Introduction
Title: Understanding 21 CFR 806: Reporting Requirements for Medical Devices
Introduction:
In the world of medical devices, ensuring safety and effectiveness is paramount. That's where Title 21 CFR Part 806 comes into play. This regulation outlines the mandatory reporting requirements for medical devices, focusing on actions like repair, modification, adjustment, relabeling, destruction, or inspection of a device.
It sets the stage for maintaining the integrity of medical devices and encompasses various entities involved in their distribution and use.
The FDA, responsible for public health assurance related to medical devices, enforces these regulations to manage the risks associated with device safety and effectiveness. With the staggering statistics of over 1.7 million injuries and 83,000 deaths potentially linked to medical devices in the United States over a 10-year period, it's clear why these regulations are critical.
In this article, we will explore the key aspects of 21 CFR 806, including who must comply, types of reportable events, when to report to the FDA, recordkeeping requirements, FDA's access to records, public availability of reports, class definitions for recalls, evaluating safety risks, and compliance program guidance. By understanding these crucial elements, stakeholders in the medical device industry can navigate the landscape of regulatory compliance and contribute to the continued safety and efficacy of medical devices.
Join us as we delve into the details of 21 CFR 806, providing accurate and detailed information in a formal and professional manner.
Overview of 21 CFR 806
Title 21 CFR Part 806 expands on the obligatory reporting criteria for medical equipment, specifically concentrating on activities such as repair, modification, adjustment, relabeling, destruction, or inspection of an apparatus. This section clarifies that such corrections do not require physically relocating the equipment from its point of use. The term 'consignee' refers to entities such as hospitals, surgical facilities, nursing homes, or outpatient treatment centers that have received an item subject to a cease distribution order.
The regulation is crucial in maintaining the integrity of medical instruments and encompasses various entities involved in the distribution and use of these tools, excluding lay individuals or patients. For example, healthcare experts such as doctors, nurses, and pharmacists are classified as 'instrument user facility' when it comes to their role in utilizing instruments for human use. Furthermore, the expression 'initial importer' encompasses importers who promote the marketing of a product to the ultimate consumer without altering its packaging or labeling.
The FDA, in charge of the public health assurance pertaining to medical instruments, enforces these regulations to handle risks connected with instrument safety and effectiveness. Based on a 2018 FDA investigation, during a span of 10 years, medical equipment were potentially associated with over 1.7 million injuries and 83,000 deaths in the United States, underscoring the crucial importance of these regulations.
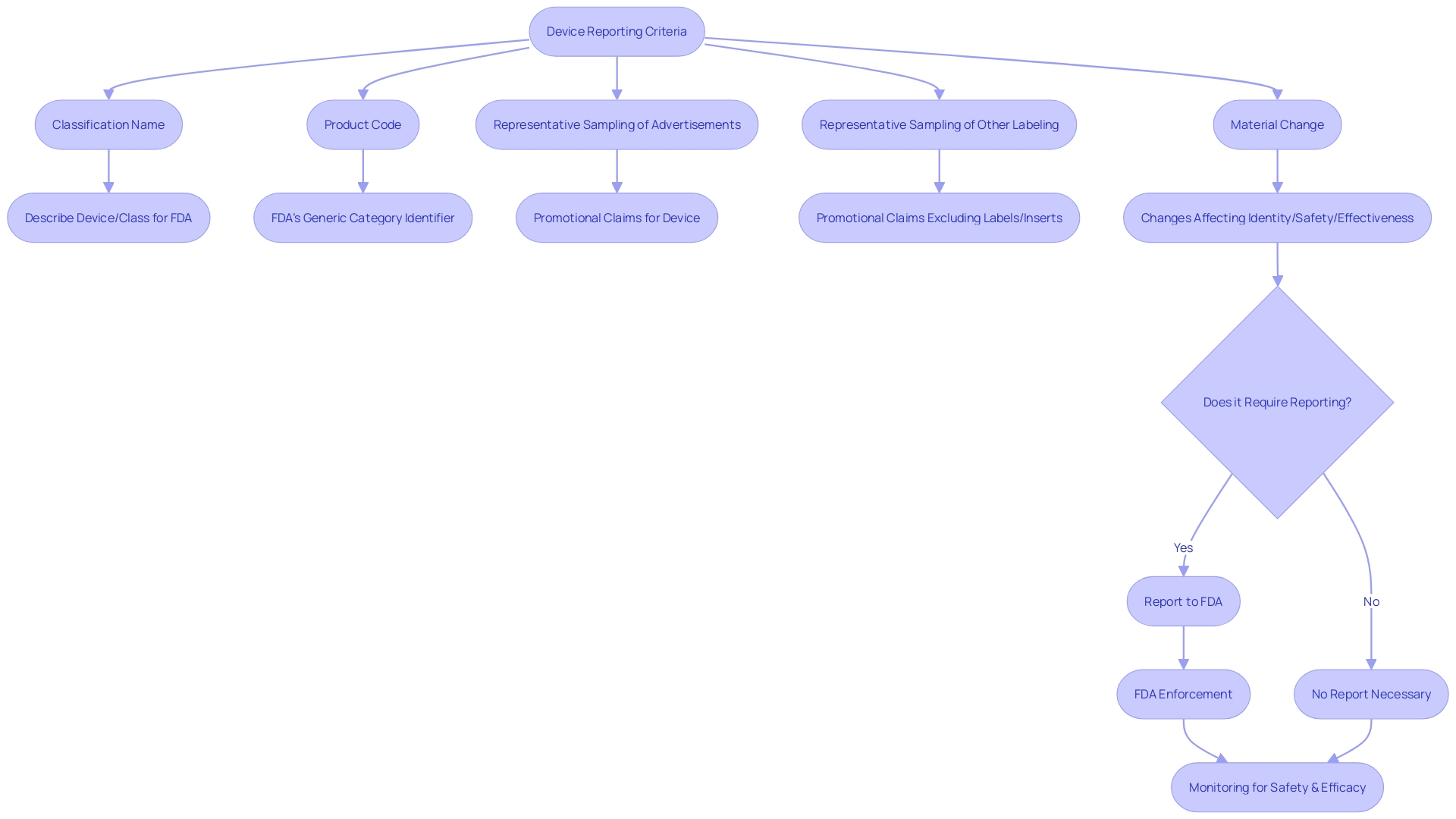
The 'classification name' and 'product code' employed by the FDA are also outlined within this part, assisting in the categorization for regulatory purposes. Furthermore, a 'representative sampling of advertisements' and other labeling materials, excluding labels and package inserts, must reflect the promotional claims made for the product. Significantly, any substantial alteration that impacts the identity or efficacy and soundness must be reported under this regulation.
Continual monitoring of evidence from different data sources is an essential element of the FDA's approach to identifying concerns related to medical devices. This proactive approach to monitoring is an essential component of ensuring the continued safety and efficacy of medical instruments in the healthcare system.
