Introduction
In the realm of clinical trials, Serious Adverse Events (SAEs) are untoward medical occurrences that pose a significant threat to a participant's life or well-being. These events, which can result in severe outcomes like hospitalization or disability, are meticulously monitored through systems like the Vaccine Adverse Event Reporting System (VAERS). VAERS exemplifies the importance of surveillance mechanisms in clinical trials, ensuring the safety and trustworthiness of medical interventions.
Evaluating the seriousness of SAEs involves considering the severity of the event, resulting consequences, and the need for medical intervention. The complexity of determining SAEs is exemplified by real-world cases and underscores the necessity for strategic safety assessments. Accurate reporting of SAEs is not only a regulatory obligation but a scientific imperative that contributes to transparency, reproducibility, and the collective understanding of medical safety.
Regulatory bodies and investigators play crucial roles in reporting SAEs, upholding participant safety, and maintaining the integrity of clinical trials. Best practices for documenting and reporting SAEs involve meticulous data collection, standardized forms, quality control measures, and a culture of safety and transparency. By adhering to these practices, researchers contribute to the advancement of medical knowledge while protecting the well-being of study participants.
Definition of Serious Adverse Events
In the realm of clinical trials, a Serious Adverse Event (SAE) encompasses any untoward medical occurrence that is both unexpected and grave, posing a significant threat to a participant's life or well-being. Such events may lead to dire consequences, including death, hospitalization, the onset of disability, or other severe outcomes. The criticality of SAEs in clinical research is underscored by systems like the Vaccine Adverse Event Reporting System (VAERS), a key component of the U.S. national vaccine safety framework. VAERS's robust mechanisms for monitoring vaccine safety epitomize the vigilance required in clinical trials, drawing from a multitude of peer-reviewed studies and expert testimonies to identify and investigate potential safety issues.
Safety monitoring is an integral aspect of evaluating medical interventions and their implications on public health. In the context of vaccine evaluation, for instance, the effectiveness and potential risks of a product are inextricably linked to the specific 'Indication' for which the product is developed. This connection shapes the entire development plan, with market need and size being paramount considerations for leading pharmaceutical companies. The VAERS system, having effectively pinpointed safety concerns, demonstrates the importance of such surveillance mechanisms in maintaining the integrity and trustworthiness of clinical research. As the landscape of clinical trials evolves, continuous efforts are made to enhance transparency and data sharing, thereby advancing the collective understanding of sales and their management.
Criteria for Identifying Serious Adverse Events
Evaluating the seriousness of an adverse event in clinical research hinges on specific criteria. These criteria include not only the severity of the event but also the resulting consequences, such as hospitalization, an extension of existing hospital stays, or persistent and significant disabilities. Moreover, the necessity for medical intervention to stave off severe outcomes is equally considered. For instance, the Vaccine Adverse Event Reporting System (VAERS) serves as an integral part of the national vaccine safety system in the United States, ensuring that vaccines' risks are meticulously monitored. VAERS allows various stakeholders, including patients, healthcare providers, and pharmaceutical companies, to report medical events following vaccination. This system has been vital in identifying safety issues, which sometimes lead to changes in clinical recommendations or even halting vaccine administration.
The significance of thorough planning for the safety assessment of drugs cannot be overstressed. This involves meticulous planning to evaluate key risks during confirmatory trials to enhance the quality and reliability of the safety data. Such an approach necessitates precise definitions of key risks and detailed plans for their ascertainment. The challenge of analyzing adverse outcomes extends beyond mere proportions of affected participants, requiring a nuanced approach that accounts for treatment discontinuations and data from multiple trials.
An exemplar of the complexity of determining serious adverse events (SAEs) is the case of Maddie, a participant in the Pfizer Covid-19 vaccine trial for 12-to-15-year-olds. Maddie's severe illness post-vaccination led to questions about the trial's transparency and the handling of her case by Pfizer and the FDA. This incident underscores the necessity for rigorous safety monitoring in clinical trials, especially when they involve high stakes, such as vaccine development during a global pandemic.
