, government entities, or non-profit organizations, delve into advancing scientific knowledge and enhancing our medical understanding without the end goal of commercial gain. Such research initiatives, known as Research INDs (Investigational New Drug applications), are pivotal as they contribute to the foundational insights that underpin further medical breakthroughs and innovations. These studies may encompass a vast array of inquiries, from the ethics of integrating human brain organoids into animal models to the intricacies of wildfire risk management. As highlighted by a case study that required approximately 65 hours of meticulous research, interviews, and authorship, the goal is to elevate safety standards and protocols, reflecting a commitment to the public good over profit motives.
In the context of experimental research, the distinction between basic, applied, and experimental research is essential. Basic research serves as the bedrock for acquiring new knowledge on fundamental aspects of phenomena without specific applications in mind. In contrast, applied research targets new knowledge with a direct practical aim, such as enhancing a particular crop's genome. Experimental development, an iterative and creative process informed by existing knowledge and practical experience, aims to either forge new products and processes or improve upon existing ones. This tier of research may involve the generation and testing of prototypes or scaled demonstrations of systems which, post-demonstration, could potentially undergo further refinement based on feedback.
Understanding these nuances is essential for directing research efforts appropriately and navigating the complexities associated with human subjects research. Whether it's distinguishing between delayed start and delayed onset in study design or grappling with the ethical considerations of groundbreaking research, the integrity of the scientific process is ensured through careful planning and adherence to regulatory guidelines. This is especially significant when research activities are deemed not to fall under the traditional scope of research, which may bypass certain approval processes, yet still require robust ethical considerations and disclosures as part of the publication process.
Emergency Use INDs
In situations where patients face life-threatening conditions without any existing satisfactory treatments, Emergency Use Investigational New Drugs (INDs) serve as a critical pathway for accessing investigational medical products. These INDs are granted on an individual basis after rigorous review by regulatory authorities, such as the U.S. Food and Drug Administration (FDA), and require the concurrence of an independent ethics committee. The FDA's Center for Drug Evaluation and Research (CDER) plays a pivotal role, leveraging its scientific expertise to evaluate the safety and efficacy of new drugs, including those proposed for emergency use.
CDER's yearly approvals cover a range of new molecular entities and innovative therapeutic biological products, some of which may have never been previously administered in medical settings. In the context of an emergency response, certain products might be dispensed with emergency use instructions as part of a government-led effort to prepare for or respond to a public health crisis. Medtronic plc, a leading healthcare technology company, exemplifies the commitment to innovating technologies that address pressing health challenges, potentially contributing to the pool of products considered for emergency use authorization.
The process of reviewing Emergency Use INDs is also transparent, with provisions for public comments and considerations regarding the confidentiality of submitted information. Recent studies, including a systematic review from 2023, underscore the importance of consistent methodologies in evaluating medical interventions. Such robust scrutiny ensures that only products meeting the highest standards of safety and efficacy are approved for emergency use, reflecting the FDA's mandate to safeguard public health.
Treatment INDs (Expanded Access INDs)
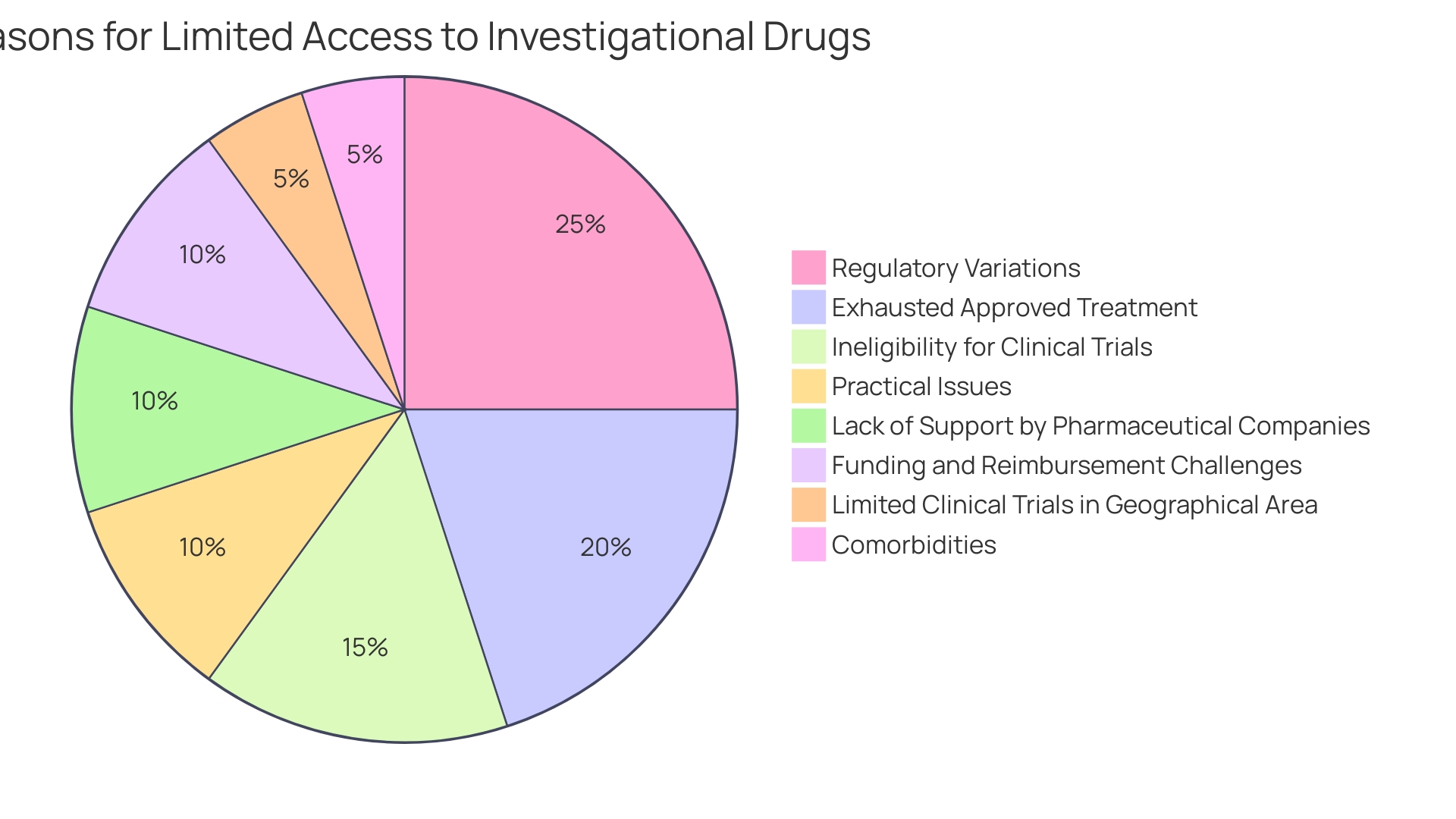