Who Must Comply with 21 CFR 806
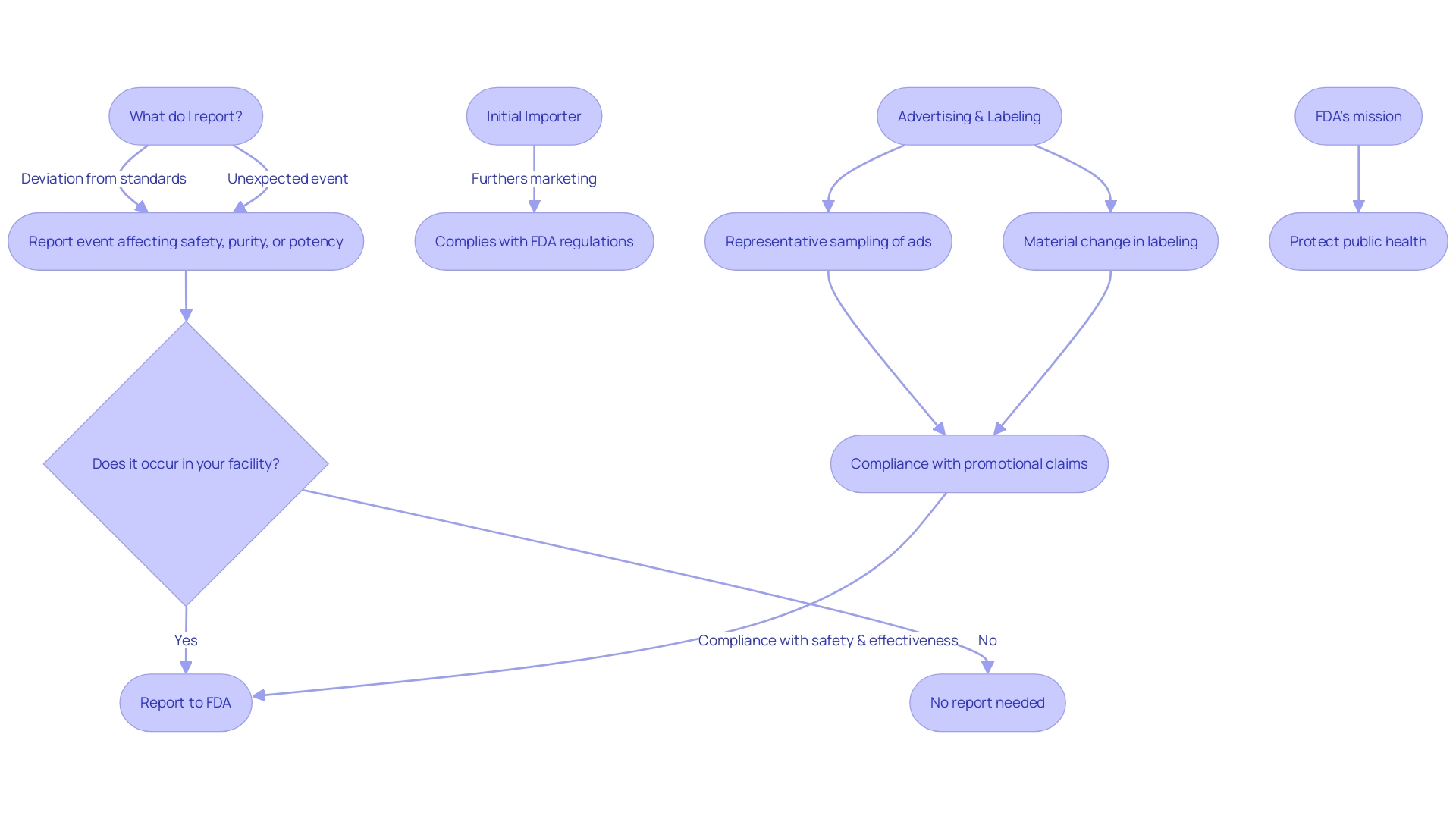
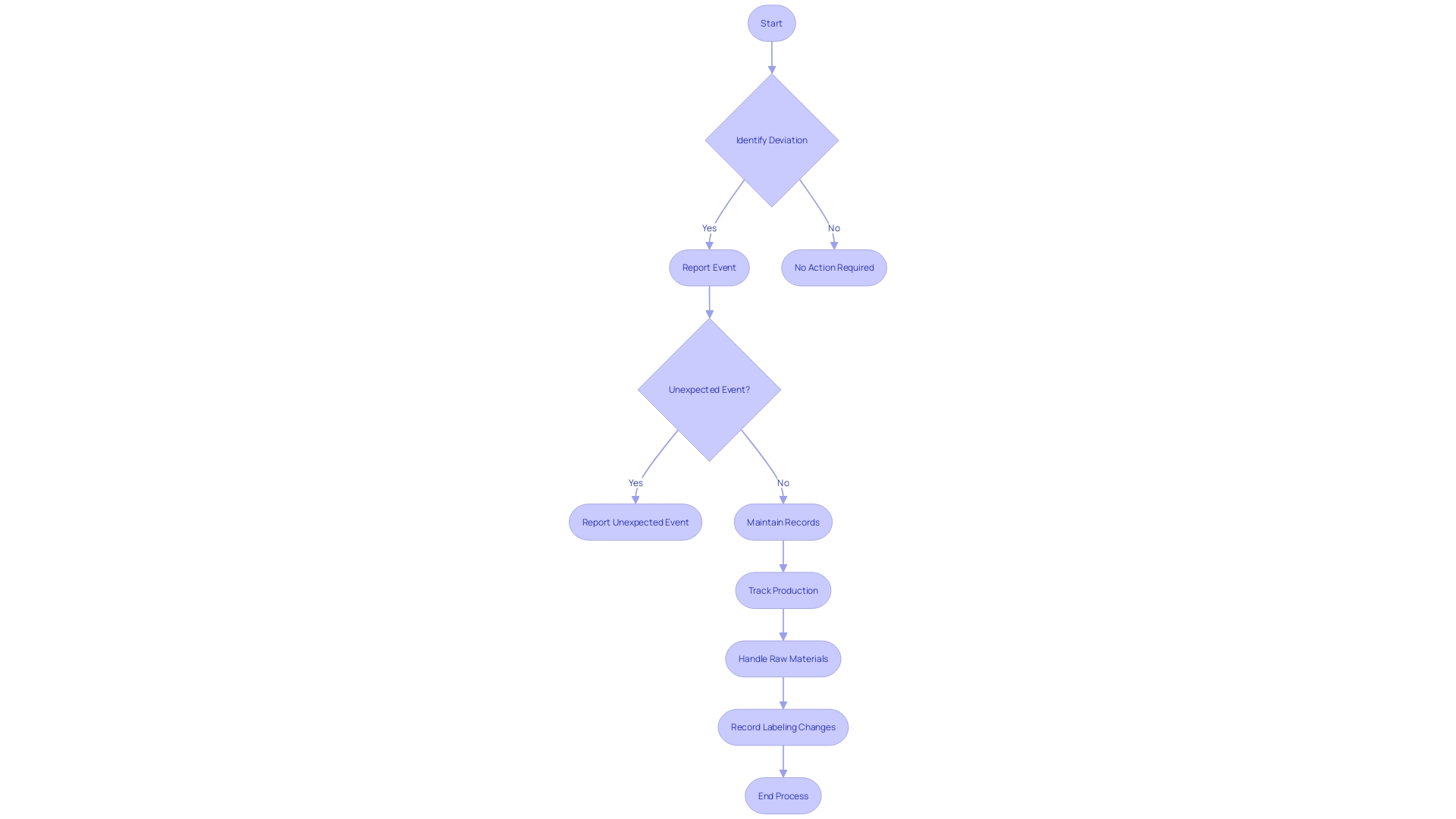
Title 21 CFR 806 outlines specific responsibilities for different entities involved in the life cycle of medical equipment. These obligations are not uniform but rather tailored according to the role each entity plays in bringing products to market. For example, manufacturers are obligated to disclose any deviations from the current standards of good manufacturing practice or any unforeseen incidents that could affect the safety, purity, or effectiveness of a product. The criteria for reporting encompass events related to manufacturing, testing, processing, packing, labeling, storage, holding, or distribution, provided they occur within the manufacturer's facilities or those under contract.
