Introduction
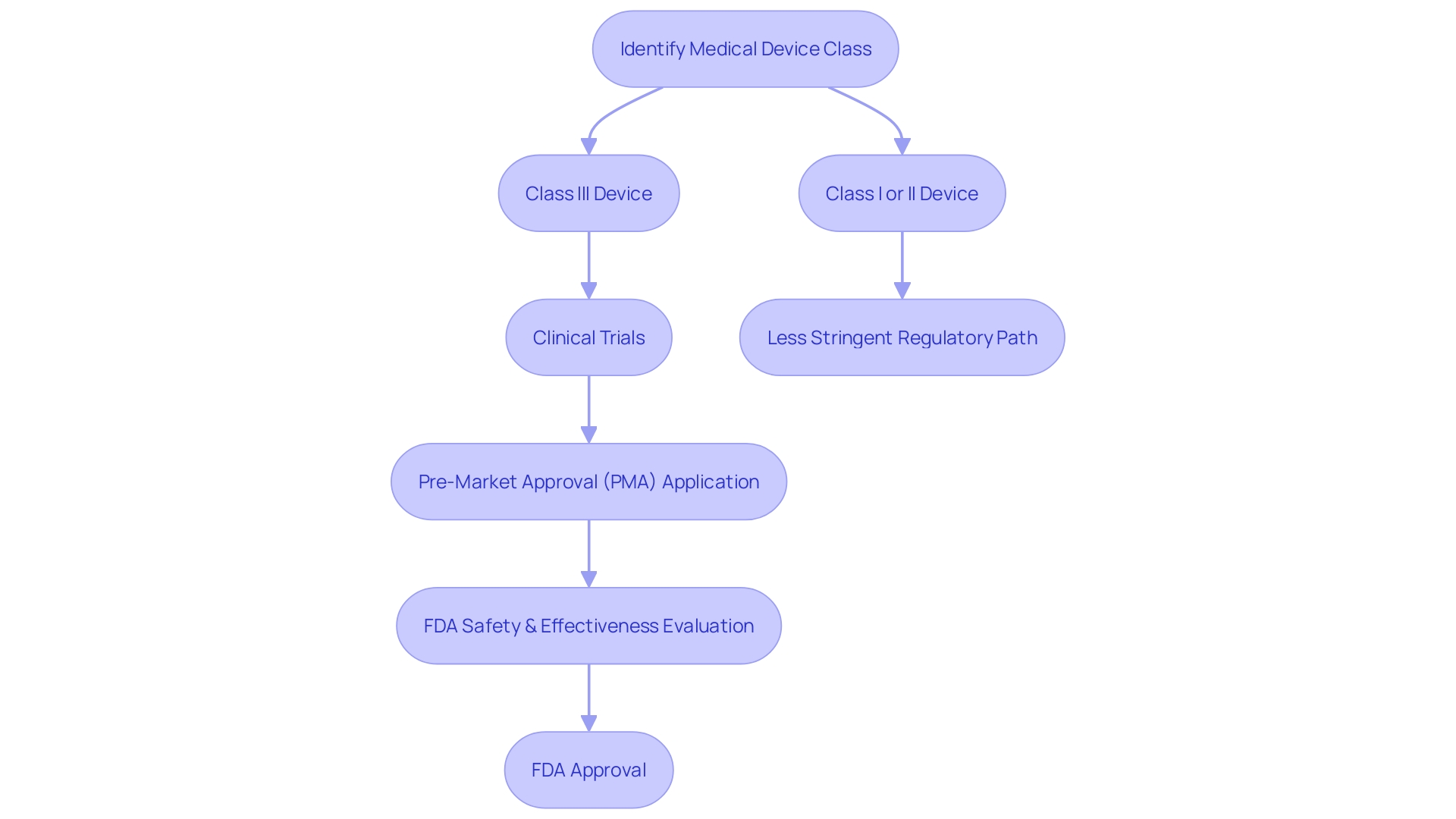
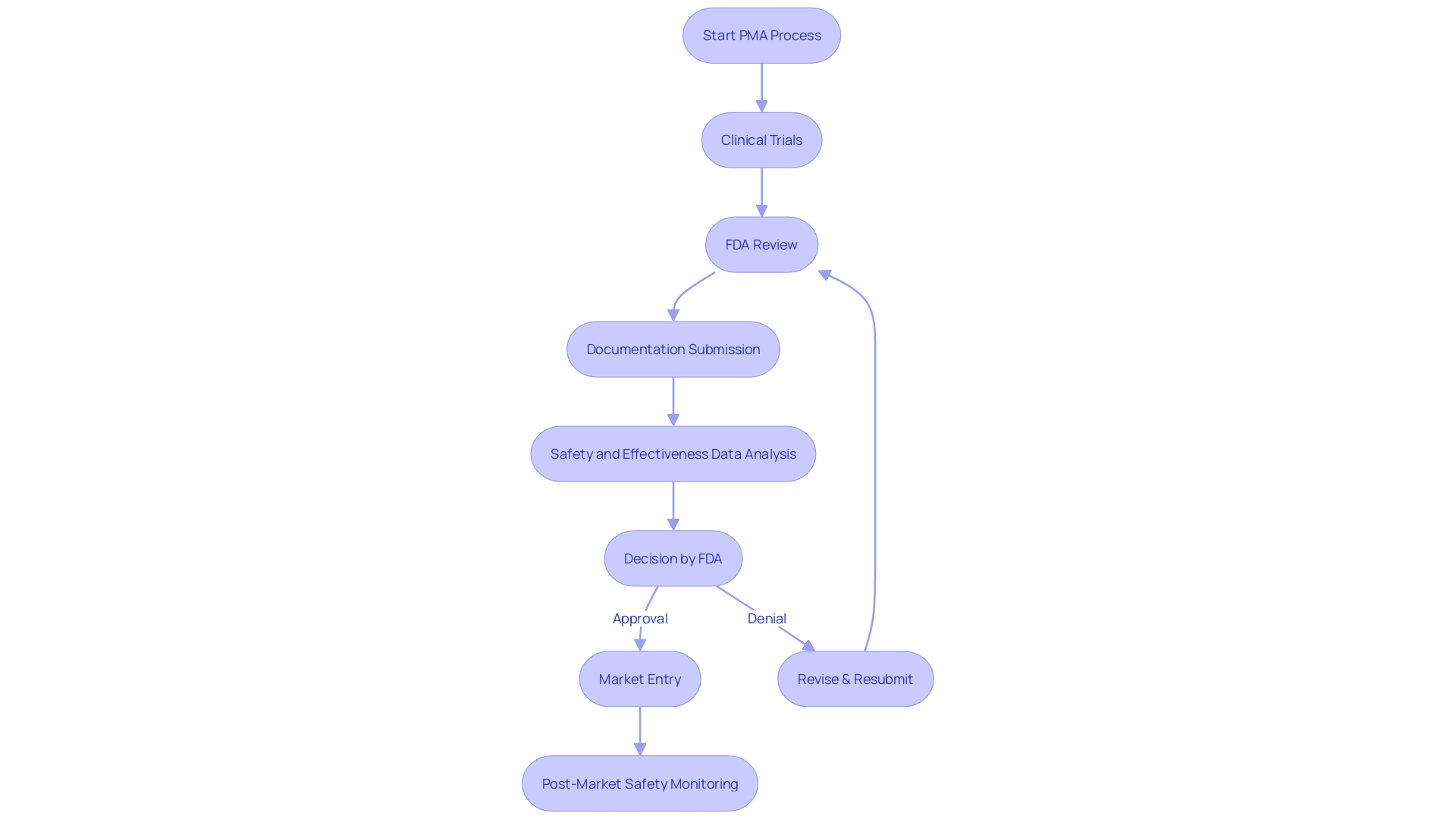
The Pre-Market Approval (PMA) process is a critical regulatory pathway enforced by the U.S. Food and Drug Administration (FDA) for high-risk medical devices. Unlike the 510(k) clearance, which allows for expedited review for devices that are substantially equivalent to a predicate, the PMA process requires manufacturers to provide substantial scientific and clinical evidence to demonstrate the safety and efficacy of their device. Devices that fall under the Class III category, representing about 10% of devices overseen by the FDA, typically go through this stringent PMA process.
They are subject to rigorous evaluation due to their potential to pose significant risk to patients, and as a result, often experience longer approval times. Before embarking on the PMA pathway, it's essential for manufacturers to thoroughly understand the device's intended use and user interaction, as well as to conduct a comprehensive analysis of the competitive landscape and predicate devices. The FDA's commitment to public health requires that any medical device, whether it aims to diagnose, prevent, or treat conditions, must adhere to the highest standards of safety and effectiveness.
The recent push for more streamlined regulatory pathways, particularly in the wake of COVID-19, reflects the ongoing efforts to address urgent medical needs without compromising on these rigorous standards.