Investigational New Drugs (INDs), also referred to as expanded access INDs, play a vital role in providing investigational treatments to patients with severe or life-threatening conditions who are unable to participate in trials. These individuals usually have tried all available approved treatments and are not qualified for ongoing trials due to factors like the lack of trials in their area, specific inclusion criteria, or comorbidities that disqualify them. Furthermore, the use of investigational substances through treatment INDs may represent the last line of defenseâultimum remediumâagainst their condition, especially when standard treatments have failed. As the FDA's Center for Drug Evaluation and Research (CDER) approves a range of new medications and biological products annually, some of which have never been used in clinical practice, the agency underscores the importance of providing clear guidance on study designs and data required for medication applications, thereby enabling a thorough assessment of these innovative treatments.
Although oncology sees a significant share of expanded access cases, with 53% of PubMed publications on the topic reflecting this field, the challenges associated with the expanded access process can impede its implementation. These challenges include the complexity of application procedures, restrictive hospital policies, and limited support from pharmaceutical companies. Furthermore, obtaining funding and reimbursement for investigational medications continues to be a challenging endeavor. Despite these obstacles, an online survey reveals that a majority of oncologists—75%—are familiar with expanded access protocols, indicating a widespread awareness among healthcare professionals who are vital in navigating these regulatory pathways.
In light of these considerations, the FDA's commitment to public health encompasses not only ensuring the safety and efficacy of drugs but also facilitating access to potentially life-saving treatments for those with no other options. This commitment is evident in the agency's oversight of over 300 Early Access Programs (EAPs) worldwide, many of which target rare conditions, including rare cancers. These programs are designed for patients who are unable to take part in experiments and have no other approved treatments, thus offering a regulated and ethical way to obtain investigational medicinal products. Moreover, CDER's annual approvals showcase the progress of healthcare by introducing new treatment options, reflecting the FDA's role in bringing innovative therapies from clinical studies to patients in need.
Key Components of an IND Application
The IND application is an important dossier that provides a detailed snapshot of the investigational medication and the studies it is intended to undergo. This includes an investigational plan describing the proposed study's objectives and methodology, and detailed chemistry, manufacturing, and controls (CMC) information that outlines the medication's composition, the specifications of each component, and the manufacturing and packaging processes involved. Additionally, nonclinical study data provide insights into the compound's pharmacology and toxicology, while clinical trial protocols delineate the execution plan for human studies.
To guarantee the safety and effectiveness of the investigational product, the application must contain a comprehensive list of all components used in the medication product's manufacture, even those not present in the final formulation, accompanied by a statement of the medication product's composition. Specifications for each component, the names and addresses of manufacturers, descriptions of manufacturing and packaging procedures, in-process controls, and the necessary tests and analytical procedures are also mandated to confirm the product's identity, strength, quality, purity, potency, and bioavailability. This may also include alternative approaches to meet these requirements, ensuring flexibility in the regulatory process.
Notably, the application process is supported by the FDA's Center for Drug Evaluation and Research (CDER), which provides guidance on study design and the data needed for a complete assessment. CDER's expertise stems from a deep understanding of the scientific principles underlying new products, testing, and manufacturing procedures, as well as the conditions the products aim to treat. Each year, CDER approves a diverse array of new medications and biological products, some of which may be pioneering treatments.