Similarly, initial importers have distinct responsibilities. Their responsibility is to promote the marketing of products from international manufacturers to local customers, without modifying the packaging or labeling. The description of an initial importer extends to any entity that makes a product available for final delivery or sale to the end-user.
The CFR also provides specific definitions to ensure clear communication of responsibilities. A 'limited tool' refers to any object subject to sale, distribution, or use restrictions by FDA regulations, premarket approval conditions, or performance standards. The 'classification name' is the FDA's term for describing an apparatus or class of apparatus, while the 'product code' identifies the generic category of an apparatus. When it comes to advertising and labeling, entities are required to offer a 'representative sampling' that precisely mirrors the promotional claims made for the product, along with any 'material changes' that might impact its identity or well-being and efficacy.
This regulatory framework is essential to the FDA's mission to protect public health by ensuring the safety, effectiveness, and security of medical equipment. It also underpins the agency’s oversight of the nation's food supply, cosmetics, dietary supplements, electronic products, and tobacco products. The recent FDA publication on 'Direct-to-Consumer Prescription Drug Advertisements' further underscores the importance of clear and neutral presentation of information in media, reinforcing the agency's commitment to consumer-friendly communication.
Types of Reportable Events
Under 21 CFR 806, specific occurrences associated with medical equipment must be carefully recorded and reported to the FDA. These events primarily include actions such as corrections or repairs, removals, and recalls of medical equipment from the market. A correction may involve fixing, altering, adjusting, relabeling, destroying, or inspecting an instrument, which does not necessarily require physically relocating the instrument from its point of use. Removals often involve withdrawing a device from the market when its defects or risks outweigh the benefits. Recalls happen when an item is discovered to present considerable health risks and must be returned or rectified. The reporting standards are strict, guaranteeing that any deviation from current good manufacturing practices or unforeseen incidents that may impact the purity, efficacy, or integrity of an item are brought to the attention of the FDA. This thorough supervision is a component of the FDA's dedication to protecting public health by ensuring the effectiveness and reliability of medical equipment. The agency's responsibility covers a broad array of items, encompassing human and veterinary drugs, biological items, and medical devices, guaranteeing they adhere to the utmost standards for the public's well-being.
When to Report to FDA
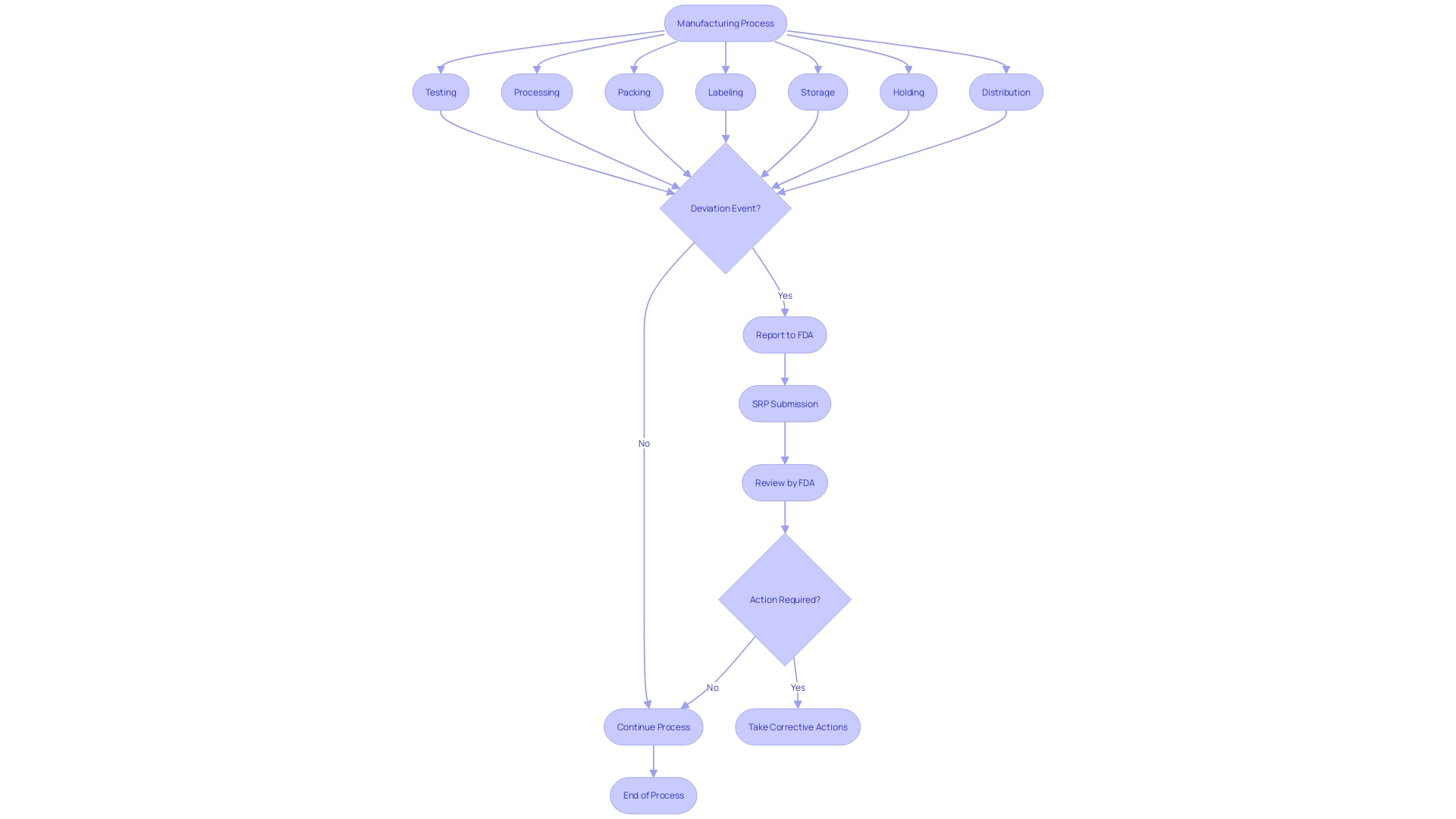
For clinical trials and FDA-regulated items, strict timelines govern the reporting of certain events. If any violations of good manufacturing practices, regulations, standards, or specifications arise, they must be reported to the FDA if they have the potential to affect the purity, potency, or integrity of an item. This includes unexpected events during the manufacturing process such as testing, processing, packing, labeling, storage, holding, or distribution stages. The Reporting Portal (SRP) is a critical tool in this procedure, allowing manufacturers, health care professionals, researchers, and the public to submit reports that contribute to the monitoring of America's food and medical items.