With the introduction of the new Clinical Trials Information System (CTIS) in the EU, transparency in clinical trials has been significantly enhanced. The CTIS ensures the earlier availability of information about authorized trials, benefiting patients and healthcare professionals. This transparency is critical, as the cost of bringing a new drug to market is approximately $1 billion, factoring in the high failure rate of clinical trials. The new system eliminates the deferral mechanism that allowed trial sponsors to delay publishing certain trial data, thereby contributing to an environment where safety data is more readily accessible and scrutinizable.
Types of Serious Adverse Events
Adverse events in clinical trials can range from mild to severe, with serious adverse events (SAEs) being particularly concerning due to their potential to cause lasting harm or even death. Examples of Sales include but are not limited to acute hypersensitivity reactions, organ dysfunction, critical cardiovascular incidents, neurologic complications, and severe responses to pharmaceutical products. A profound understanding of Sales is imperative for their prompt detection and subsequent reporting.
One such case that illustrates the significance of recognizing and addressing SAEs involved a young woman named Velaye, whose encounter with a severe allergic reaction was chronicled on social media. After dyeing her hair black at 18, Velaye experienced such intense swelling that within four days, her eyes were completely shut, a stark depiction of the potential severity of allergic reactions as Sales. This incident echoes similar reports, including those of individuals who suffered fatal allergic reactions from food products, highlighting the critical nature of Sales in both clinical and everyday contexts.
The complexity of SAEs is further underscored by news from the pharmaceutical industry, where companies like Fulcrum Therapeutics grapple with the challenges of advancing drug candidates through clinical trials. These trials are fraught with risks and uncertainties, including adverse reactions that can lead to trial modifications or even discontinuations, emphasizing the need for meticulous safety assessments and planning.
Experts in the field stress the importance of strategic planning for safety evaluations in confirmatory trials to bolster the quality and reliability of collected safety data. VAERS, the Vaccine Adverse Event Reporting System, stands as a testament to the efficacy of such proactive safety monitoring systems, demonstrating a robust track record in identifying vaccine-related safety concerns and reinforcing the indispensability of SAE vigilance in clinical trials.
As the medical community continues to innovate and evaluate new treatments, it is imperative to uphold the integrity of safety monitoring systems and ensure the thorough investigation of all adverse events to safeguard the wellbeing of patients and maintain public trust in healthcare advancements.
Importance of Reporting Serious Adverse Events
The criticality of reporting serious adverse events (SAEs) in clinical trials cannot be overstated. This process is a cornerstone in safeguarding patient well-being and maintaining the integrity of research. By meticulously documenting Sales, researchers and sponsors provide regulatory authorities with vital data that informs the ongoing evaluation of investigational drugs or interventions. It's essential for ensuring that the risks to future participants are minimized and that the study's direction is correctly shaped by the emerging safety profile of the intervention.
The landscape of clinical research has seen a surge in industry involvement, which has brought about a call for greater transparency in clinical trials. Despite many trials being registered in databases like ClinicalTrials.gov, the information is often insufficient for comprehensive transparency. Full disclosure would entail making trial protocols, statistical analysis plans, and raw data publicly available. However, the reality is that many industry-sponsored trials are analyzed internally, and the raw data remain inaccessible to the broader scientific community. This situation underlines the importance of accurate and inclusive reporting of SAEs, as it contributes to the overall transparency and reproducibility of clinical research.
Recent initiatives aimed at enhancing data sharing in clinical trials underscore the need for improvement. For instance, the International Committee of Medical Journal Editors' push for data statements in journal publication policies is a step towards more open access to raw data. Yet, the current implementation remains less than ideal, with many trial results not available in the public record. A study reviewing Canadian clinical trials from 2009 to 2019 found that many results were not publicly documented, emphasizing a gap in reporting that affects the reliability of clinical research.
Patients, who bear the brunt of the challenges associated with clinical trial participation, are the human face behind these statistics. Their commitment, often amidst managing multiple health conditions, is driven by the hope of advancing treatment options for future generations. This dedication further highlights the ethical imperative for transparent and timely reporting of SAEs, as it directly impacts the safety and trust of trial participants.