The diligence in preparing an IND application is reflected in the forward-looking statements of companies like Intellia, which express expectations regarding the safety, efficacy, advancement, and ultimate commercialization of their investigational therapies. These statements emphasize the dedication to thorough medical tests and compliance with regulatory demands, while also recognizing the dangers and uncertainties inherent in pharmaceutical development. For example, Intellia's program for NTLA-2001 aimed at treating transthyretin (ATTR) amyloidosis with cardiomyopathy is based on the thoroughness of its applications and IND submissions.
The precision and clarity in the presentation of clinical trial data are also paramount, as healthcare providers' understanding of data displays is vital for accurate interpretation and informed decision-making. Studies have shown that complex data displays, such as survival curves or bar graphs in prescription medicine advertisements, can be challenging to interpret, indicating a need for plain language restatements and detailed study disclosures.
In conclusion, the IND application is a foundational element in the continuum of pharmaceutical development, facilitating a clear and comprehensive evaluation of investigational substances, ensuring patient safety, and enabling medical breakthroughs.
How to Prepare and Submit an IND Application
The intricacies of submitting an Investigational New Drug (IND) application are significant, encompassing a thorough compilation and analysis of extensive data and documentation to meet stringent regulatory standards. The meticulous process requires a multidisciplinary effort, uniting researchers, sponsors, and regulatory consultants to ensure that every component of the IND applicationâfrom the medication composition and manufacturing details to the quality control measuresâis precisely documented. Notably, the application must detail all constituents used in manufacturing, even those not present in the final product, and it must address specifications, manufacturing, and packaging procedures, as well as in-process controls. Moreover, the application should include stability data and proposed expiration dating, all of which contribute to establishing the medication's identity, strength, purity, and bioavailability. After finishing, the IND application goes through a thorough evaluation by the relevant regulatory authority, a crucial stage towards advancing clinical progress.
IND Review Process
Finding the way through the complex system of regulations in the creation of new medicines is crucial to guarantee the safety and efficacy of innovative treatments. The Investigational New Drug (IND) application is a cornerstone of this journey, indicating the shift from preclinical research to human trials. Crafting a meticulous IND application is paramount, as it embodies a comprehensive compendium of preclinical data that underscores the potential safety profile of a new therapeutic. This dossier features extensive pharmacological, toxicological, and chemistry, manufacturing, and controls (CMC) information. It's not uncommon for multiple species to be examined during this stage to gather the requisite safety data.
As new therapies advance through the process of creating medications, it is the IND stage that sets the stage for trials. Success in this phase is measured by the ability to demonstrate the promise of a drug's safety and the scientific rigor behind its development. Without any data available at this point, the emphasis is on preclinical evidence to gain regulatory confidence.
The IND review process unfolds in a series of meticulous stages, starting with the initial submission, followed by a detailed examination that may lead to a hold before activation. Once experiments are in progress, the continuous assessment and supervision are crucial for upholding adherence and guaranteeing patient well-being during all study stages.
Drawing on insights from case studies like Intellia's program for NTLA-2001, which targets transthyretin (ATTR) amyloidosis, can be instructive. Despite the complexities and potential for unforeseen challenges, such as those articulated in Intellia's forward-looking statements, the goal remains to shepherd investigational therapies through to ultimately initiate pivotal studies, like the anticipated global Phase 3 study for NTLA-2001.
In the context of today's fast-paced biopharmaceutical landscape, the stakes couldn't be higher. With McKinsey reporting an increase in the duration of medical experiments and a staggering 80 percent of experiments falling behind schedule, the need for effectiveness is evident. Equally pressing is the urgency in development, as companies vie to be the first to market amidst increasing competition and legislative changes like the US Inflation Reduction Act.
The United States remains a center for experiments, with a significant majority of Phase 3 experiments including at least one US site. Nevertheless, the infrastructure supporting medical research encounters obstacles, such as the high turnover of healthcare staff and the economic models that undervalue research activities. These challenges underscore the importance of a well-navigated IND process, which is not only vital for regulatory approval but also pivotal for maintaining the tempo of innovation and delivering new treatments to patients with unmet medical needs.