Understanding the PMA Process
The Pre-Market Approval (PMA) pathway is a crucial regulatory pathway enforced by the U.S. Food and Drug Administration (FDA) for high-risk medical instruments, such as those that are life-sustaining or life-supporting. In contrast to the 510(k) clearance, which allows for expedited review for items that are substantially identical to a predicate, the PMA procedure necessitates manufacturers to provide substantial scientific and clinical evidence to demonstrate the safety and efficacy of the product. These items falling under the Class III category, which make up approximately 10% of the devices overseen by the FDA, typically undergo this stringent PMA process. They are subject to rigorous evaluation due to their potential to pose significant risk to patients, and as a result, often experience longer approval times.
Prior to starting the PMA pathway, manufacturers must have a deep understanding of the intended purpose and user engagement of the product, in addition to performing a thorough analysis of the competitive environment and previous models. The FDA's dedication to public health necessitates that any instrument, whether it aims to diagnose, prevent, or treat conditions, must adhere to the highest standards of safety and effectiveness. The recent push for more streamlined regulatory pathways, particularly in the wake of COVID-19, reflects the ongoing efforts to address urgent healthcare needs without compromising on these rigorous standards.
Key Features and Requirements of the PMA Process
Understanding and maneuvering through the FDA's regulatory landscape is an essential part of bringing medical products to market. Class III instruments, which encompass life-sustaining technologies like pacemakers, represent the highest risk category and necessitate a Pre-Market Approval (PMA) due to their significant role in patient health. This arduous process is necessary for approximately 10% of devices overseen by the FDA and involves comprehensive scrutiny, including clinical trials to ensure patient safety and equipment efficacy.
To start the PMA pathway, the precise categorization of the medical equipment is crucial. Once the risk level of the equipment is determined, manufacturers must show the safety and effectiveness of the product, often requiring extensive clinical data. This is different from less risky Class I and II products, which may qualify for the 510(k) clearance, a less strict procedure based on preceding products.
The FDA's commitment to public health extends beyond the realm of medical devices, as demonstrated by recent regulations that enhance the clarity and transparency of direct-to-consumer drug advertisements. These standards, mandating transparent, obvious, and unbiased presentation of a drug's major side effects, reflect the agency's dedication to informed patient decisions—a principle that resonates deeply within the healthcare equipment industry.
Project managers in this domain must integrate patient-centered considerations and regulatory knowledge to navigate this complex environment successfully. Striking a balance between project management skills and regulatory acumen, with a relentless focus on patient outcomes, is the cornerstone of a successful path to FDA approval.
Clinical Data
The Premarket Approval (PMA) process stands as a pivotal gateway for high-risk healthcare instruments, ensuring their safety and effectiveness before they can be marketed. Dissimilar to the 510(k) clearance, which is based on comparing to an existing product, the PMA procedure is founded on the thorough examination of clinical data. Such data is collected from carefully designed and carried out human clinical trials, which aim to clearly demonstrate the reliability of the apparatus and its ability to safely fulfill its intended medical requirement.
One illustrative example of this procedure is a spinal fusion apparatus, which obtained FDA approval supported by a persuasive clinical study covering 57 patients over a median duration of 30 months. The study yielded a notable 96% fusion rate, a figure independently verified by a core imaging laboratory, with 79% of patients reporting a significant reduction in pain. This emphasizes the PMA's essential function in protecting patient health by requiring a comprehensive demonstration of a product's benefit-risk balance.
The FDA classifies objects into three categories, with category three objects like pacemakers, which are crucial for maintaining life, going through the PMA process. While making up approximately 10% of FDA-regulated products, these high-risk interventions require a more extensive regulatory oversight. As the regulation governing healthcare equipment advances, there has been a concerted effort to streamline the approval pathways, particularly for tools that fulfill unmet medical needs or are at the forefront of innovation in areas like digital health.
To uphold the integrity of healthcare equipment after it is released in the market, Post-Market Surveillance (PMS) plays a vital role. It extends beyond pre-market testing to continuously evaluate and report on an object's performance in real-world scenarios. For example, passive and active monitoring systems, in addition to the utilization of electronic health records, provide valuable insights into the long-term safety and efficacy of healthcare instruments.
Regulatory agencies and industry stakeholders aim to accelerate approval procedures while upholding rigorous safety and effectiveness standards. The FDA, along with other international standards organizations, promotes creativity and uniformity, ensuring that new healthcare instruments meet the highest quality benchmarks and fulfill their intended roles in advancing patient care.
Risk Assessment
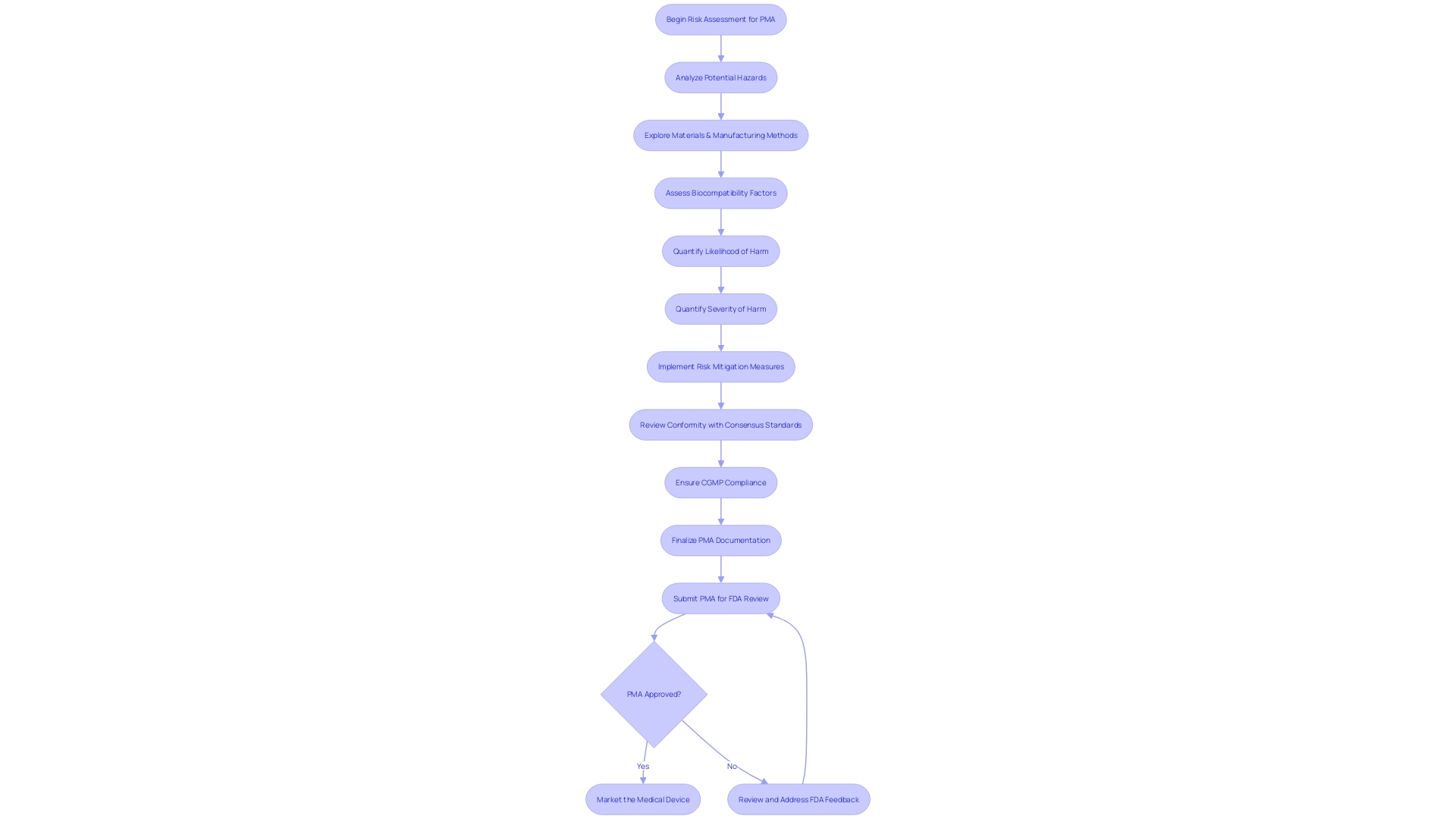
The pre-market approval (PMA) for medical instruments, particularly those falling under the high-risk Class III category, is a meticulous and stringent procedure aimed at guaranteeing patient safety and instrument efficacy. During this process, manufacturers must carry out an exhaustive risk assessment, critically analyzing potential hazards associated with the equipment's intended use. This entails an in-depth exploration of the materials, processing techniques, and manufacturing methods, in addition to assessing the geometry, mechanical forces, and surface properties that may affect biocompatibility.