In response to these challenges, regulatory bodies like the European Medicines Agency (EMA) are enhancing features in their CTIS public portal to improve user-friendliness and accessibility. Such updates aim to strike a balance between transparency and the protection of commercially sensitive information, ultimately serving patients, sponsors, and healthcare professionals alike by providing early access to key clinical trial information.
In conclusion, the reporting of SAEs is a fundamental aspect of clinical research that extends beyond regulatory compliance. It is a moral obligation to the participants and a critical element for the credibility and advancement of medical science. As we continue to navigate the complexities of clinical trials, the role of accurate SAE reporting remains a beacon for ethical and effective medical research.
Components of a Serious Adverse Event Narrative
In the meticulous process of clinical trial management, documenting serious adverse events (SAEs) is paramount. An SAE narrative is a methodical recount of an adverse incident that a participant incurs, which is pivotal for post-trial evaluations. The narrative encompasses a participant's demographic details, medical antecedents, concurrent medications, and a thorough account of the adverse incident. Additionally, it includes pertinent laboratory data, diagnostic results, therapeutic measures undertaken, and the eventual outcome of the event. The Vaccine Adverse Event Reporting System (VAERS) serves as an exemplar of an early-warning mechanism that underscores the importance of such meticulous documentation in monitoring vaccine safety. VAERS, through its systematic collection and analysis of data, has been instrumental in identifying safety signals and informing subsequent clinical recommendations. This system has been a key component in maintaining public trust and ensuring the safety of medical interventions.
In the context of vaccine safety and adverse event reporting, the FDA's recent initiative to enhance the clarity and visibility of major side effects in direct-to-consumer prescription drug advertisements is a significant development. This effort aims to present the major risks of drugs in a way that is transparent and easily comprehensible to the public, which mirrors the ethos of thorough SAE narratives in clinical trials. Such initiatives reflect the ongoing commitment of health authorities to safeguard public health through rigorous safety monitoring and transparent communication.