Submission and Initial Review
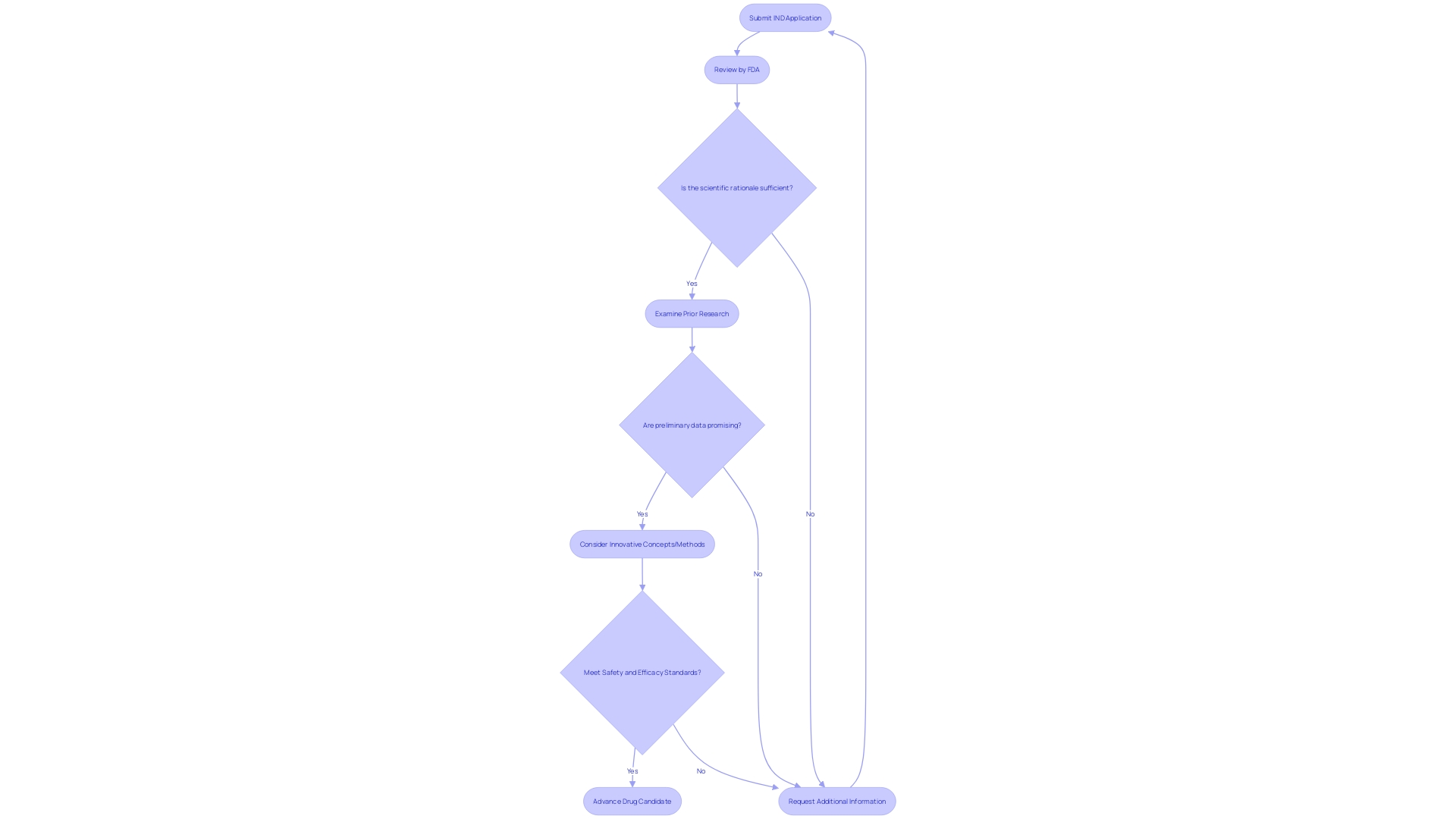
When an Investigational New Drug (IND) application is submitted, it triggers a rigorous review process by the regulatory authority. The authority meticulously evaluates the IND submission to confirm that it is complete and the information provided therein is sufficient. This evaluation includes a thorough examination of the scientific rationale for the proposed study, the robustness of the scientific underpinnings such as prior research and preliminary data, and whether these justify moving forward with the study. If the proposed work features innovative concepts, methods, or technologies, or if it applies existing ones in novel ways, this could significantly amplify the project's impact, a factor that is also taken into consideration during the review.
The process not only looks for innovation but also for the scientific quality and feasibility of the proposed work. If the application successfully addresses a critical knowledge gap or has the potential to resolve a significant issue or deliver a valuable advancement, these elements strengthen the authority's assessment.
Additionally, the review process may involve interactions with the applicant for clarifications or additional information, to rectify any deficiencies or omissions identified. This collaborative aspect is crucial, as emphasized by industry news, such as press releases from biopharmaceutical companies like Intellia, which often discuss the progress and expectations surrounding their IND applications, experimental programs, and the associated regulatory milestones.
Moreover, the importance of velocity and effectiveness in medical advancement cannot be emphasized enough. With an estimated 80 percent of experiments failing to complete on time, and the increasing competitive pressure to be first to market, the IND review process plays a pivotal role in advancing drug candidates through the necessary phases toward approval. The regulatory body, through its review, seeks to ensure that new therapies not only show promise but also meet the stringent safety and efficacy standards necessary for patient use.