Manufacturers must quantify the likelihood and potential severity of harm, drawing on a wealth of sources such as prior experience with similar materials, literature reviews, and data from other manufacturers. This all-encompassing strategy for managing risk is crucial, as highlighted by alarming data showing more than 1.7 million injuries and 83,000 fatalities in the US potentially linked to healthcare devices throughout a ten-year period.
Additionally, considering the COVID-19 outbreak accelerating automation and digitalization across various sectors, the incorporation of these technologies into assembly of healthcare equipment is not only expected but crucial. This evolution brings with it a complex regulatory landscape that manufacturers must navigate with precision. The incorporation of automation and AI within the sector is thus not just a matter of technological capability but also regulatory compliance.
According to Bijan Elahi, an experienced specialist in safety risk management for healthcare equipment, a comprehensive grasp of risk management is not only limited to familiarization with standards such as ISO14971, but also the utilization of practical recommendations and illustrations to navigate the intricacies of the field. The PMA process, therefore, is not only about ticking off a checklist but ensuring a product's safety profile through robust risk management strategies that align with regulatory expectations.
Manufacturing Controls
To guarantee the uniform excellence and effectiveness of healthcare equipment, producers must comply with strict manufacturing controls. This involves the development of quality management systems, which are vital in maintaining the high standards anticipated in the instrument industry. Regular inspections and adherence to good manufacturing practices (GMP) are also crucial elements of this framework, as they help to minimize the risk of failures or defects in the equipment.
The intricacy of the regulatory framework for healthcare equipment, especially with the integration of automation and AI, requires a comprehensive comprehension of legal obligations. As the COVID-19 pandemic has sped up the digitalization of industries, including healthcare, the significance of automation in the assembly of healthcare equipment has become increasingly crucial. Manufacturers must therefore ensure they have the appropriate tools and partnerships to navigate this complex environment effectively.
Furthermore, the FDA has specified current good manufacturing practice (CGMP) requirements in their quality system regulation, which governs the methods and controls used for the design, manufacture, packaging, labeling, storage, installation, and servicing of all finished products intended for human use. These requirements are designed to guarantee the safety and effectiveness of medical instruments, in accordance with the Federal Food, Drug, and Cosmetic Act.
Errol Erturk, Vice President of Engineering and Product Development for Life Sciences, emphasizes the importance of starting with a company's requirement specification. This initial step is crucial in determining the targeted manufacturing performance and ensuring the successful implementation of automation technologies.
Nevertheless, the credibility of the manufacturing can be jeopardized if untrustworthy data from third-party test labs are submitted to the FDA. The organization has noticed a concerning pattern of submissions containing made-up or untrustworthy information, which obstructs the authorization procedure and ultimately affects patient availability to new gadgets and the stability of gadget supply chains.
Finally, manufacturers must consider the OECD Due Diligence Guidance for Responsible Supply Chains of Minerals from Conflict-Affected and High-Risk Areas, particularly when dealing with materials such as tin, tantalum, and tungsten, to avoid contributing to conflict through their sourcing practices. Comprehending the particular market and regulatory environment for each product is vital, as certain regulations are limited to individual regions. Therefore, maintaining global regulatory compliance is often the most strategic approach for manufacturers aiming to mitigate the risks associated with changing market strategies and regulations.
Labeling and Instructions for Use
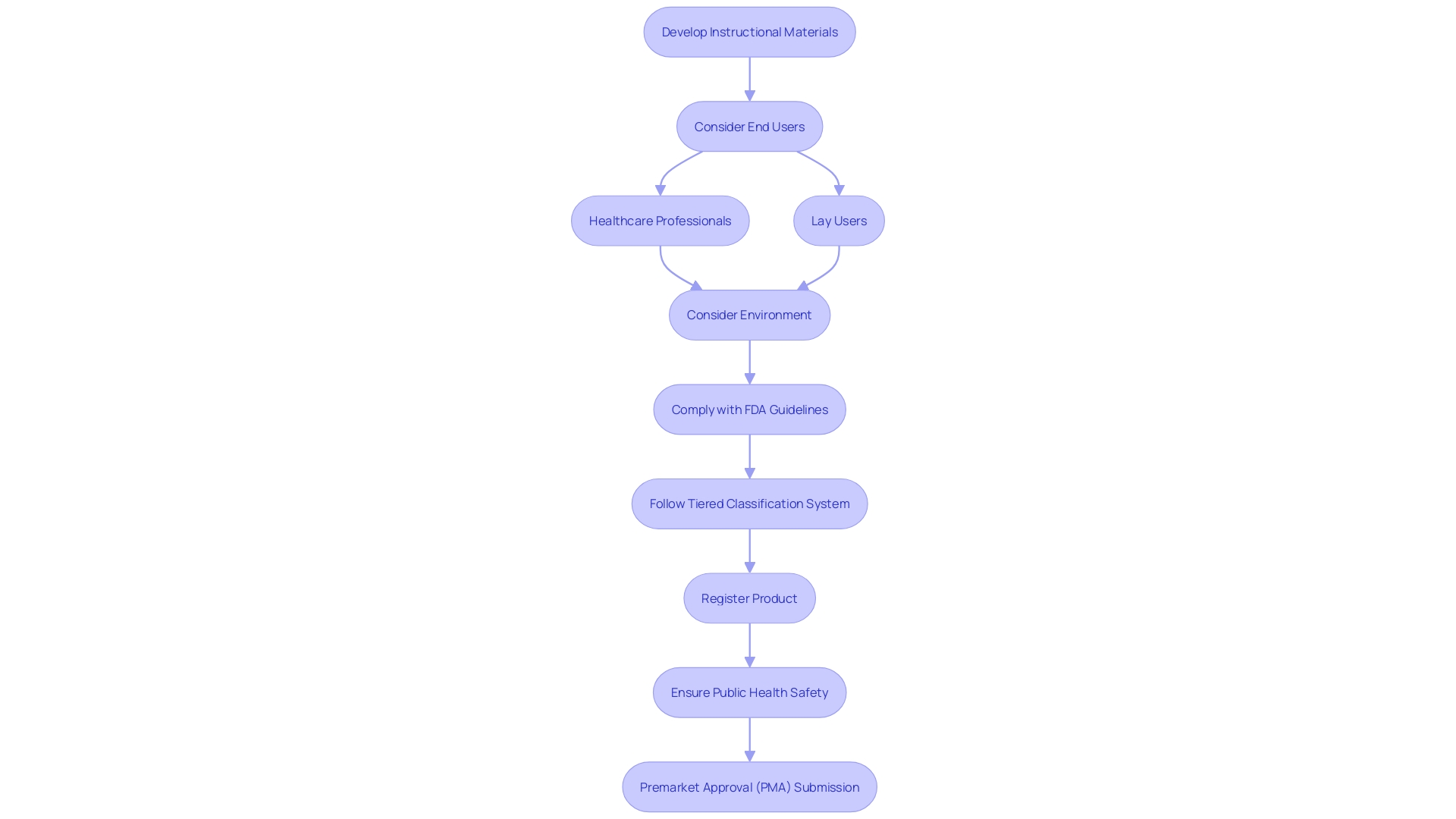
Ensuring that healthcare equipment arrives with concise, detailed labeling and instructions for utilization (IFU) is a vital element of the Premarket Approval (PMA) submission procedure. To protect the well-being of the general population and ensure the proper utilization of healthcare instruments, the FDA requires that these substances sufficiently provide information regarding the intended purpose, indications, contraindications, warnings, and precautions, while also presenting clear instructions for the operation and upkeep of the instrument. When developing these instructional materials, it's essential to consider the end users—whether healthcare professionals or lay users—and their environments, as these factors dictate both the format of the instructions and the complexity of the content.
Healthcare providers such as surgeons, nurses, and medical technicians, despite their higher education and training, may encounter stress, fatigue, and time constraints when using healthcare instruments, necessitating instructions that are easily accessible and comprehensible even under demanding circumstances. On the other hand, lay users, including patients and caregivers, require information that addresses their varying levels of health literacy and accommodates any health conditions they may have.
The FDA's watchfulness in overseeing the safety and effectiveness of healthcare equipment is demonstrated by recent findings where third-party test labs have produced unreliable data, which has resulted in significant consequences for equipment approvals and market access. This emphasizes the significance of integrity and accuracy in every facet of the PMA procedure, encompassing the creation of IFUs.