The FDA, under the U.S. Department of Health and Human Services, is responsible for ensuring the well-being of the public by supervising the effectiveness and soundness of drugs, medical devices, and other items. Reports on the security aspect are an essential part of the FDA's surveillance system, which also incorporates the Vaccine Adverse Event Reporting System (VAERS), renowned for its function in identifying concerns regarding the well-being of vaccines and upholding their safety.
All safety-related communications with the FDA should be done in English, and any discrepancy in meaning between translated materials and the English version will defer to the English version as official. It's crucial to remember that comments and reports submitted electronically will be made publicly available and should not contain confidential information. If confidentiality is required, reports should be submitted in written or paper form following the detailed instructions provided by the agency. Stakeholders are responsible for the content of their comments, including the exclusion of sensitive personal or business information.
Reporting Process
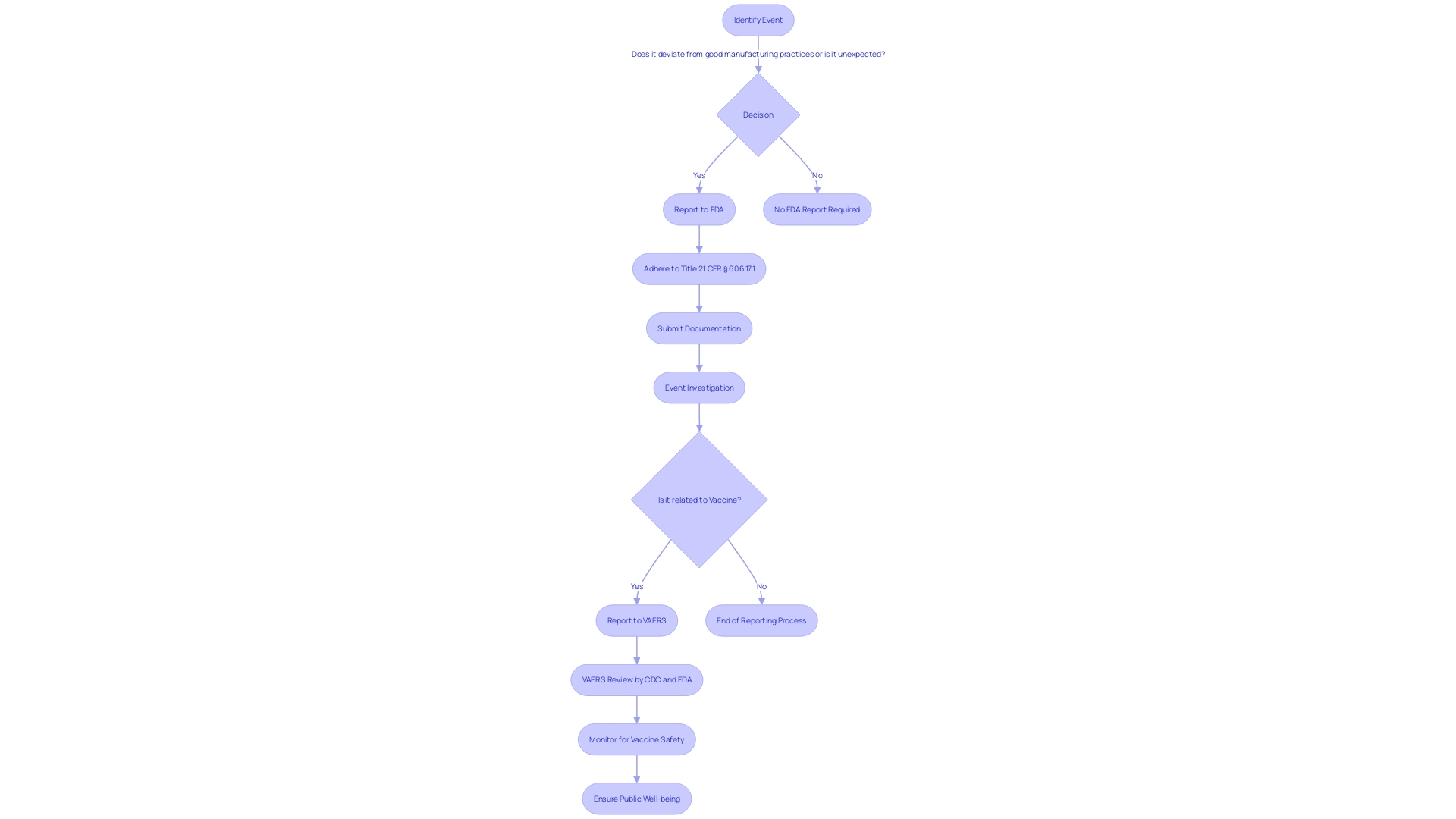
When reporting events regarding the safety, purity, or potency of biological items, it is crucial to provide comprehensive documentation to the FDA. This guarantees adherence to Title 21 of the Code of Federal Regulations (CFR), which is crucial for preserving the integrity of the nation's drug and biological supplies. The reporting process is clearly outlined under § 606.171 and other relevant sections. It requires the submission of any event associated with the manufacturing stages, including testing, processing, packing, labeling, or storage, as well as the holding or distribution of a licensed product, under the condition that the event deviates from established good manufacturing practices or represents an unexpected event that could impact product quality.
This reporting mechanism is a part of the Vaccine Adverse Event Reporting System (VAERS), a critical component of the national vaccine security system. VAERS's role in identifying issues of well-being is well-documented, with a track record of contributing to the evaluation of new vaccines and the monitoring of existing ones. Given that vaccines are essential in preventing diseases and saving lives, the system in place to ensure their well-being is multi-layered, with VAERS serving as an early warning system. It allows various stakeholders, including patients, health professionals, and manufacturers, to report adverse events post-vaccination. The collaborative efforts of the CDC and FDA to review these reports are vital for the continued trust in the integrity of vaccines and the prompt response to potential health concerns.