Clinical Hold and Activation
The process of testing new pharmaceuticals is a vital part of their development, however, it may face regulatory obstacles. An Investigational New Drug (IND) application may be subject to a clinical hold if the Food and Drug Administration (FDA) identifies safety or ethical issues with the proposed studies. This hold is a precautionary measure, pausing the start or progress of experiments until the concerns are addressed to the satisfaction of the regulatory body. The resolution of these matters is crucial for the continuation of the experiments, as evidenced by Rapt's proactive approach to investigate the incident that led to the hold on their application. They worked diligently to meet the FDA's requirements, as detailed in their Quarterly Report and subsequent SEC filings. Cardinal Health's case study further illustrates the significance of creating a thorough regulatory approach, which can result in prompt and well-coordinated IND submissions, ultimately facilitating the advancement of clinical evaluations. In the ever-evolving landscape of drug development, the FDA continues to provide guidance to ensure that new drugs and biological products meet the necessary standards for a comprehensive assessment. As per CDER's annual reports, this meticulous process is essential to introducing innovative treatments that can significantly improve patient care.
IND Phases: Overview of Clinical Trials
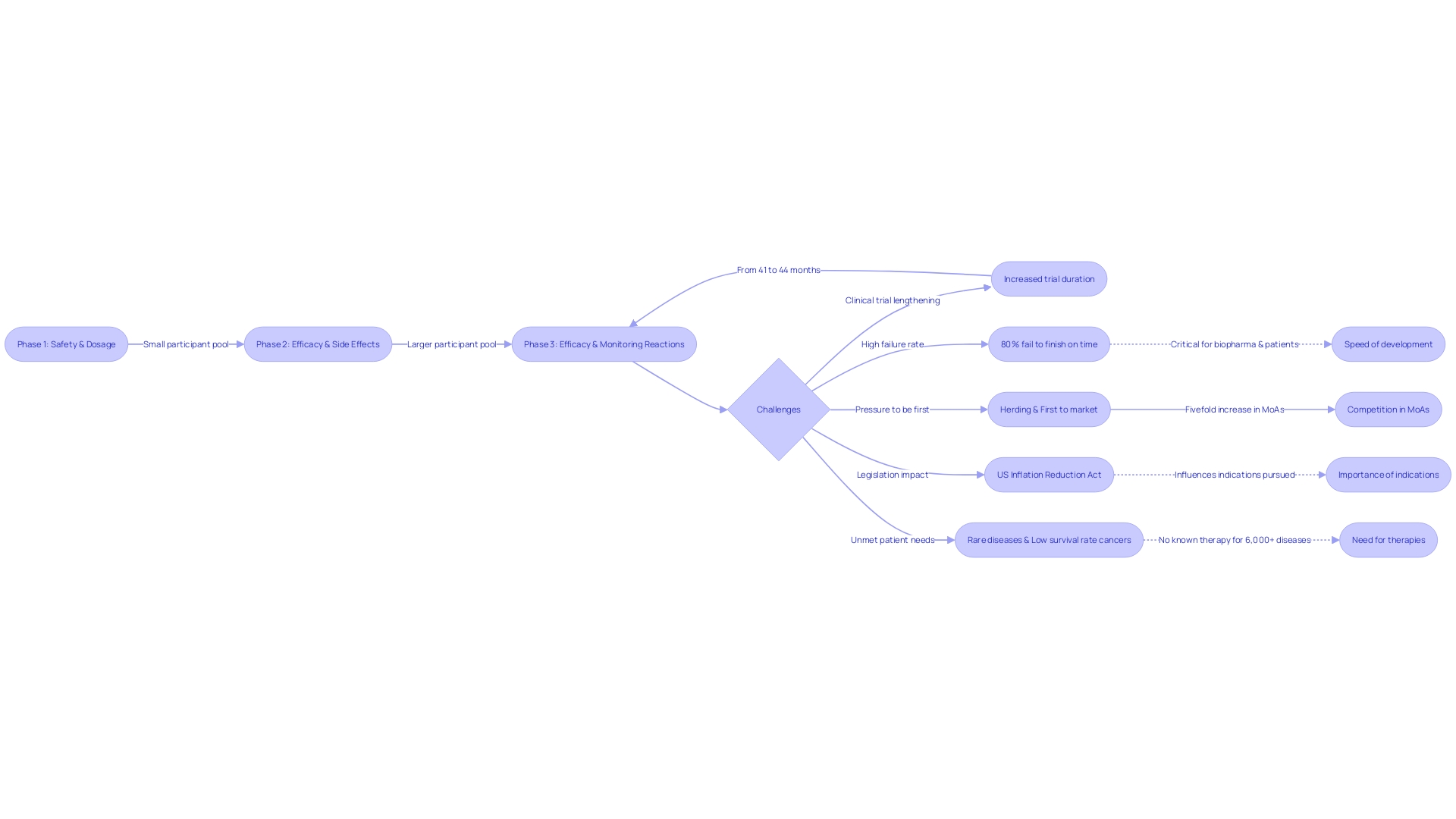
Clinical experiments are an essential part of medical research, organized into stages that gradually aim to establish the safety and effectiveness of investigational products. Phase 1 studies start with a small group of volunteers to evaluate safety and side effects. During the progression to Phase 2, the participant pool expands, with a focus on efficacy and optimal dosing, while still monitoring safety. Phase 3 then further expands the number of participants to solidify the data gathered on the investigational product's effectiveness and monitor adverse reactions over a longer period.