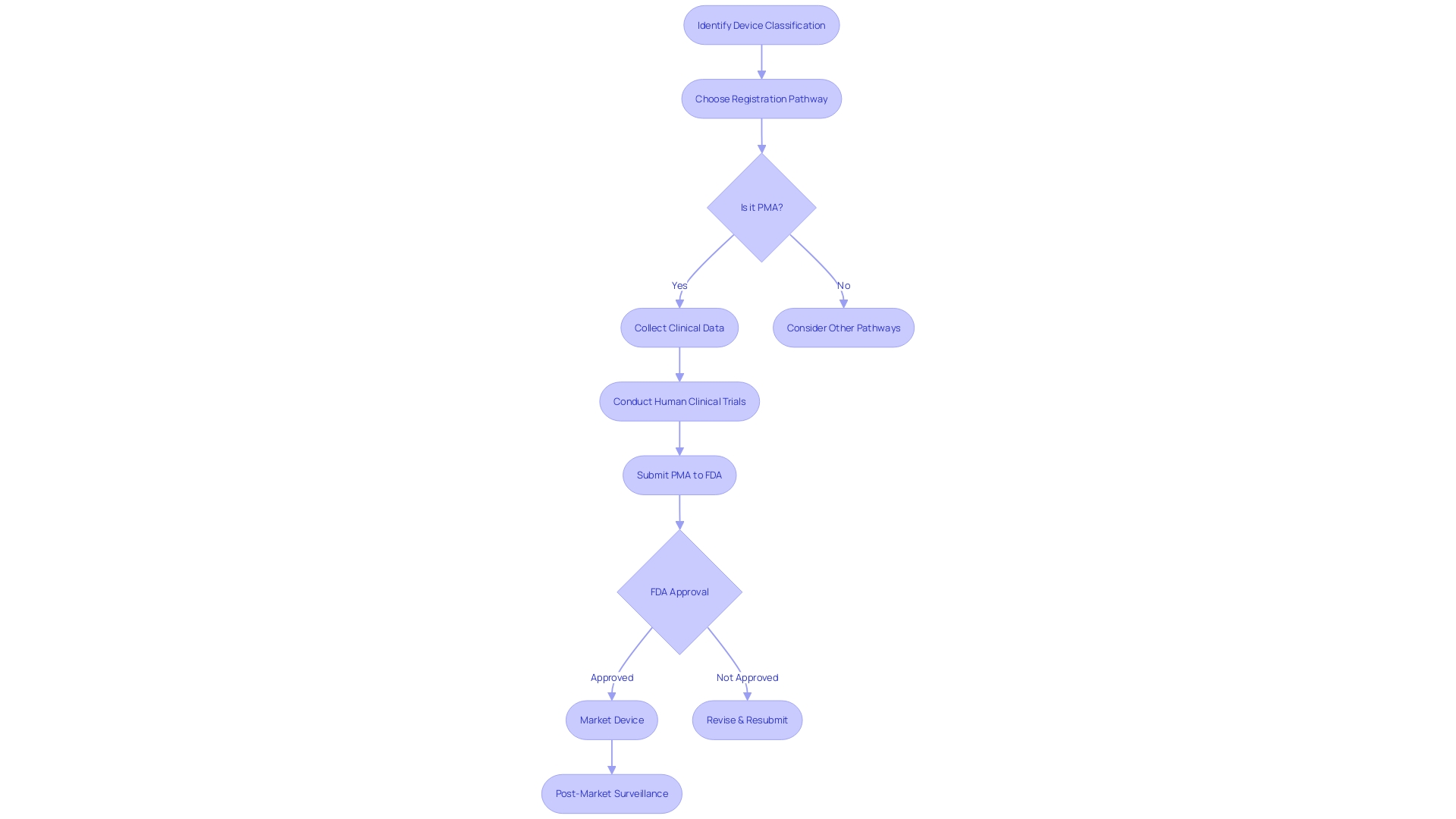
As healthcare instruments differ in intricacy and hazard, the FDA utilizes a tiered classification system to ascertain the suitable registration pathway. Whether it is a 510(k) premarket notification, a PMA, or the De Novo process, the chosen pathway must align with the classification and risk profile of the product to ensure that, once on the market, it is considered safe and effective for patient use.
Considering these factors, manufacturers are encouraged to give priority to creating user-friendly instructional materials that comply with FDA guidelines, thus promoting the secure and efficient utilization of healthcare equipment and furthering the overall objective of the FDA to safeguard public health.
Differences Between PMA and 510(k)
Comprehending the categorization and the governing route for healthcare equipment is crucial for manufacturers. The FDA classifies medical equipment into three categories based on the level of control required to ensure the safety and efficacy of the product. Class I objects are considered low risk and are subject to the least regulatory control, while Class III objects are high risk, supporting or sustaining human life, and are subject to the highest level of regulatory control.
Class III products, which include life-sustaining implants like pacemakers, often require a Pre-Market Approval (PMA), the most stringent type of item marketing application required by the FDA. The PMA procedure varies greatly from the 510(k) notification, which is commonly used for Class I and II equipment. The 510(k) is a premarket submission that shows that the product to be marketed is at least as safe and effective, that is, substantially equivalent, to a legally marketed item that is not subject to PMA.
The PMA evaluation includes a comprehensive scientific and regulatory assessment to assess the safety and efficacy of Class III medical devices. Unlike the 510(k) pathway, where a reference tool is used for comparison, PMA applications must include scientific evidence, often including clinical trial data, to support claims of safety and effectiveness. This strict procedure guarantees that patients are given groundbreaking life-saving technology that has been carefully assessed.
The FDA's responsibility extends beyond just ensuring safety and effectiveness; it also includes assuring the security of medical equipment. Manufacturers should be well-informed of the different pathways and the expectations for each to navigate the regulatory landscape effectively. As depicted in the documentary 'The Bleeding Edge', the 510(k) process has been examined for allowing products to be expedited without necessitating clinical trials in certain instances, resulting in concerns regarding patient safety.
Consequently, manufacturers must strive for a profound comprehension of the apparatus and its intended utilization, encompassing user needs and the competitive landscape. This entails constructing a comparative table for potential substitute devices and performing a gap analysis with contract manufacturers to guarantee preparedness for production.
In summary, while the 510(k) and PMA are both FDA regulatory pathways, they are applicable to different product classes and involve distinct levels of scrutiny. Manufacturers must select the appropriate pathway based on classification, which has implications for the time and resources needed to bring a product to market.
Level of Evidence
Understanding the distinct pathways for FDA approvals and what evidence is necessary for each is crucial in navigating the regulatory landscape. The Premarket Approval (PMA) process is considerably more stringent than the 510(k) notification, focusing on a comprehensive demonstration of a product's safety and effectiveness. Unlike the 510(k) route, which determines if a new product is substantially equivalent to an existing one (a predicate), PMA involves a thorough examination of clinical data. This often includes pivotal studies, which are critical in determining whether a Class III deviceâthose with the highest risk to patientsâcan be deemed safe and efficacious for its intended use.
In the case of PMA, the evidence doesn't solely rest on pre-market studies. Post-market surveillance (PMS) plays a crucial role in continuously monitoring medical equipment once they are in use by the public. This stage is crucial for identifying potential safety concerns that may not have been evident during pre-market testing and for ensuring that equipment continues to function as anticipated over time. The procedure of PMS utilizes a range of techniques, including passive surveillance systems such as spontaneous reporting and active surveillance through registries, leveraging electronic health records and administrative databases to collect essential safety and performance data in real-world settings.
The significance of these regulatory differences was emphasized in the 2018 documentary 'The Bleeding Edge,' which disclosed that not all gadgets undergo the same level of examination before reaching the market. In reality, specific equipment have been expedited through the 510(k) pathway without the requirement for clinical experiments, resulting in patient harm in certain instances. These revelations highlight the importance of rigorous clinical data and post-market monitoring in ensuring safety of the equipment.
With the ongoing progress of healthcare technology, the FDA's categorization system and endorsement procedures guarantee the introduction of products with a thorough comprehension of their risk profiles and the essential supervision to safeguard patient safety. The PMA pathway, with its focus on comprehensive clinical evidence, represents a crucial stage in confirming that high-risk healthcare tools are both secure and advantageous for their intended purposes.
Time and Cost
The Premarket Approval (PMA) pathway, while more intricate and time-consuming than the 510(k) clearance, is a vital measure in ensuring the safety and effectiveness of Class III medical instruments, which include life-sustaining implants like pacemakers. Manufacturers are required to perform extensive clinical trials, which are frequently a crucial stage in proving the safety and efficacy of a product. The trials, in addition to the comprehensive documentation and regulatory submissions needed, contribute to the increased costs linked with the PMA procedure. Class III products, constituting approximately 10% of the items supervised by the FDA, cannot be launched into the market without this thorough assessment, which is designed to prioritize patient safety above everything.
Comprehending the purpose of the healthcare equipment, its users - including clinicians and patients - and the competitive environment are essential for manufacturers maneuvering through the PMA journey. This requires a careful comparison of the new equipment to existing ones, ensuring it meets the rigorous criteria for a unique healthcare tool that can greatly enhance patient outcomes. The classification system of the FDA highlights the different levels of examination that devices undergo depending on their potential risks, with the PMA procedure being designated for those with the greatest risk to patients, thus requiring longer approval periods. However, this thoroughness is in the pursuit of delivering cutting-edge technology that is not only groundbreaking but also secure and dependable, ultimately improving the quality of life for patients.
