Introduction
Title: Understanding 21 CFR Part 820: Ensuring Quality and Compliance in the Medical Device Industry
The medical device industry plays a pivotal role in improving patient outcomes, but ensuring the safety and effectiveness of these devices is of utmost importance. Enter 21 CFR Part 820, also known as the Quality System Regulation (QSR), which outlines the regulatory framework established by the U.S. Food and Drug Administration (FDA) to oversee the design, production, and distribution of medical devices.
In this article, we will delve into the comprehensive scope and applicability of 21 CFR Part 820, the specific quality system requirements it entails, and the personnel requirements and training necessary for compliance. We will also explore common mistakes and best practices for ensuring compliance, the importance of record keeping and documentation, and the harmonization of this regulation with ISO 13485 and other standards.
Understanding and adhering to 21 CFR Part 820 is crucial for medical device manufacturers to guarantee the safety, effectiveness, and compliance of their products. Join us as we explore the key aspects of this regulation and its significance in the ever-evolving landscape of the medical device industry.
What is 21 CFR Part 820: Quality System Regulation (QSR)
Title 21 of the Code of Federal Regulations (CFR) Part 820, also referred to as the Quality System Regulation (QSR), embodies the regulatory framework by the U.S. Food and Drug Administration (FDA) for overseeing the design, production, and distribution of medical devices. These regulations are pivotal for clinical trials, as they ensure medical devices meet safety and efficacy standards before reaching the market.
Specifically, the QSR outlines Current Good Manufacturing Practice (CGMP) requirements, which dictate the methods, facilities, and controls employed in manufacturing medical devices for human use. CGMP standards are designed to confirm that devices are consistently produced and controlled according to quality standards to ensure their safety and effectiveness. Manufacturers are required to comply only with the regulations that apply to the operations they are engaged in.
For Class I devices, for instance, design controls are mandated only for devices listed in § 820.30(a)(2).
The FDA's commitment to public health extends beyond medical devices to the assurance of safety and effectiveness of human and veterinary drugs, biological products, and more. For example, the recent 'Direct-to-Consumer Prescription Drug Advertisements' rule demands clarity and neutrality in TV and radio drug advertisements, emphasizing the importance of understandable consumer language and the concurrent presentation of audio and text information.
In clinical research, establishing bioequivalence (BE) for generic drugs is crucial as it ensures no significant differences in bioavailability parameters between the test drug and a reference drug. Adaptive trial designs and cross-over studies are among the methods used to demonstrate BE, which often involve log-transformed values to derive a geometric mean ratio (GMR).
Moreover, non-interventional studies, which include observational cohort and case-control studies, play a role in clinical trials by evaluating marketed drugs in routine medical practice without the need for an Investigational New Drug (IND) application. Nevertheless, requirements for informed consent and Institutional Review Board review remain applicable.
Understanding FDA regulations is essential for initial importers, manufacturers, and others involved in the marketing and distribution of medical devices. This understanding ensures adherence to restrictions on sale and distribution, proper classification of devices, and the maintenance of representative sampling of advertisements and labeling, which are all critical in maintaining the integrity and compliance of medical products in the market.