It is through the lens of these anecdotes, gathered from a multitude of healthcare professionals and patients, that we glean insights into the real-world implications of adverse events. The aggregation of these experiences, far from being dismissed as mere anecdotes, constitutes a powerful narrative that informs and shapes our understanding of medical safety. As we continuously evolve our methods of capturing and analyzing SAEs, it is important to recognize the value of both formal reporting systems and the informal communication channels that pervade the healthcare landscape. Collectively, they form a robust network that enhances our ability to respond to and prevent adverse outcomes, thereby ensuring the efficacy and safety of medical treatments for all.
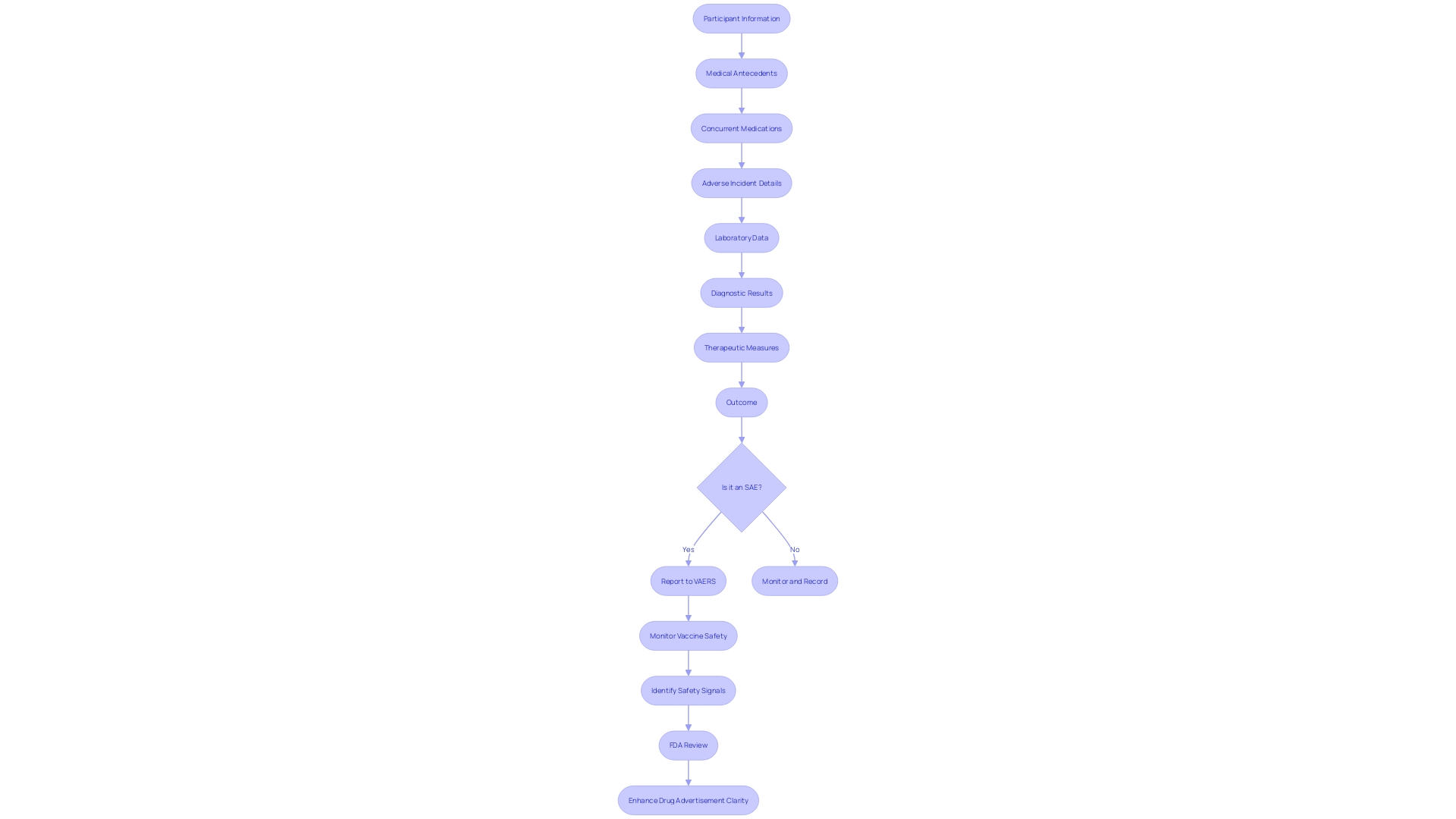
Guidelines for Writing a Serious Adverse Event Narrative
Documenting serious adverse events (SAEs) in clinical trials is a meticulous process that must adhere to stringent guidelines to furnish a clear, objective, and comprehensive narrative. These narratives serve as crucial documents for regulatory bodies like the FDA, ensuring the safety and efficacy of new treatments and interventions. For instance, consider a patient in a placebo-controlled trial for lecanemab, an Alzheimer's disease medication, who experiences severe headaches post-infusion, leading to days in bed, and subsequent memory impairment described as brain fog. This case highlights the necessity of recording Sales in a systematic manner, detailing the chronological sequence of events without subjective interpretation or speculation.
To foster transparency and accountability, the FDA requires that all comments and adverse event reports submitted by stakeholders are made accessible to the public. This open approach emphasizes the importance of excluding any confidential information from these narratives. Factual presentation of such narratives, without personal identifiers or protected health information, is essential for upholding patient privacy while contributing to the collective understanding of a drug's safety profile.
In the context of vaccine safety, systems like the Vaccine Adverse Event Reporting System (VAERS) exemplify the critical role of SAE reporting. VAERS's track record in identifying vaccine-related safety concerns underscores the value of rigorous adverse event documentation practices, which are universally applicable across all clinical trial phases.