Device Modifications
The FDA's PMA pathway ensures that high-risk medical instruments adhere to stringent safety and effectiveness standards before they are available in the market. In contrast to the more relaxed 510(k) clearance, which permits specific modifications after clearance without extra FDA approval, the PMA procedure necessitates manufacturers to acquire approval for any notable alterations to an apparatus. This includes modifications that impact the safety or effectiveness of the apparatus, necessitating a new PMA submission or a separate approval process. For example, the change in classification of ultrasound cyclodestructive instruments from class III to class II was influenced by feedback that supported less strict regulations, aiming to enhance patient availability of these instruments once they have been demonstrated to be safe through initial more stringent measures.
The FDA's vigilance in regulating medical equipment modifications is reflected in recent statements, emphasizing the agency's commitment to public health and safety. This regulatory framework ensures that any significant changes undergo rigorous review to maintain the integrity of the approval process. As such, manufacturers must navigate these regulations wisely, anticipating the need for continued compliance and potential submission of new PMA applications to accommodate significant alterations to the equipment.
Understanding the FDA's classification system is crucial for manufacturers, as it dictates the regulatory pathway required for market entry. The three-level classification system is based on the risk posed to patients, with class III instruments, like those requiring PMA, representing the highest risk. The difference between FDA terms like Registered, Cleared, Approved, and Granted is important, and regulatory professionals must be knowledgeable in these nuances to ensure correct classification and selection of regulatory pathway.
In the ever-changing realm of regulations concerning healthcare instruments, forward-thinking statements regarding business strategies, product effectiveness, and expected authorizations have a crucial impact. However, they carry inherent uncertainties, and the industry is advised to exercise caution, recognizing the potential for variations in actual outcomes based on emerging risks and assumptions. The FDA's function goes beyond healthcare equipment to a wide array of public health obligations, further emphasizing the significance of adhering to its comprehensive regulatory standards.
Choosing the Right Regulatory Pathway
Choosing the appropriate regulatory route for a medical instrument is essential, considering different factors like the instrument's objective, technical characteristics, and level of risk. For example, in the United States, the FDA categorizes equipment into three classifications, reflecting the level of risk they present to patients. Lower risk equipment (class one and two) might be suitable for the 510(k) clearance, where a comparison is made to an existing, approved apparatus. Conversely, higher risk instruments (class three), which are frequently essential for supporting life, such as pacemakers, require a more extensive Premarket Approval (PMA) procedure.
It's crucial to investigate and comprehend the particular regulations for the markets in which the product will be sold, as some are distinctive to certain regions, and adherence to global standards can be a complex endeavor. The swift progress in healthcare technology, such as digital health tools and personalized medicine, have prompted regulatory bodies to streamline approval pathways, reducing time to market for critical innovations.
Recent initiatives, such as the UK's new regulatory roadmap for healthcare equipment, stress the safety of patients and strive for increased international alignment. The plan pledges to strengthen the UK's status as a prominent ecosystem for technology innovation in healthcare, guaranteeing patients' availability to new tools without unwarranted delays. This approach to regulation reflects a broader shift towards accommodating rapid technological progress while maintaining rigorous safety standards.