The eCFR provides an accessible format for referencing the requirements, with paragraphs structured to reflect the hierarchy of the document, aiding in the understanding of the reporting process. Aligned with the FDA's objective to safeguard public health, the agency supervises a variety of items, guaranteeing their effectiveness, reliability, and security. As part of this oversight, the FDA emphasizes the importance of accurate and timely reporting of deviations and adverse events, which is a shared responsibility among manufacturers, healthcare professionals, researchers, and the public.
Reports should be submitted in English, and for those seeking additional guidance, the FDA Safety Reporting Portal provides comprehensive information, with sections translated into Spanish for broader accessibility. It's important to follow the instructions carefully when submitting comments or reports to ensure confidentiality and compliance with regulations.
Recordkeeping Requirements
21 CFR 806 outlines particular requirements for recordkeeping in connection with the events associated with the manufacturing of licensed biological items, including testing, processing, packing, labeling, storage, holding, or distribution. The regulation mandates the reporting of any event that deviates from the current good manufacturing practice, applicable regulations, applicable standards, or established specifications impacting the item's safety, purity, or potency. Such events also encompass those that are unexpected or unforeseeable and occur within the manufacturer's facility or a contracted facility.
Records must be meticulously maintained to trace all stages of manufacture, as outlined in the eCFR, which is structured to mirror the document's hierarchy for clarity and ease of access. This includes the sequential coding of each production aggregate, allowing for the identification of the product and establishment, along with detailed tracking of the year, day, and specific period of packing, in addition to the handling of raw materials used.
Furthermore, the FDA requires that any material change in labeling or advertisements that could affect the identity or effectiveness of an object be recorded. A 'material change' refers to any alteration in the promotional claims or information provided that could have implications for the product's safety and efficacy.
In terms of regulatory compliance, it is critical for industry professionals to stay abreast of these recordkeeping obligations, ensuring that all necessary information is accessible to FDA inspectors as required. The eCFR's automated display process aids in this endeavor by providing a user-friendly representation of these complex regulations, although it is important to note that this does not modify the intent of the agency.
Comments and feedback on these regulations can be submitted to the FDA, with an emphasis on maintaining confidentiality of sensitive information. The FDA promotes the submission of comments that can improve the quality, utility, and clarity of the information collected under these regulations, including adverse experience reporting for licensed biological items, as part of the broader 21 CFR Part 600.
The FDA's notice of the proposed collection of information, as required under the Paperwork Reduction Act of 1995, invites public input on several aspects including the necessity of the information for FDA's functions, the accuracy of the burden estimate, and suggestions for minimizing the burden of information collection. These efforts support the implementation of statutory and regulatory authorities governing adverse experience reporting and are pivotal for the medical device industry's preparedness to meet regulatory demands.
FDA Access to Records
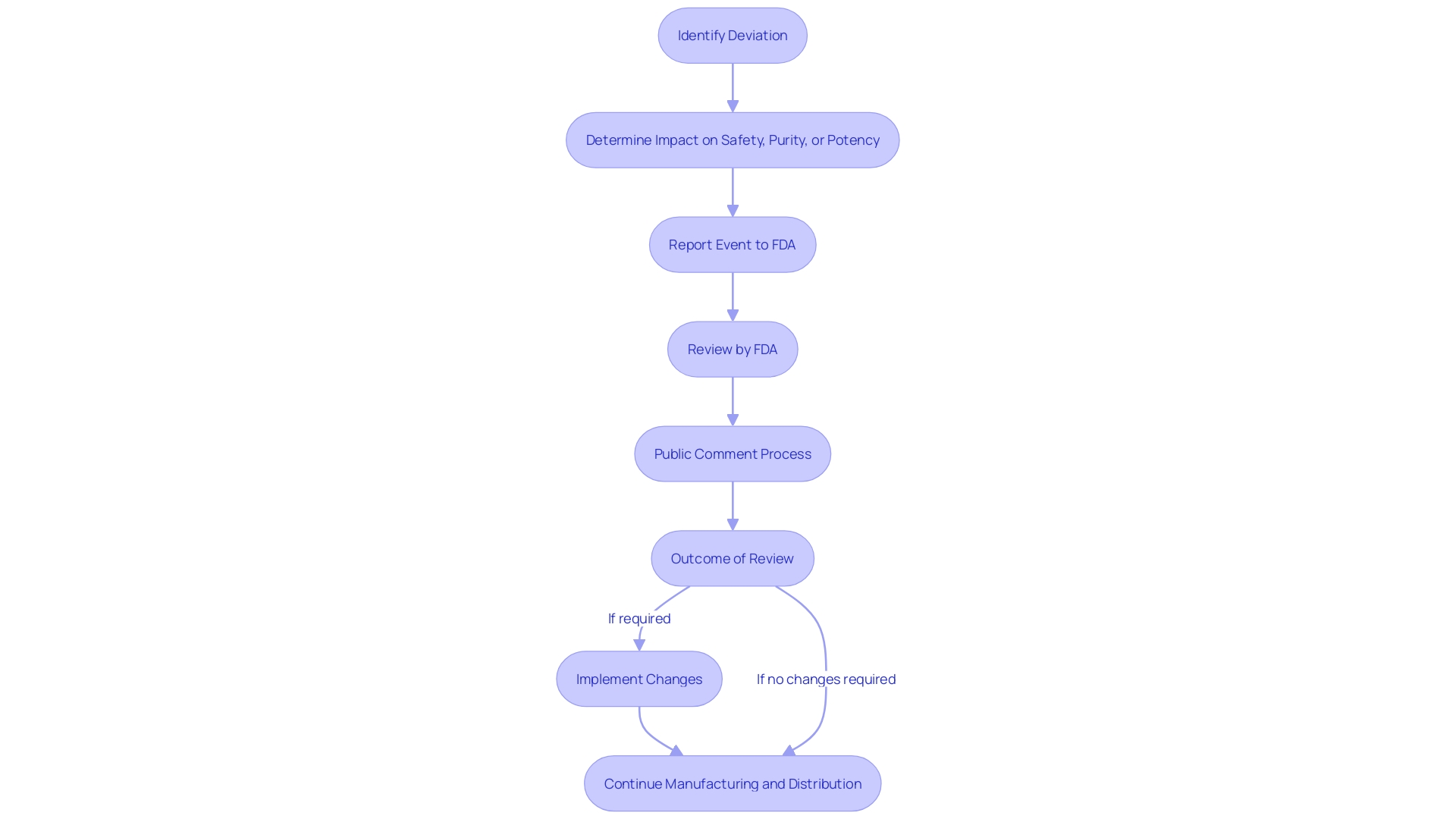
Title 21 of the Code of Federal Regulations is a crucial document for any entity involved in clinical trials and FDA-regulated activities. Among its many requirements, it mandates that certain events associated with the manufacturing and distribution of licensed biological items must be reported if they deviate from current good manufacturing practice, regulations, standards, or specifications and could impact the safety, purity, or potency of the item.