Recent analyses by McKinsey reveal a noticeable increase in the duration of experiments, with Phase 3 studies now averaging 44 months and Phase 2 studies 41 months. This extension in trial duration, alongside the finding that up to 80% of trials fail to meet their original timelines, underscores the challenges faced by biopharma companies. Concurrently, the competitive nature of the industry, characterized by a phenomenon known as 'herding,' places immense pressure on these companies to be first to market, especially for high-potential therapies. The urgency is further compounded by the introduction of legislative measures like the US Inflation Reduction Act, which aims to reduce drug prices, potentially altering the strategic focus of companies.
For patients, especially those facing rare diseases with no existing therapies and cancers with low five-year survival rates, the need for accelerated medical development is critical. As the scenery of experiments develops, so do the resources accessible to patients. Internet platforms have greatly simplified the procedure of discovering and registering in medical experiments, offering tools that screen opportunities by different criteria, such as illness stage and location.
Comprehending the framework and objective of the phases of experiments is essential for those contemplating involvement. It is also important to be mindful of the potential expenses associated with participating in a study, which may involve travel costs and taking time off from work. Knowing that taking part in clinical experiments contributes to essential medical progress, people can make informed choices about their engagement in research that has the potential to revolutionize healthcare and enhance lives globally.
Phase 1: Safety and Dosing Studies
The beginning human studies, called Phase 1 studies, have a crucial role in the advancement of new medical treatments. These experiments are formulated to evaluate the safety and tolerance of a new experimental medication, with an emphasis on determining the appropriate dosage. They are often conducted with a select group of participants, who can be healthy volunteers or patients, depending on the nature of the treatment and its intended target population. The objective is to identify the highest dose that can be administered without causing unacceptable side effects, often referred to as the maximum tolerated dose (MTD).
In the context of modern medicine, the traditional approach to Phase 1 evaluations, which was developed during the time when cytotoxic therapies were prevalent, has come under scrutiny. This traditional method aimed to find the MTD based on the onset of dose-limiting toxicities (DLTs), operating under the assumption that increased doses would enhance both efficacy and toxicity. However, this may not hold true for newer classes of therapies such as targeted treatments and immunotherapies, which may demonstrate a plateau in efficacy beyond certain dosage levels, without necessarily reaching a traditional MTD.
With improvements in medical practices, the International Council for Harmonization (ICH) has introduced ideas like 'estimands' in their ICH E9(R1) guidance to enhance the process of risk-benefit evaluation in medical experiments. Estimands are statistical quantities that are estimated during the study and are critical for optimizing treatment decisions. They incorporate various factors including efficacy, toxicity, and patient-reported quality of life.
Moreover, the presence of software tools has simplified the development and execution of dose-optimization experiments, incorporating intricate statistical models and utility functions to assist in medical decision-making. These models take into account the probability of adverse effects, therapeutic response, and other outcomes as functions of the dose or treatment schedule and patient-specific factors.
With the continuous evolution of research and technology, the approaches employed in Phase 1 assessments are also progressing, enabling more intricate and patient-centered methods for drug advancement and safety evaluation.
Phase 2: Efficacy and Side Effects
Phase 2 clinical evaluations are a critical step in the development of new medical treatments, where the focus shifts toward assessing the treatment's effectiveness in a more diverse patient population. These experiments are crucial as they provide initial data on effectiveness and further clarify side effects observed during initial testing. In these experiments, researchers often focus on specific patient groups to gain insights into how well the treatment works within the intended demographic, and refine dosing regimens based on collective responses. Furthermore, Phase 2 experiments are crucial in highlighting ethical, legal, and social considerations, ensuring that the advancement of medical technologies proceeds with a conscientious understanding of the implications across various sectors. The management of these experiments is carefully organized around guiding questions and historical contexts, which offer a comprehensive perspective of the technology's trajectory and its societal impact over time. For instance, a study focused on cachexia in cancer patients demonstrated the potential of a targeted treatment to improve body weight, muscle mass, and overall quality of life, marking a hopeful advancement for this debilitating condition. Such experiments not only promote medical knowledge but are also regulated by frameworks that take into account market incentives, intellectual property, and a multitude of other factors that guide the development of emerging medical technologies. The data acquired from these experiments is crucial for stakeholders, including healthcare professionals, investors, and patients, who depend on this information to make informed decisions about the future of healthcare products and investments.
Phase 3: Larger-Scale Safety and Efficacy Studies
Phase 3 clinical studies are crucial evaluations that rigorously assess the safety, efficacy, and overall benefit-risk balance of the investigational product. Carried out on a significant scale, these experiments generally involve a diverse group of participants distributed across different geographic locations. The experiments are designed to provide conclusive evidence that is crucial for regulatory bodies when considering the product for approval and subsequent market authorization. By validating the product's efficacy, continuously monitoring potential side effects, and determining its impact on patient health outcomes, Phase 3 studies play an essential role in ensuring that only beneficial and safe medical advancements reach the public domain.