Essentially, deciding whether to opt for a 510(k) clearance or a PMA pathway for healthcare instruments requires a comprehensive grasp of the instrument's classification, an in-depth examination of its intended purpose and technological characteristics, and a thoughtful evaluation of the regulatory landscapes in the desired markets. This, coupled with the industry's dynamic regulatory atmosphere, makes it imperative for manufacturers to stay informed and adaptable.
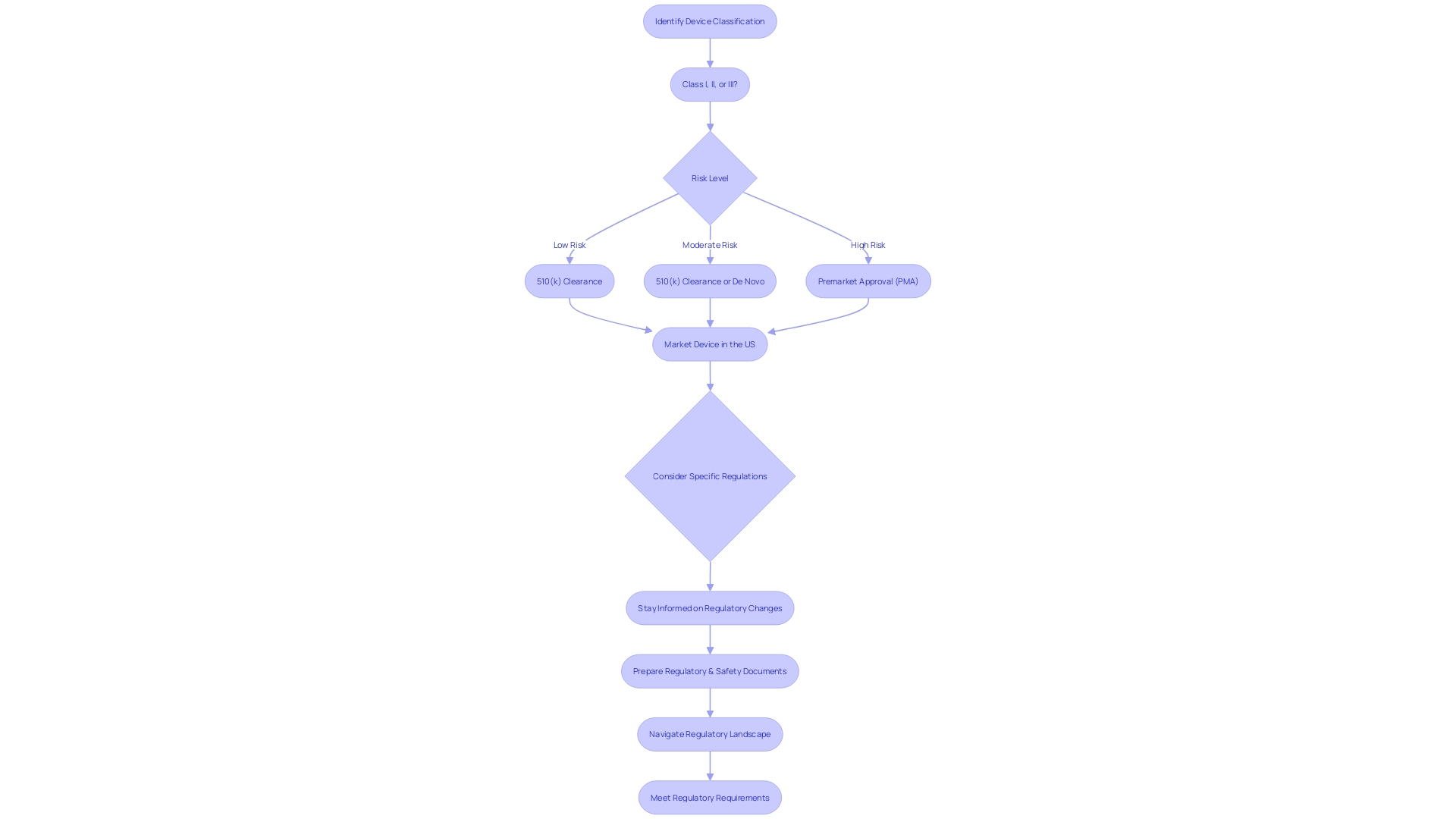
Factors to Consider When Deciding Between PMA and 510(k)
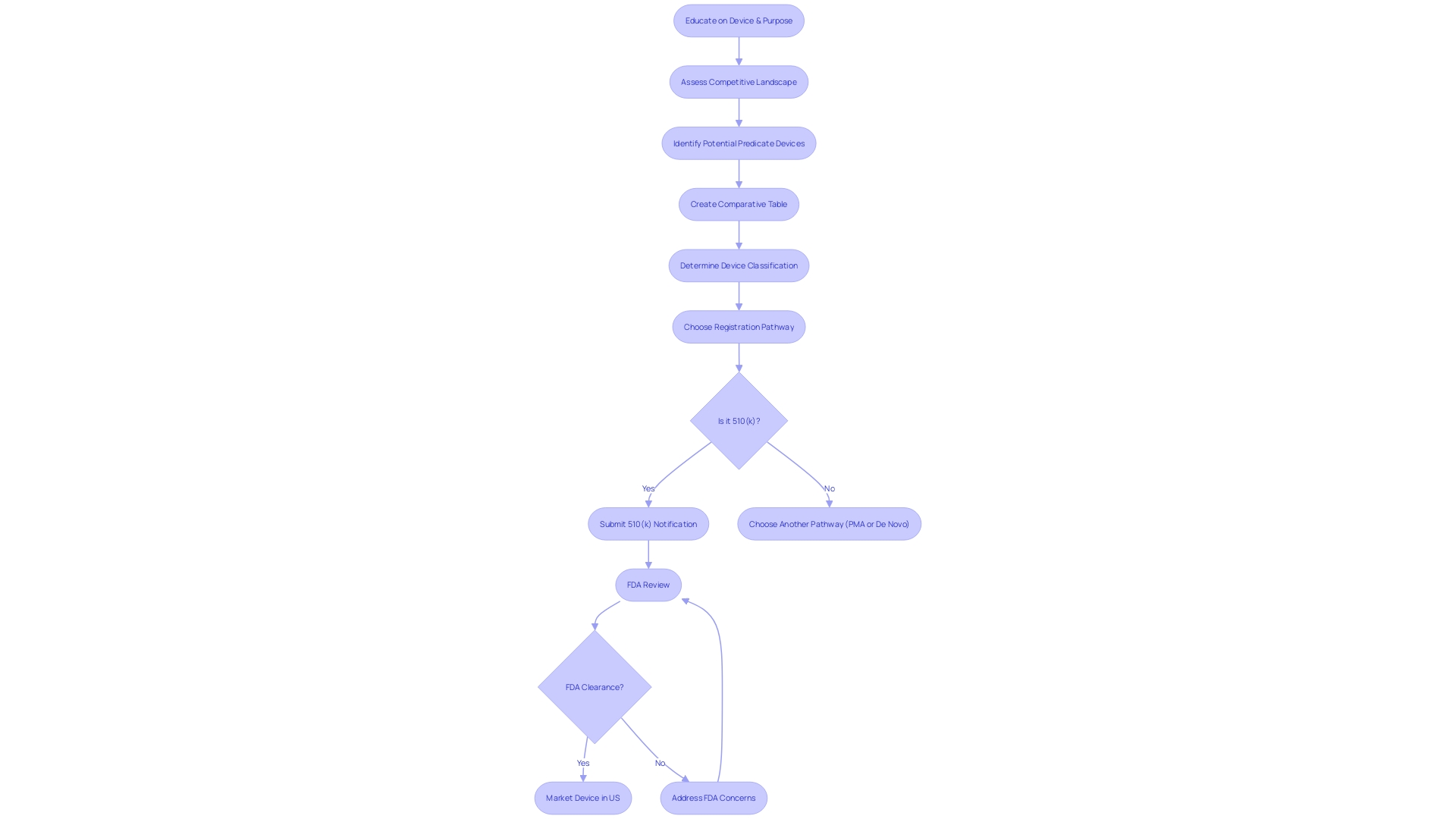
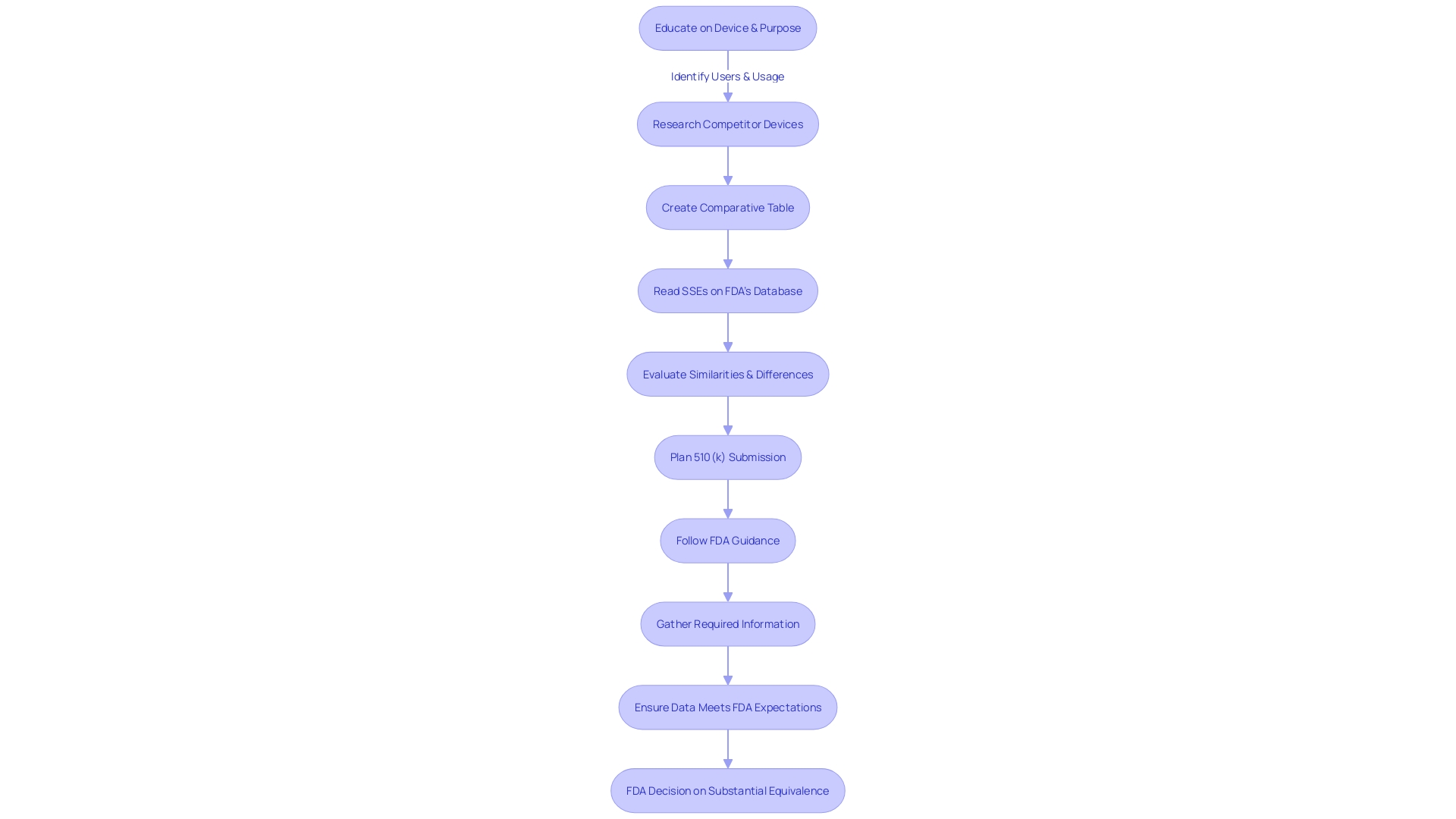
To make an informed decision between pursuing the Pre-Market Approval (PMA) process and the 510(k) clearance for medical instrument regulation, manufacturers must consider a multitude of factors. Comprehending the purpose of the instrument and its users, including clinicians and patients, is essential. Manufacturers should explore the competitive environment, examining rival products through accessible research literature, clinical studies, and marketing materials to identify potential precedent items with similar intended uses and technological characteristics.
For Class III products, which encompass those essential for sustaining life like pacemakers, the regulatory demands are more strict, representing approximately 10% of the items overseen by the FDA with longer approval durations. On the other hand, Class I and II products may be appropriate for the 510(k) process, which permits quicker market access by showing substantial similarity to a previously cleared product.
Recent regulatory updates highlight the ongoing transformation in the medical equipment approval landscape. For example, the MHRA in the UK has provided a roadmap for new regulations targeting patient safety, timely access to equipment, and improved integration with global standards. This includes the recognition of approvals from international bodies such as the FDA, which could influence strategic decisions for market entry.
In light of these advancements, it is crucial for manufacturers to be adaptable, shaping their expectations and production processes to market feedback without overwhelming the initial launch. This approach ensures a safe and effective product is brought to market, with the potential to scale up production based on demand and success.
Ultimately, the selection between PMA and 510(k) pathways is nuanced, necessitating a profound comprehension of the product, its market, and the developing regulatory environment. By prioritizing patient safety and quality, manufacturers can navigate this complex terrain, aiming for successful outcomes that might range from acquisitions to strategic alliances.
Device Complexity and Novelty
For medical instruments that are highly complex or integrate novel technologies, the Pre-Market Approval (PMA) pathway is often the most suitable registration option. Items that belong to this classification are typically those that are regarded as high-risk, such as Class III devices, which are vital in sustaining or supporting life. These equipment necessitate a thorough assessment to guarantee their security and efficiency. The FDA's PMA procedure is intended to meticulously evaluate these categories of instruments through a thorough examination that involves the submission of clinical trial data, guaranteeing a high degree of safeguard for patients. The PMA is the FDA's most stringent type of marketing application and is required for products that represent a significant risk of illness or injury or that are deemed not substantially equivalent to already legally marketed items. This procedure is crucial for upholding patient safety and promoting advancement in the healthcare equipment sector, enabling the implementation of cutting-edge healthcare technologies that can greatly enhance patient results.