Entities must also be prepared for the FDA's authority to review their records. This guarantees openness and responsibility in the production and storage of biological items. The FDA's access to these records is not arbitrary; it is typically triggered by reported events that suggest a deviation from established practices or unexpected events that may influence the item's integrity. When such reports are submitted, they become part of a public docket, subject to review and comment by any interested party.
For the well-being of the general population, the FDA, as a component of the U.S. Department of Health and Human Services, guarantees the effectiveness and security of diverse goods, which encompasses the thorough supervision of vaccine well-being through systems like the Vaccine Adverse Event Reporting System (VAERS). VAERS has been crucial in identifying concerns regarding well-being and is a testament to the multi-layered approach the U.S. employs to monitor vaccine well-being.
To facilitate public participation and maintain transparency, the FDA provides clear instructions for submitting comments on proposed information collections. These comments are made publicly available, though submitters are cautioned to exclude confidential information. Moreover, the FDA solicits feedback on the utility, accuracy, and clarity of the information collected, as well as suggestions for minimizing the burden of information collection on respondents.
These regulations demonstrate the FDA's dedication to protecting public health by ensuring that the procedures involved in bringing biological items to market are thoroughly monitored and subject to public scrutiny.
Public Availability of Reports
Understanding the intricacies of 21 CFR 806 is crucial for maintaining the integrity of clinical research and ensuring public transparency. Reports submitted under this regulation provide information on deviations from good manufacturing practices or unexpected events that may impact the purity, potency, or integrity of biomedical items. These reports are not just procedural; they serve as a vital checkpoint in the life cycle of an item, from manufacturing through distribution.
Public inspection listings play a key role in legal research and regulatory oversight. They offer a snapshot of such reports, albeit with certain limitations, such as the potential absence of graphics or the presence of non-substantive markup language. Thus, it is imperative to cross-reference these documents with their official editions in the Federal Register to ensure legal accuracy and compliance.
Clinical trials and their outcomes, including security and effectiveness studies, form part of the data that may be encompassed within these reports. It's a diligent process where every piece of data, from manufacturing changes to distribution figures, is scrutinized for its impact on the product's profile.
The wider consequences of 21 CFR 806 reach into the domain of vaccine well-being and the strength of the National Vaccine Safety System. VAERS, for example, underscores the importance of having a transparent reporting mechanism that not only flags concerns about well-being but also reinforces the confidence in medical interventions.
Essentially, the public availability of reports under 21 CFR 806 enhances the collective understanding of item safety and efficacy, which is indispensable for the continuous advancement of healthcare and the protection of public health.
Class Definitions for Recalls
Having knowledge of the categorizations of recalls under 21 CFR 806 is essential for any organization involved with FDA-regulated items. A recall is a method of removing or correcting items that are in violation of laws administered by the Food and Drug Administration (FDA). Recalls fall into three classes based on the potential risk to health they pose:
- Class I: Situations where there is a reasonable probability that the use of, or exposure to, a violative product will cause serious adverse health consequences or death. Class II: Situations where use of, or exposure to, a violative item may cause temporary or medically reversible adverse health consequences, or the probability of serious adverse health consequences is remote.
- Class III: Situations where use of, or exposure to, a violative item is not likely to cause adverse health consequences.
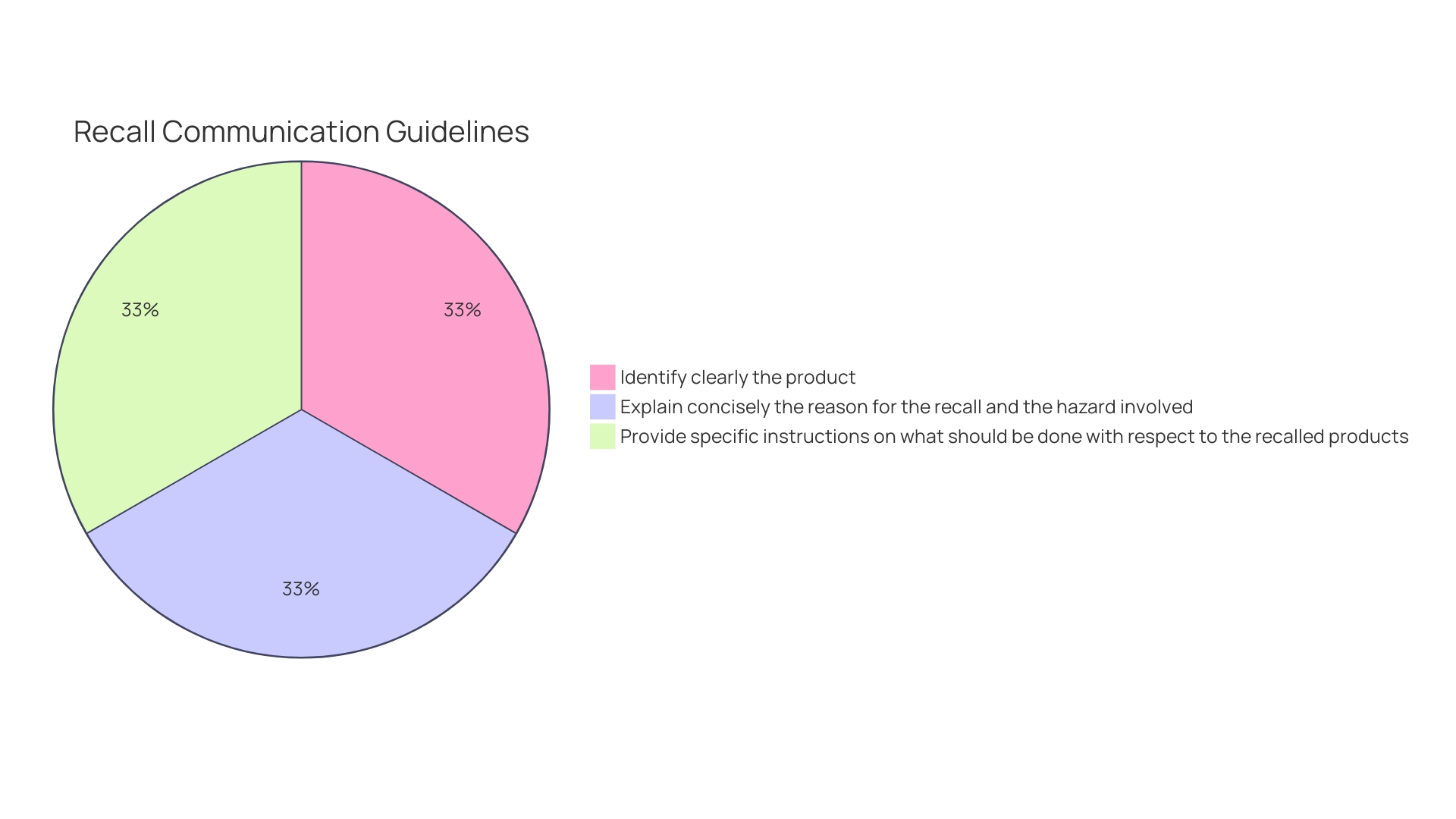
Recall communications should be clear and concise, identifying the item, lot number(s), and any other pertinent information for immediate identification. The cause for the recall and any possible dangers should be clarified, with detailed guidance on what measures should be taken with the recalled items. To facilitate responses, a mechanism should be provided for recipients to report to the recalling firm if they have the product.