Such experiments take into account various factors, including market incentives and intellectual property rights, which can greatly impact the development of emerging technologies and treatments. Moreover, the ethical, legal, and social implications of these technologies are examined through case studies and historical contexts, providing a comprehensive understanding of their development and governance across sectors. These case studies often feature vignettes and guiding questions to illustrate the nuanced ethical issues prevalent in today's medical landscape.
The importance of Phase 3 studies is further underscored by the fact that there are over 2,000 ongoing clinical investigations seeking to enroll individuals with diabetes alone, exploring a wide array of interventions from new insulins to telehealth services. The experiments cover a range of new therapies, including medications that could be granted orphan-drug status, giving them exclusive approval for seven years after being approved by the FDA. This exclusivity is granted to promote the advancement of drugs for rare diseases or conditions, acknowledging the unique challenges in bringing such treatments to market.
Staying updated on the newest advancements in medical experiments, such as those reported by Medical Device News Magazine, is crucial for medical professionals, industry executives, investors, and patients. Staying informed enables stakeholders to make educated decisions and contribute to the advancement of medical technology and patient care. The collaborative effort of volunteers, researchers, and regulatory agencies in Phase 3 trials is a testament to the collective endeavor to improve health outcomes and introduce innovative treatments to the healthcare system.
Special Considerations
In medical research, navigating the regulatory landscape is crucial, especially when it comes to Investigational New Drugs (INDs). The Food and Drug Administration (FDA) plays a pivotal role by ensuring the safety, purity, and potency of drugs and biological products. For example, the FDA's latest ultimate recommendation on public health emergencies emphasizes the requirement for a risk-based assessment in medical experiments, dealing with practical concerns such as investigational product shipment and site monitoring. This reflects the agency's adaptive approach post-pandemic, focusing on protecting participants while maintaining the integrity of the experiment.
The complexity of regulations extends to dietary supplements and conventional foods, which are also under the FDA's purview. When clinical experiments involve endogenous compounds or live organisms, researchers must adhere to specific guidelines, ensuring that such experiments do not compromise safety or ethical standards. The FDA's guidance documents, although not legally binding, offer current thinking on a myriad of topics, including early food safety evaluations for new plant varieties and non-pesticidal proteins.
Moreover, the FDA's role in orphan-drug designation illustrates the complexity of regulatory considerations, as it involves exclusive approval periods and implications for subsequent sponsors. The assessment of promotional materials for prescription drugs also falls under the FDA's jurisdiction, ensuring that terms and phrases used are clear and do not mislead healthcare providers or the public.
Recent advancements like the DAPA-MI study demonstrate innovative trial designs that merge registry data with randomized controlled trials, aiming to draw causal inferences while leveraging real-world data. Such studies emphasize the changing nature of medical research and the significance of adhering to regulations to ensure ethical conduct and patient safety.
The FDA's comprehensive vaccine assessment process, which spans the entire lifecycle of vaccine development, is another testament to the agency's commitment to public health. This continuous scrutiny dispels myths such as the unfounded link between vaccines and autism, reaffirming the importance of evidence-based medicine.
Comprehending these diverse regulatory considerations is not a choice but crucial for researchers and sponsors aiming to navigate the experiment landscape effectively. It ensures that the research conducted adheres to the highest standards of quality and safety, ultimately benefiting public health.
Role of the Regulatory Authority in IND Review
When a new investigational application (IND) is submitted to the FDA, it undergoes a rigorous assessment process. The FDA carefully examines the application to guarantee the safety and effectiveness of the experimental drug for possible human participants in studies. This requires a comprehensive analysis of the data provided, the structure of the proposed experiments, and a meticulous evaluation of the investigational product's expected advantages versus its potential dangers. The approval of an IND is contingent on meeting the FDA's stringent criteria, which focus on protecting study participants and maintaining the scientific integrity of the research. Moreover, the FDA's guidance on these applications is advisory, offering developers an understanding of the agency's current perspectives without imposing legally binding obligations, unless specific legal or regulatory requirements are referenced. The procedure reflects a wider pattern in medical advancement, where postponements are prevalentâup to 80% of medical experiments do not finish on time. Given the competitive landscape, where being first to market can be crucial, the FDA's role is critical in facilitating timely and responsible drug development. However, the data submitted for FDA approval may not always satisfy the needs of other decision-makers, such as payors and healthcare providers, potentially leading to delays in device coverage and reimbursement post-approval. In the United States, for example, the majority of Phase 3 experiments involve at least one local location, but healthcare professionals may find it difficult to take part in research studies due to existing incentive models that undervalue research activities. This situation is made worse by the high turnover of staff in healthcare, with approximately half of the principal investigators of research studies discontinuing their involvement. As a result, the FDA's evaluation is not only a pivotal step in the development of new therapies but also a complex intersection of scientific, economic, and regulatory considerations.