Available Data
The decision between PMA (Premarket Approval) and 510(k) clearance for medical instruments rests on the extent of clinical data accessible. Devices backed by robust clinical evidence affirming their safety and effectiveness are often steered towards the PMA pathway. On the contrary, products that are capable of demonstrating substantial equivalence to an existing, legally marketed predicate item might find the 510(k) process more fitting.
Medical instruments in the U.S. are categorized by the FDA into three risk-based classifications. Class I and II products, considered lower risk, may be eligible for 510(k) clearance, which involves comparing the new item to a predicate. However, Class III objects, which include life-sustaining implants like pacemakers, must undergo the more stringent PMA process due to their high-risk nature.
The FDA's established regulatory pathways aid in swiftly introducing novel technologies to the market. A prime example is the Apple Watch's heart monitor, which garnered FDA clearance in 2018 for its low-risk functionality in detecting irregular heart rhythms.
Regulatory strategies must consider the global reach of the product, adapting to diverse rules that can be country-specific. For items with broader distribution, global regulatory compliance may be more practical given the dynamic nature of market strategies and regulations.
Moreover, recent initiatives to simplify regulatory routes and promote cooperation between agencies and industries, particularly in times of the COVID-19 crisis, have sought to expedite approvals for products addressing pressing healthcare requirements. The efficiency of regulatory processes and the intricacy of the equipment contribute to different approval timelines across regions.
The importance of active postmarket surveillance is emphasized by FDA data connecting over 1.7 million injuries and 83,000 deaths in the U.S. to healthcare instruments over a decade. This surveillance is critical in identifying and addressing safety issues that may not be reported during initial approvals.
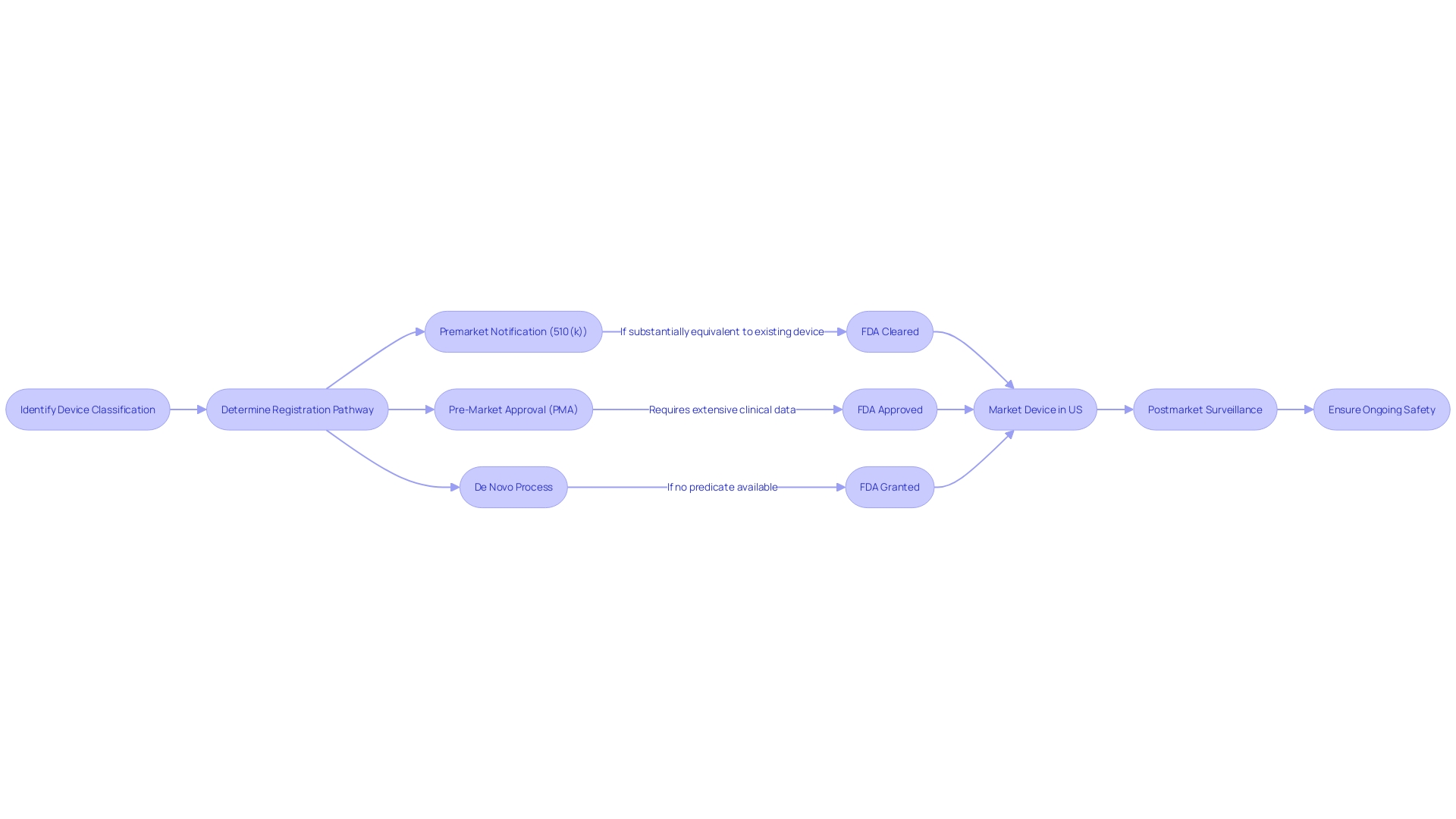
Benefits and Drawbacks of Each Pathway
Selecting the appropriate regulatory route for healthcare equipment is a crucial choice that producers must make, taking into account the advantages and difficulties involved. The Food and Drug Administration (FDA) in the US categorizes medical equipment into three risk-based categories. Class I and II devices, which present lower risks, may qualify for the 510(k) clearance pathway, permitting comparison to an established, legally marketed product, known as a predicate. This procedure can frequently be faster and more economical than the stricter Pre-Market Approval (PMA) needed for Class III equipment, which consist of life-sustaining or implantable tools such as pacemakers.
However, the PMA procedure, while more rigorous and time-consuming, ensures a higher level of scrutiny, which can be crucial for patient safety and product efficacy. The PMA involves a comprehensive evaluation of the design, manufacturing, and intended use of the product to demonstrate its safety and effectiveness.
The challenge remains to balance the need for stringent regulatory oversight to ensure safety and efficacy against the desire for a more streamlined pathway that can bring innovations to market more swiftly. Good Manufacturing Practice (GMP) standards, compliance with international standards such as ISO 13485, and FDA regulations form the backbone of the qualification process. Despite the intricacy and potential for lengthy approval times, these regulatory measures are crucial for maintaining high-quality medical equipment.
Manufacturers should also be well-informed about their product, its users, and the competitive landscape. A thorough comprehension of the purpose of the apparatus, the pertinent instructions for use, and any warnings or cautions are crucial. Moreover, recognizing possible predicate instruments with comparable intended application and technological features can assist in navigating the regulatory proceedings.
Lately, there has been a drive for more effective regulatory routes, particularly for products that tackle unaddressed health requirements. The COVID-19 pandemic has further emphasized the requirement for flexibility in regulatory procedures. Streamlining approvals while maintaining high safety and efficacy standards remains a major focus for both regulatory agencies and industry stakeholders.