The expression 'restricted equipment' denotes any apparatus for which a condition restraining sale, distribution, or utilization has been established by regulation, premarket approval, or performance standard. The classification name is used by the FDA to describe an instrument or class of instruments for classification purposes. The product code is the code used by the FDA to identify the generic category of an item.
These definitions and guidelines are critical for maintaining compliance and ensuring public well-being. According to FDA data, more than 1.7 million injuries and 83,000 deaths in the United States over a 10-year period were potentially linked to medical devices. This underscores the importance of rigorous postmarket surveillance and adherence to recall procedures to mitigate risks to health.
Evaluating Safety Risks
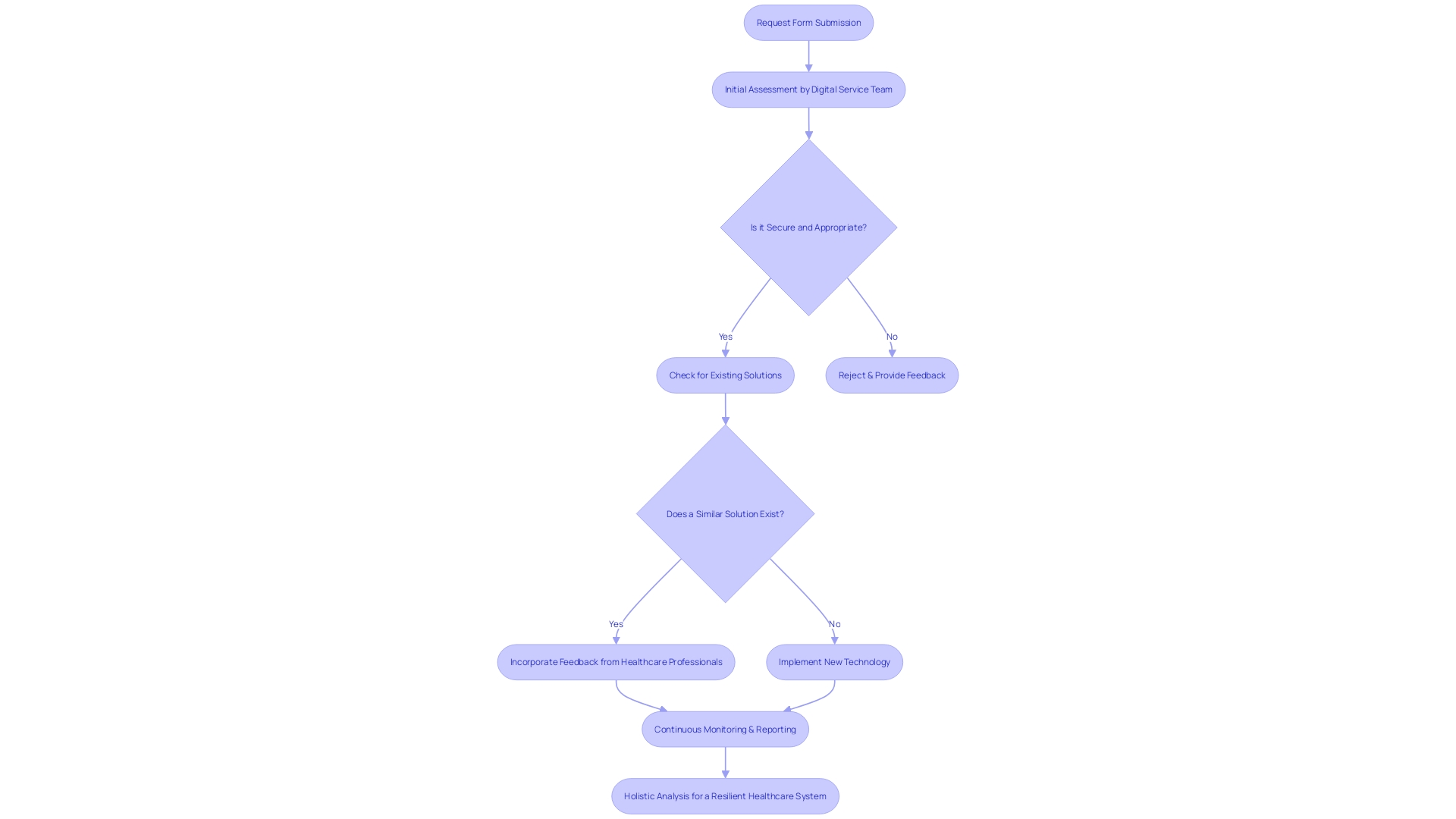
When assessing risks associated with reportable events in clinical trials, a comprehensive approach is crucial for evaluating potential harm to patients. This involves a rigorous process starting with a request form submitted by clinicians or departments seeking to adopt new technologies. The proposal undergoes an initial assessment led by a Digital Service Team to ensure the technology is secure, appropriate, and compliant with necessary approvals, while also determining if similar solutions already exist within the system.
Moreover, parallels can be drawn with the management of catastrophic wildfire risks, where a few entities cover significant areas, akin to the concentrated oversight in clinical trials. These parallels emphasize the importance of a small number of key players in managing expansive and complex security landscapes.
Incorporating feedback from healthcare professionals, such as those commending MedTech Safety's comprehensive insight into risk management, demonstrates the value of practical, hands-on examples in understanding the multifaceted nature of risk assessment. This real-world perspective is essential for developing effective mitigation strategies.
Additionally, recent news from ECRI highlights the top health technology hazards, urging manufacturers to consider usability challenges in home settings to prevent misuse and patient harm. This call to action is a reminder of the evolving nature of medical devices and the continuous need for cautious evaluations.
Statistics from the Vaccine Adverse Event Reporting System (VAERS) further underline the effectiveness of monitoring systems in identifying risks. Such systems play a crucial role in the national healthcare security network, emphasizing the importance of continuous watchfulness and reporting in upholding patient well-being.
Finally, a holistic view of the system is necessary to identify vulnerabilities. This includes analyzing people, organizations, tasks and processes, tools and technology, and the physical environment. Each element can contribute to failures, and understanding their interplay is key to fostering a safe and resilient healthcare system.