Furthermore, statistical rigor is paramount when documenting SAEs. For instance, authors are advised to forgo the presentation of intermediate analytical steps that offer limited insight and instead focus on multivariable analyses that accurately adjust for confounding factors. Such meticulous attention to detail in the statistical analysis ensures that the reported results are reliable and verifiable.
To enhance the reach and impact of clinical research findings, it is advisable for authors to craft their manuscripts with discoverability in mind. The choice of title, keywords, and abstract plays a pivotal role in the accessibility of the document through search engines. Similarly, the clarity and detail of an SAE narrative can significantly affect its utility for clinicians, researchers, and regulatory agencies.
In summary, the construction of an SAE narrative is not merely a regulatory obligation but a scientific imperative that requires precision, clarity, and a commitment to contributing to the broader knowledge base in healthcare.
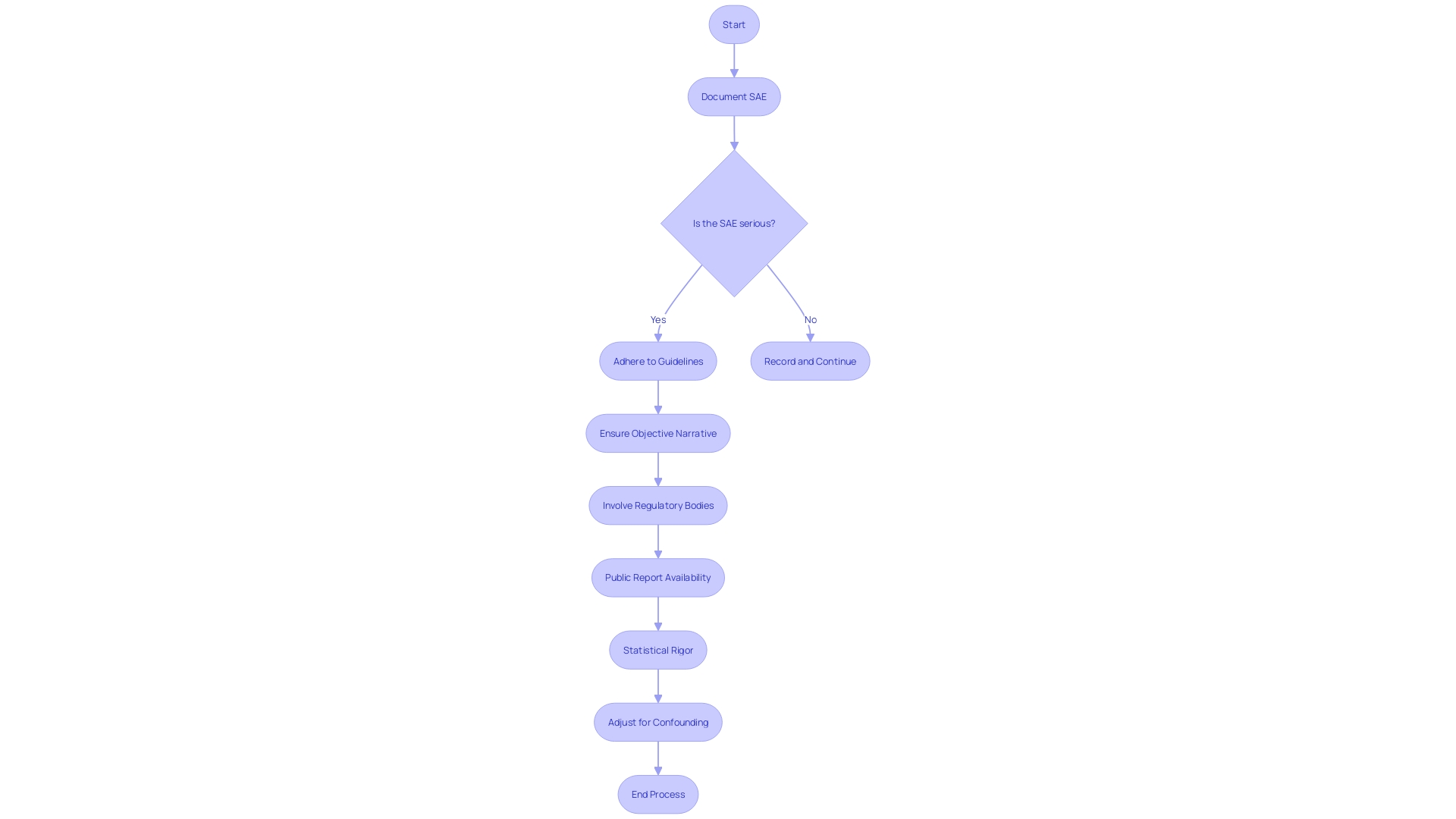
Regulatory Requirements for Reporting Serious Adverse Events
Within the realm of clinical research, the vigilant reporting of serious adverse events (SAEs) is a cornerstone of participant safety and the integrity of the trial itself. Regulatory agencies worldwide have mapped out stringent frameworks to catalogue these events, which, while variable across different jurisdictions, share a common goal: to safeguard the well-being of participants and preserve the scientific validity of the study.