Ensuring Safety and Rights of Subjects
The comprehensive assessment of Investigational New Drug (IND) applications is a crucial stage in safeguarding participants in trials. The U.S. Food and Drug Administration (FDA) plays a pivotal role in assuring the safety, efficacy, and security of new therapies. As part of this responsibility, the FDA mandates rigorous ethical and regulatory standards, such as obtaining informed consent, which is not only a regulatory requirement but a fundamental patient right. This consent must be clear, concise, and must present key information that aids participants in understanding the study's purpose, potential risks, and benefits, as well as its duration and procedures.
Moreover, the safety of participants is continually monitored throughout the study, with an emphasis on transparency and prompt reporting of adverse events. This is not only to adhere to regulatory standards but also to uphold the integrity of the experiment and the trust of the participants. The recent FDA guidance also addresses logistical concerns and emphasizes the importance of a risk-based approach to monitoring research study sites and participants, ensuring a robust safety net is in place.
Clinical experiments frequently involve vulnerable populations, and the FDA has clearly specified consent and protection requirements for minors, pregnant women, fetuses, neonates, prisoners, and mentally impaired individuals. Protecting the rights of these groups is vital, and the FDA's regulations are intended to prevent exploitation and ensure that engagement in clinical research is a well-informed and voluntary choice.
Individuals can participate in experiments for different purposes, such as personal health advantages, altruistic intentions, or the progress of medical understanding. Irrespective of their motivations, the ethical conduct of a legal process and the welfare of its participants remain paramount. The Declaration of Helsinki and the FDA's recent initiatives highlight the evolution of ethical standards over the past 60 years, reflecting a commitment to protecting participant rights while facilitating medical advances.
In the context of research ethics, it is important to acknowledge the sacrifices made by participants in experimentation. As Daniel Wikler, a Harvard University philosopher, pointedly remarks, volunteers contribute significantly to societal health, and their welfare should not be disregarded when they face harm. This emphasizes the need for ethical oversight and the moral responsibility researchers and sponsors have towards participants in experiments.
Evaluating Scientific Quality
The scrutiny of the scientific underpinnings in the Investigational New Drug (IND) review process is paramount. Regulatory bodies, like the FDA, carefully evaluate the scientific worth of the proposed studies, with emphasis on elements such as study design, endpoints, and the statistical analysis plan. The assessment is not only about ticking boxes but ensuring the scientific approach is robust enough to yield reliable, reproducible, and significant results that contribute to the advancement of medical science and the enhancement of patient care. Utilizing frameworks like GRADE, adapted for context-specific evaluations, the quality of evidence is holistically rated against criteria such as innovation, rationale, and scientific rigor.
Moreover, the scientific quality is not assessed in isolation but is also gauged against the potential to address critical knowledge gaps and create valuable advances in the field. For instance, the consideration of whether the study fills an important void in understanding or solves a pressing problem is as crucial as the methodological soundness of the research itself. This thorough assessment guarantees that the experiment can endure examination and contribute to the broader objective of enhancing health results.
With the growing intricacy of experiments and the urgency to be the first to market due to phenomena like 'herding,' the significance of guaranteeing scientific quality becomes even more pronounced. McKinsey data highlights that clinical trials are taking longer, with Phase 3 trials extending from 41 to 44 months on average. With the high stakes involved, both in terms of patient need and market competition, the meticulous evaluation of scientific quality cannot be overstated. This evaluation must also be adaptable to changing regulations, such as those introduced by the US Inflation Reduction Act, which affects drug pricing and market dynamics.
The commitment to scientific excellence is underscored by the need for studies to be discoverable and scrutinized through peer review, ensuring that they contribute meaningfully to the body of medical knowledge. With this rigorous approach to evaluating scientific quality, healthcare professionals and researchers can continue to strive for the highest standards of research excellence, as mandated by institutions such as the Canadian Institutes of Health Research (CIHR).
Conclusion
In conclusion, the Investigational New Drug (IND) application is a crucial component in the drug development process. It undergoes meticulous review by regulatory bodies like the FDA to ensure the safety, efficacy, and scientific quality of proposed studies. Different types of INDs cater to various aspects of clinical research and patient care, such as commercial INDs for market introduction and research INDs for advancing scientific knowledge.
The IND review process involves a thorough evaluation of the application, including scientific merit, safety, and ethics. It plays a pivotal role in advancing drug candidates through clinical trial phases. Clinical trials, spanning Phase 1 for safety, Phase 2 for efficacy, and Phase 3 for larger-scale studies, establish the viability of investigational products and contribute to medical advancements.
Ensuring the safety and rights of participants is paramount. Informed consent, continuous monitoring of participant safety, and protection of vulnerable populations are key considerations. The FDA's regulatory standards and ethical guidelines provide a framework for upholding these principles.
Scientific quality is rigorously evaluated, considering study design, endpoints, and statistical analysis plans. This evaluation ensures reliable and significant results and addresses critical knowledge gaps.
The IND application and review process are essential for bringing new therapies to market, protecting patient safety, and advancing medical knowledge. It requires meticulous preparation, adherence to regulatory guidelines, and a commitment to scientific excellence. By navigating the complexities of IND applications, researchers and sponsors contribute to the development of innovative treatments that improve patient care and address unmet medical needs.