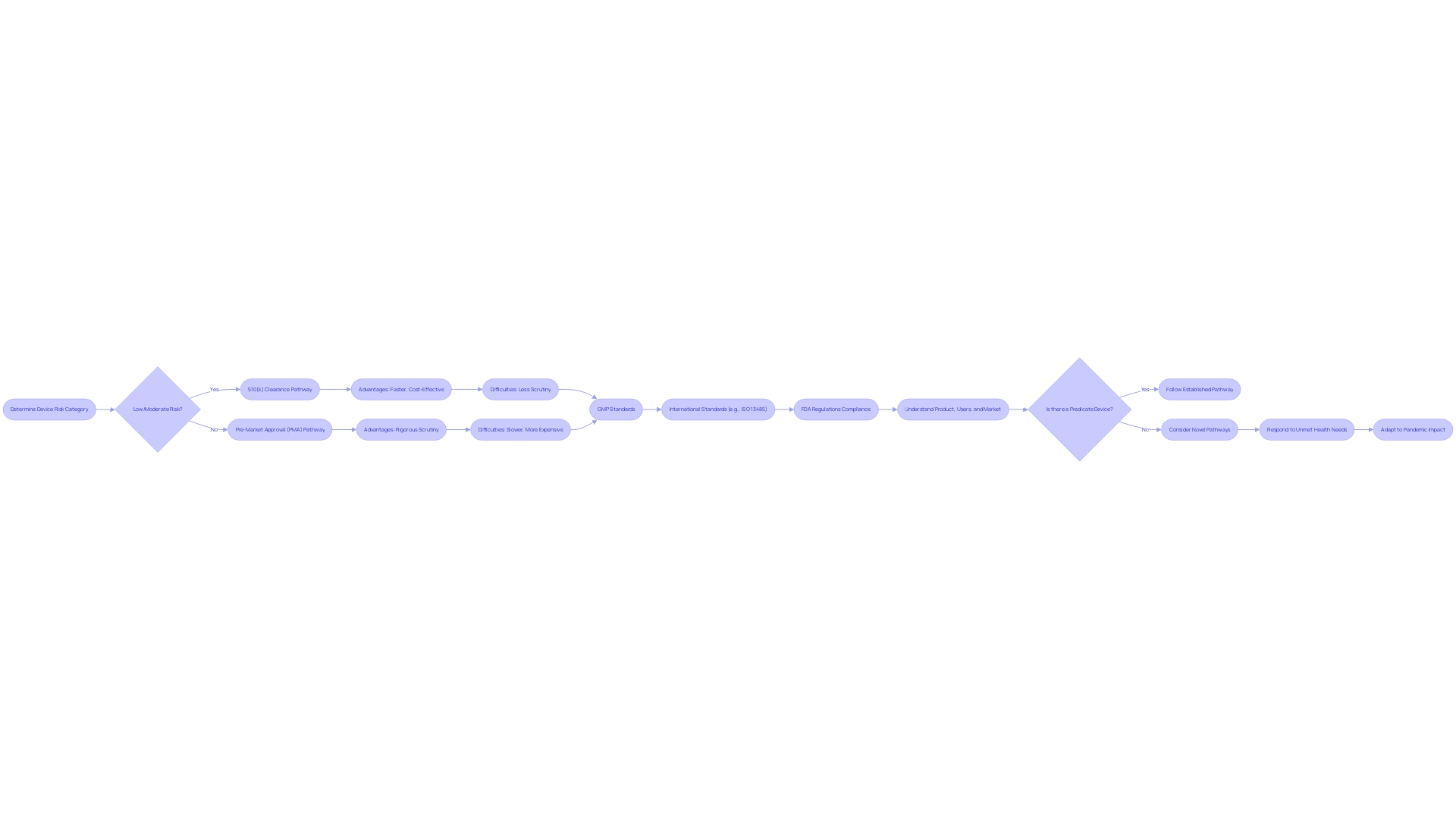
PMA Process Benefits
The Premarket Approval (PMA) process is known for its rigorous evaluation of high-risk medical equipment, such as those used for life-sustaining purposes or that incorporate groundbreaking technologies. By requiring extensive clinical data, PMA offers healthcare professionals and patients with a higher level of assurance in the safety and effectiveness of the apparatus. For instance, the Impella Connect System, which includes both software and hardware components for critical cardiac support, had to demonstrate its reliability through notifications and status updates that are vital for patient-specific information and timely alarms. These characteristics categorize it as an object under section 201(h) of the act, thus necessitating PMA. Another regulatory pathway, the De Novo classification, is available for fresh gadgets without a legally marketed predecessor, which, after approval, helps establish a new regulatory category and serves as a predicate for future gadgets. The FDA's responsibility in classifying products into three categories, with Class III being the highest risk, emphasizes the significance of comprehensive evaluation for items with a significant impact on patient health. It's essential for companies to understand these regulatory nuances and integrate them with traditional project management skills focused on patient safety and product quality, as emphasized by industry professionals.
PMA Process Drawbacks
The Pre-Market Approval (PMA) process for Class III health instruments, which includes critical life-sustaining products like pacemakers, is known for its rigor. These equipment undergo the most rigorous FDA review due to their significant role in healthcare and potential risk factors. As they constitute a small but crucial portion of FDA-regulated items, accounting for approximately 10% of regulated products, the path to market is typically lengthy. For manufacturers, this extended timeline is a result of the meticulous clinical trials and thorough documentation necessary to ensure patient safety and product efficacy. Though these steps are critical for maintaining high standards of care, they inevitably lead to increased development costs and delayed entry into the market. This effect is felt across the industry, impacting the strategic planning and resource allocation within organizations dedicated to the advancement of healthcare technology.
(k) Process Benefits
In the case of medical equipment, the 510(k) clearance pathway is a quicker way to bring products to market, typically used for items that have minimal variations from already existing products, referred to as predicate devices. This regulatory approach is critical as it allows for innovation and the introduction of new technologies while maintaining a focus on safety and efficacy. Understanding the competitive landscape and identifying a precedent instrument are crucial first steps in this process. By comparing the new product to one already in use, manufacturers can create a comparative table to illustrate substantial equivalence.
The FDA classifies healthcare equipment into three categories, with Category I and II usually qualifying for 510(k) approval. This procedure is considerably less time-consuming than the Pre-Market Approval (PMA) necessary for Class III equipment, which encompass life-sustaining products such as pacemakers. The PMA procedure is more rigorous due to the higher risks associated with these instruments, which comprise approximately 10% of all FDA-regulated medical equipment. In spite of the efficiency of the 510(k) procedure, the significance of thorough education regarding the use, users, and potential risks of the equipment cannot be emphasized enough.
Recent examination, like the 2018 documentary 'The Bleeding Edge,' has emphasized that certain products expedited through the 510(k) procedure have resulted in patient injuries, emphasizing the importance of maintaining a equilibrium between swift market entry and patient safety. In response, the FDA has been exploring enhancements, such as predetermined change control plans for AI- and ML-enabled healthcare instruments, to improve compliance while fostering innovation.
In the field of medical equipment, market success can be unpredictable, requiring a focused approach to the initial launch. A successful strategy involves streamlining the production process to deliver a safe and effective product without overcomplicating the launch. As the market evolves and the product proves successful, production can then be scaled accordingly.
(k) Process Drawbacks
Understanding the complex classifications and pathways set forth by the FDA is crucial when navigating the regulatory landscape for medical technologies. Medical equipment is classified into three different categories, representing different levels of risk to patients. Identifying the categorization of the equipment is a crucial initial stage, as it determines the subsequent route for registrationâwhether it is the 510(k) Premarket Notification, Premarket Approval (PMA), or De Novo pathway. Each pathway serves a distinct function, with the 510(k) procedure enabling a faster route to the market by demonstrating substantial equivalence to a legally marketed prototype.
Nevertheless, the dependence on predicate instruments in the 510(k) procedure can have both positive and negative effects. While it enables manufacturers to leverage existing safety and effectiveness data, it may inadvertently stifle innovation. It's crucial to acknowledge that not all equipment cleared through this pathway are supported by extensive clinical trial data; a fact emphasized in the 2018 documentary 'The Bleeding Edge,' which brought to attention cases where the absence of mandatory clinical trials led to severe patient injuries.
The FDA has acknowledged the need for modernization within this framework. In response, they've introduced initiatives aimed at refining the review process, including the development of best practices for selecting predicates. These efforts are encapsulated in a draft guidance document released in September 2023, emphasizing the importance of selecting a legally marketed instrument with the same intended use and comparable technological characteristics that do not raise new safety or effectiveness questions.
As professionals in the field of healthcare technology, it is our responsibility to thoroughly comprehend the instruments we work with, including their intended use, the clinical and competitive landscape, and any potential risks. By creating a comparative table of predicate instruments and analyzing their Summaries of Safety and Effectiveness, we can make informed decisions that prioritize patient safety while navigating the complexities of FDA clearance.
In light of these challenges, the FDA's continuous push for regulatory clarity and efficiency is crucial. It is a reflection of the agency's commitment to protecting public health while fostering innovation—a balance that is paramount in the evolving field of medical device development.
Conclusion
The Pre-Market Approval (PMA) process is a stringent regulatory pathway enforced by the FDA for high-risk medical devices. It requires manufacturers to provide substantial scientific and clinical evidence to demonstrate device safety and efficacy. Devices falling under Class III typically undergo this rigorous process, which involves longer approval times.
Manufacturers must thoroughly understand the device's intended use, analyze the competitive landscape, and prioritize safety and effectiveness. The FDA's commitment to public health necessitates stringent regulatory pathways, even in the face of COVID-19, to address urgent medical needs without compromising on safety.
Choosing the right regulatory pathway depends on factors like device complexity, available clinical data, and risk assessment. The PMA process offers benefits but can be time-intensive and costly. The 510(k) clearance pathway allows for faster market entry but may limit innovation and require careful consideration of patient safety.
Manufacturers must stay informed, prioritize safety, and comply with global standards to navigate the regulatory environment successfully. By understanding pathway requirements and considering device classification, manufacturers can make informed decisions, ensuring safe and effective medical device use and supporting the FDA's mission to protect public health.