These frameworks are brought into stark relief by real-world scenarios such as the experiences of participants in Pfizer's vaccine study for children, where allegations emerged regarding the handling of a severe illness in a young participant. Such cases underscore the critical nature of transparent reporting systems like the Vaccine Adverse Event Reporting System (VAERS), which, despite its challenges, serves as an essential tool for tracking post-vaccine safety signals.
The recent actions of Florida's surgeon general, calling for a suspension of Covid vaccines amidst safety concerns, further highlight the dynamic and sometimes contentious landscape within which SAE reporting operates. This is compounded by the FDA's evolving stance on reporting requirements, showcased by exemptions granted for events identified via real-world data sources and registries.
The essence of these reporting obligations is captured by the Electronic Code of Federal Regulations (eCFR), which delineates the responsibilities of researchers and organizations in clear, hierarchical documentation. Consent and protection requirements for vulnerable populations, definitions of investigational products, and the conditions for research on specific groups are all meticulously outlined, ensuring clarity and consistency in the execution of clinical trials.
Statistics from comprehensive surveys, such as the one conducted by Mr. Keyes and the Taskforce, provide a macroscopic view of the state of clinical trial reporting in the U.S., revealing the sheer volume and diversity of trials being registered. This highlights the importance of robust reporting mechanisms to maintain the flow of reliable data, essential for informed decision-making in patient care.
Clinical trial participants, like those battling transthyretin-mediated amyloidosis, embody the human dimension of SAE reporting. Their participation, often marked by personal sacrifice and the burden of disease, is a potent reminder of why meticulous SAE reporting is not just a regulatory mandate but a moral imperative, as articulated by researchers and policy advocates like Gamertsfelder and Osipenko. Their experiences and testimonies advocate for a clinical trial ecosystem that is both ethically sound and scientifically rigorous, ensuring that future generations reap the benefits of today's research while minimizing harm.
Role of Investigators and Sponsors in Reporting SAEs
The accurate identification and documentation of serious adverse events (SAEs) are critical responsibilities of investigators in clinical trials. Their role involves meticulous tracking and reporting of any occurrences that may meet the criteria for being classified as serious. These activities are not only crucial for patient safety but also for the integrity of the trial itself. Sponsors, on the other hand, are tasked with the equally important duty of reporting these events to regulatory authorities within strict timelines. This process is guided by robust planning and a comprehensive understanding of the drug's safety profile, necessitating detailed operational definitions of key risks and plans for their ascertainment.
Moreover, the analysis of safety data, particularly adverse event data, demands a nuanced approach. It is not simply about enumerating the proportion of participants experiencing an event; it involves complex statistical considerations, such as the treatment of discontinuations, the incorporation of data across multiple trials, and the selection of appropriate statistical methods to both estimate incidence and gauge the surrounding uncertainty.
The collaboration between investigators and sponsors is further underscored by the success of systems like the Vaccine Adverse Event Reporting System (VAERS), which serves as a testament to the effectiveness of vigilant safety monitoring. VAERS has been instrumental in identifying safety issues, reinforcing the importance of a multilayered approach to monitoring that encompasses various medical interventions.