Compliance Program Guidance
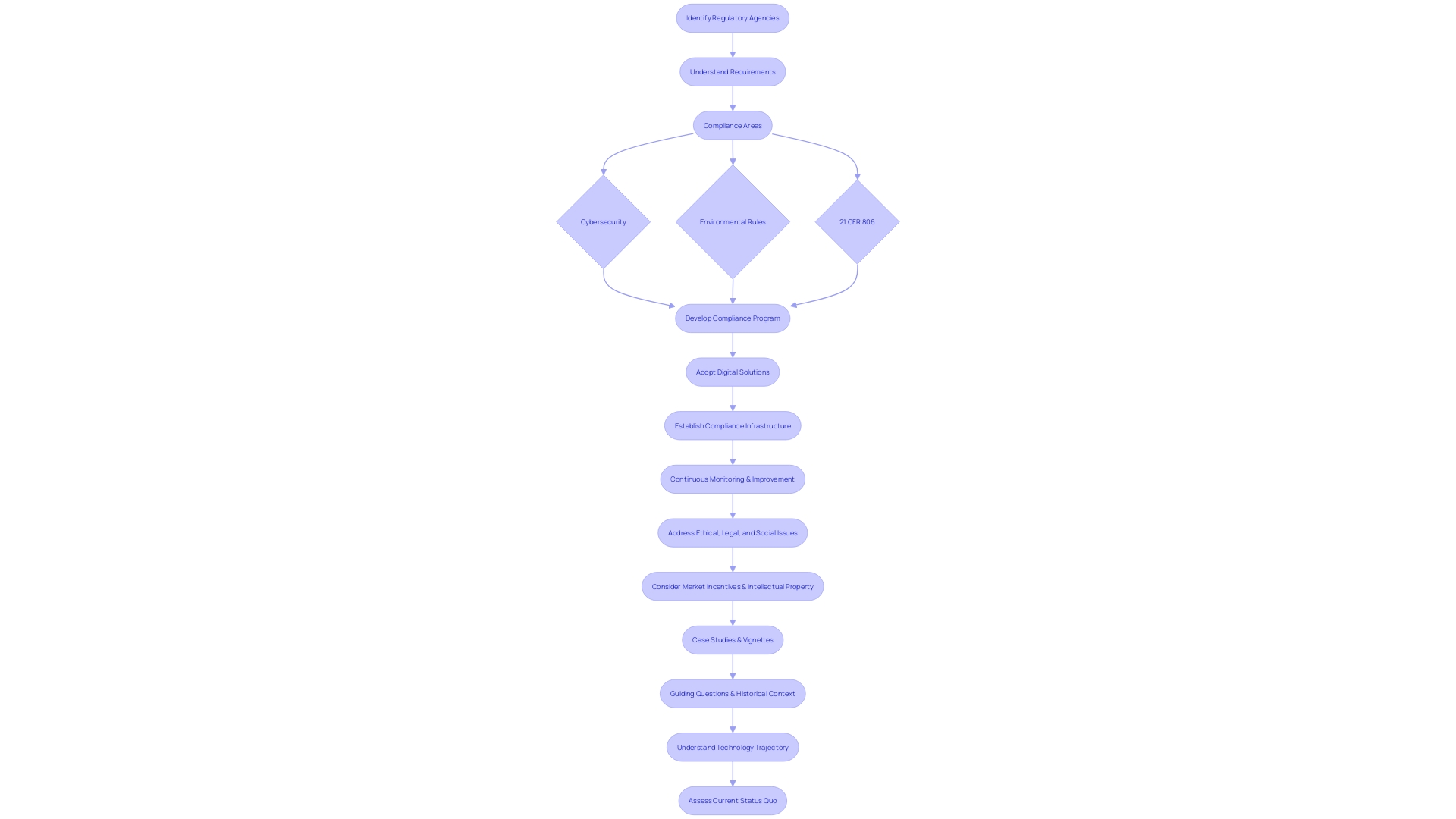
Understanding the landscape of regulatory compliance is essential for any Director of Clinical Research. It begins by identifying the specific agencies you're working with and comprehending their unique requirements. For instance, in the realm of cybersecurity for IT contracts or environmental rules for construction projects, specific standards must be met.
For compliance within the realm of 21 CFR 806, clear reporting is vital. This includes recording any deviations from the standard that may affect the product's well-being, purity, or potency, such as incidents in manufacturing, testing, or distribution. It also requires understanding the definitions provided by the FDA, such as 'restricted device' or 'classification name,' which are crucial for accurate reporting and compliance.
The importance of a robust compliance program cannot be overstated, as evidenced by the success of the FAA's Compliance Program. This initiative fostered a culture of safety in the aviation industry by encouraging active participation in resolving safety issues without resorting to punitive measures, opting instead for educational or corrective actions.
Similarly, the healthcare sector is rapidly evolving, with a growing emphasis on leveraging digital solutions to streamline management and enhance patient care. The adoption of virtual care platforms is not only about convenience but also about maintaining compliance in a digital age where regulations are becoming increasingly complex.
However, establishing a comprehensive compliance program is a complex task. It requires a meticulous approach to developing policies and procedures that align with the specific regulations of each market. As Cristina Revelo highlights, evaluating the frequency of manual approvals and internal control overrides can indicate whether your compliance culture needs strengthening or if your processes require recalibration.
In the end, it's about creating a compliance infrastructure that's both reliable and efficient, capable of adapting to the ever-changing regulatory environment. By doing so, you ensure that your organization can continue to focus on its primary goal: improving patient outcomes through rigorous and responsible clinical research.
Conclusion
In conclusion, compliance with 21 CFR 806 is vital for maintaining the safety and integrity of medical devices. This regulation outlines mandatory reporting requirements for actions like repair, modification, adjustment, relabeling, destruction, or inspection of devices. The FDA enforces these regulations to manage risks associated with device safety and effectiveness.
Entities involved in the life cycle of medical devices must fulfill specific responsibilities outlined in the regulation. Clear definitions for terms like "restricted device," "classification name," and "product code" aid in effective communication and device categorization.
Timely reporting of events related to medical devices is crucial, following stringent criteria to ensure product safety and efficacy. Meticulous recordkeeping is required, and the FDA has the authority to access these records to ensure transparency and accountability.
Reports submitted under 21 CFR 806 are made publicly available, enhancing transparency and promoting a collective understanding of product safety. The regulation also provides classifications for recalls based on potential health risks, facilitating clear communication and appropriate actions.
Evaluating safety risks associated with reportable events requires a comprehensive approach, including assessing potential harm to patients and implementing effective mitigation strategies. Compliance with regulatory requirements is crucial, and organizations should develop robust compliance programs that align with agency requirements and foster a culture of safety.
By understanding and adhering to 21 CFR 806, stakeholders in the medical device industry can navigate regulatory compliance and contribute to the continued safety and efficacy of medical devices, ultimately safeguarding public health.