In light of recent news highlighting the consequences of inadequate data integrity in women's health research and image data in clinical research, the need for meticulous reporting and transparency becomes even more apparent. Issues such as the lack of a priori trial protocols and statistical analysis plans being made publicly available are a concern, as these are essential for ensuring research transparency and the ability to reproduce findings.
As the industry continues to play a dominant role in clinical research, the variability in commitments to trial transparency, the suboptimal implementation of data-sharing requirements, and the challenges brought on by the COVID-19 pandemic highlight the pressing need for improved practices. This includes the wider sharing of raw data beyond the confines of industry-based statistical analysis, which is not yet a routine occurrence despite numerous initiatives over the past decade aimed at enhancing data sharing.
In conclusion, the interplay between investigators and sponsors in the reporting of Sales is a cornerstone of clinical trial conduct. It ensures not only the safety of participants but also the credibility and reliability of the data generated, ultimately contributing to the advancement of medical science and patient care.
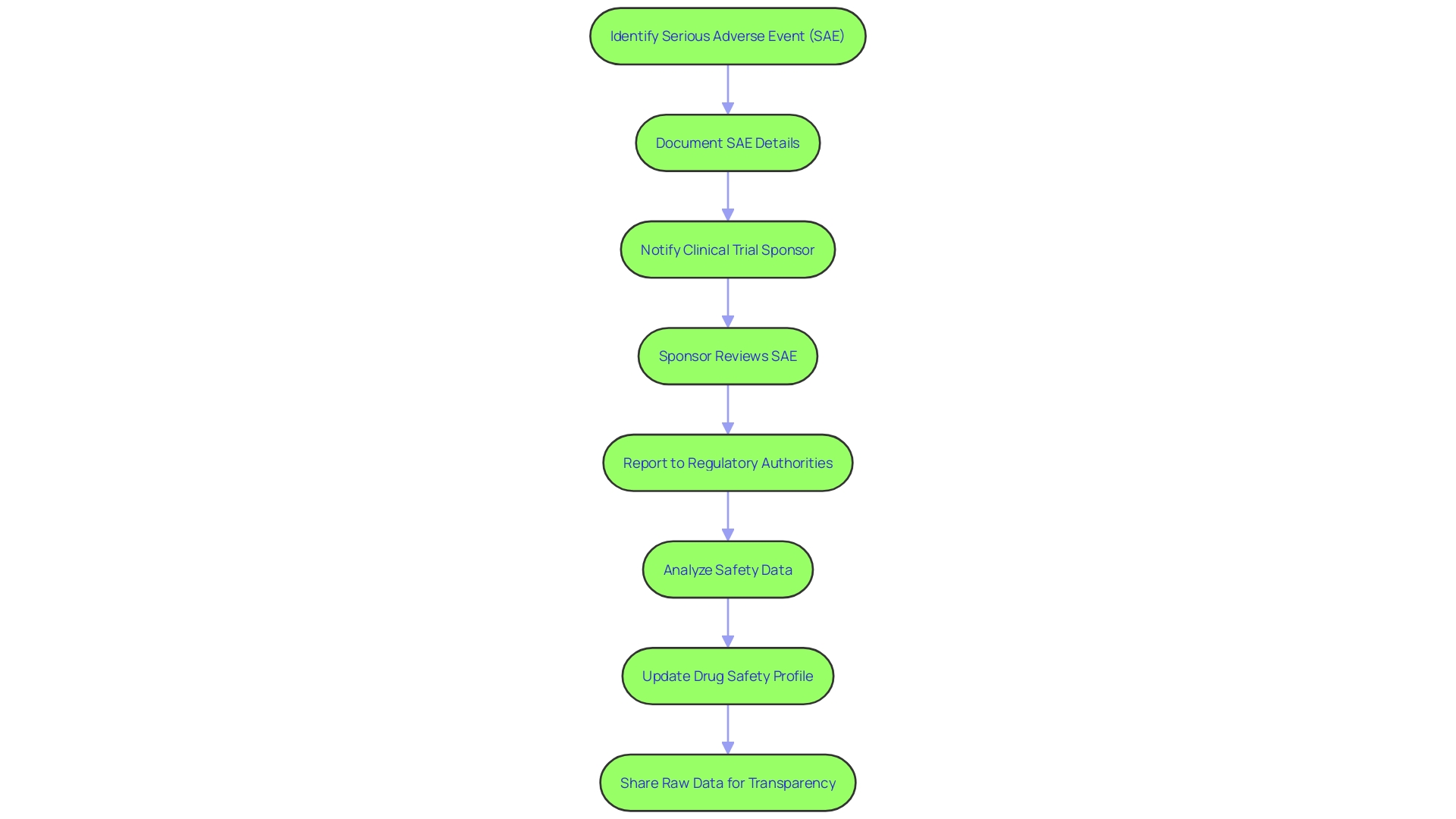
Best Practices for Documenting and Reporting SAEs
Accurate documentation and reporting of serious adverse events (SAEs) are vital for maintaining the integrity of clinical research and safeguarding participant safety. A Development Safety Update Report (DSUR) serves as a structured tool for consolidating safety information, comprising an introduction, updates on marketing authorization, clinical study status, a safety data summary, and a risk-benefit assessment.
The safety data summary, a core component of the DSUR, includes a concise account of Sales and adverse reactions observed during the study period. It's essential to use standardized forms and maintain organized records to ensure this information is accurately captured and reported. Regular training for researchers and staff is key to familiarize them with these forms and the importance of thorough data collection.
Quality control measures are also a critical aspect of this process, as they help identify and address any potential issues promptly. For instance, the image data integrity analyst Jana Christopher MA indicates that up to 35% of manuscripts may be flagged for image-related problems, often unintentional duplications due to the complexity of image data.
To foster a culture of safety and transparency, it's important to establish an environment where raising concerns is encouraged and protected. For example, an effective whistleblower policy can ensure that staff feel secure in reporting any breaches in protocol or safety without fear of repercussions. This approach aligns with the regulatory guidance provided by bodies like the FDA, EU, and EMA, which emphasize a risk-based evaluation in clinical trials to balance innovation with participant safety.
Amidst the regulatory evolution and technological advancements in clinical trials, such as the integration of AI, maintaining the highest standards of safety reporting remains a cornerstone of ethical and effective clinical research. By adhering to these best practices, researchers not only comply with regulatory requirements but also contribute to the advancement of medical knowledge while protecting the well-being of study participants.
Conclusion
Accurate reporting of Serious Adverse Events (SAEs) in clinical trials is essential for participant safety and research integrity. SAEs are untoward medical occurrences that pose a significant threat to a participant's life or well-being. Systems like the Vaccine Adverse Event Reporting System (VAERS) exemplify the importance of surveillance mechanisms in clinical trials.
Best practices for documenting and reporting SAEs involve meticulous data collection, standardized forms, quality control measures, and a culture of safety and transparency. Researchers and sponsors play a crucial role in upholding participant safety and maintaining the integrity of clinical trials.
Transparency in reporting SAEs is critical for maintaining public trust and advancing medical science. Systems like the Clinical Trials Information System (CTIS) and VAERS contribute to the accessibility and availability of clinical trial data.
The construction of an SAE narrative requires precision, clarity, and a commitment to contributing to the broader knowledge base in healthcare. Adhering to guidelines and statistical rigor ensures the reliability and verifiability of reported results.
Reporting SAEs is a moral obligation and scientific imperative. By adhering to best practices, researchers protect participant well-being and contribute to the advancement of medical knowledge. Accurate reporting fosters transparency, reproducibility, and the collective understanding of medical safety.
In conclusion, accurate reporting of SAEs is crucial for participant safety, research integrity, and the credibility of medical science. Adhering to best practices and upholding the highest standards of safety reporting contribute to the advancement of medical knowledge while protecting the well-being of study participants.