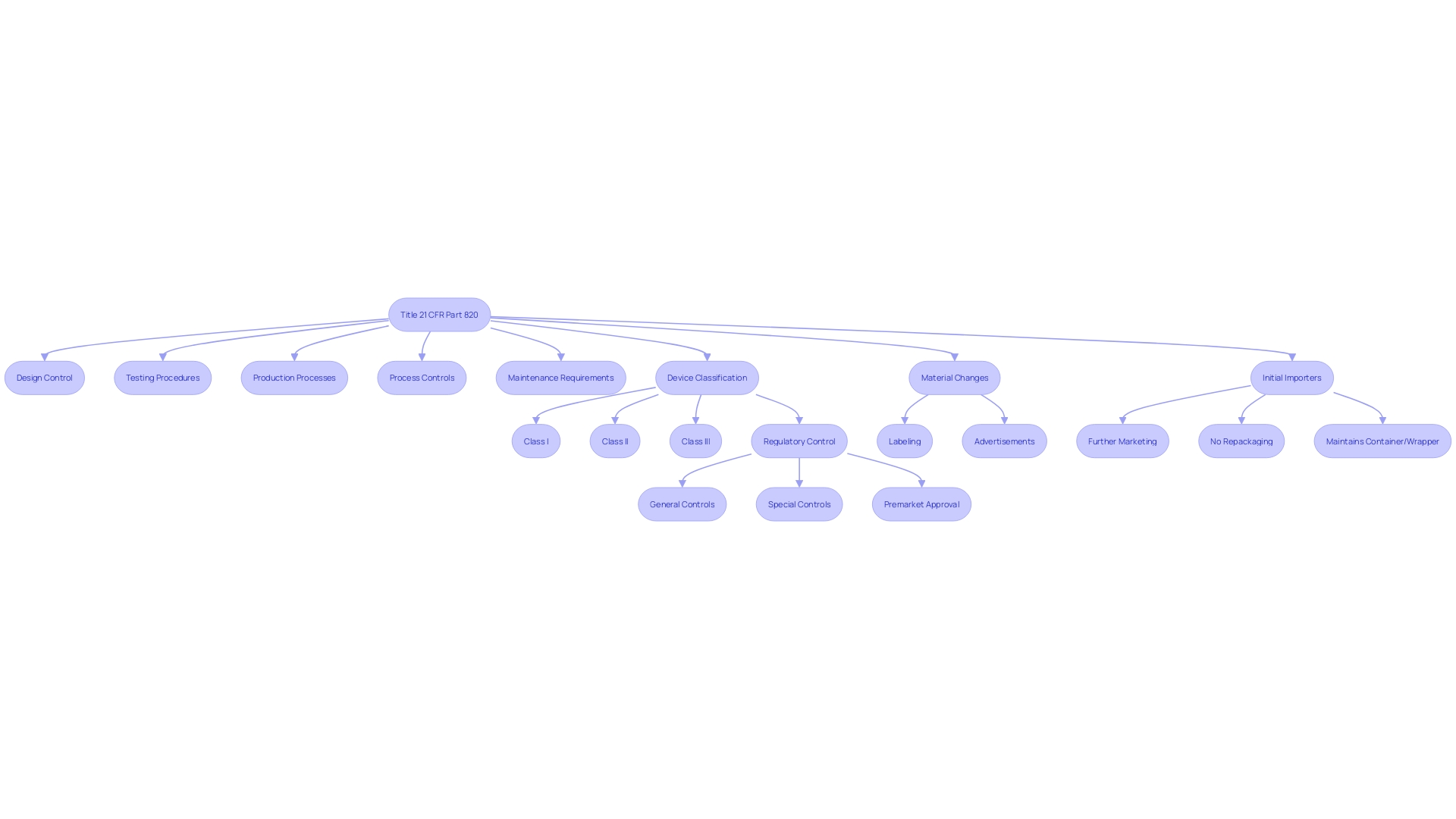
Scope and Applicability of 21 CFR Part 820
Title 21 CFR Part 820 delineates regulations for quality systems governing the methods and documentation of the design, testing, production, processes, controls, and maintenance of medical devices. The scope of these regulations is extensive, applying to any organization involved in the manufacturing and distribution of medical devices intended for use in the United States.
The regulations define a 'restricted device' as one subject to specific sales, distribution, or usage limits set by FDA regulation or order as a condition of premarket approval, or by a performance standard. This can include a variety of devices, each identified by a 'product code' that signifies its generic category.
Additionally, the term 'classification name' refers to the FDA's designation for a device or class of devices used in the classification process under section 513 of the act. This classification helps determine the level of regulatory control necessary to ensure the safety and effectiveness of a device and includes three regulatory classes: I, II, and III, with increasing levels of regulatory control from I to III.
Initial importers, who facilitate the marketing of a device from a foreign manufacturer to the final consumer without altering its packaging or labeling, also fall under the purview of these regulations.
It's important to note that any 'material change' to a device's labeling or advertisement, which could impact its identity or safety and effectiveness, must be carefully considered. Such changes could encompass modifications to the device's name, ingredients, intended use, contraindications, warnings, or usage instructions.
Understanding these definitions and requirements is crucial for compliance with the FDA's regulatory framework and ensuring that medical devices are safe and effective for public use.

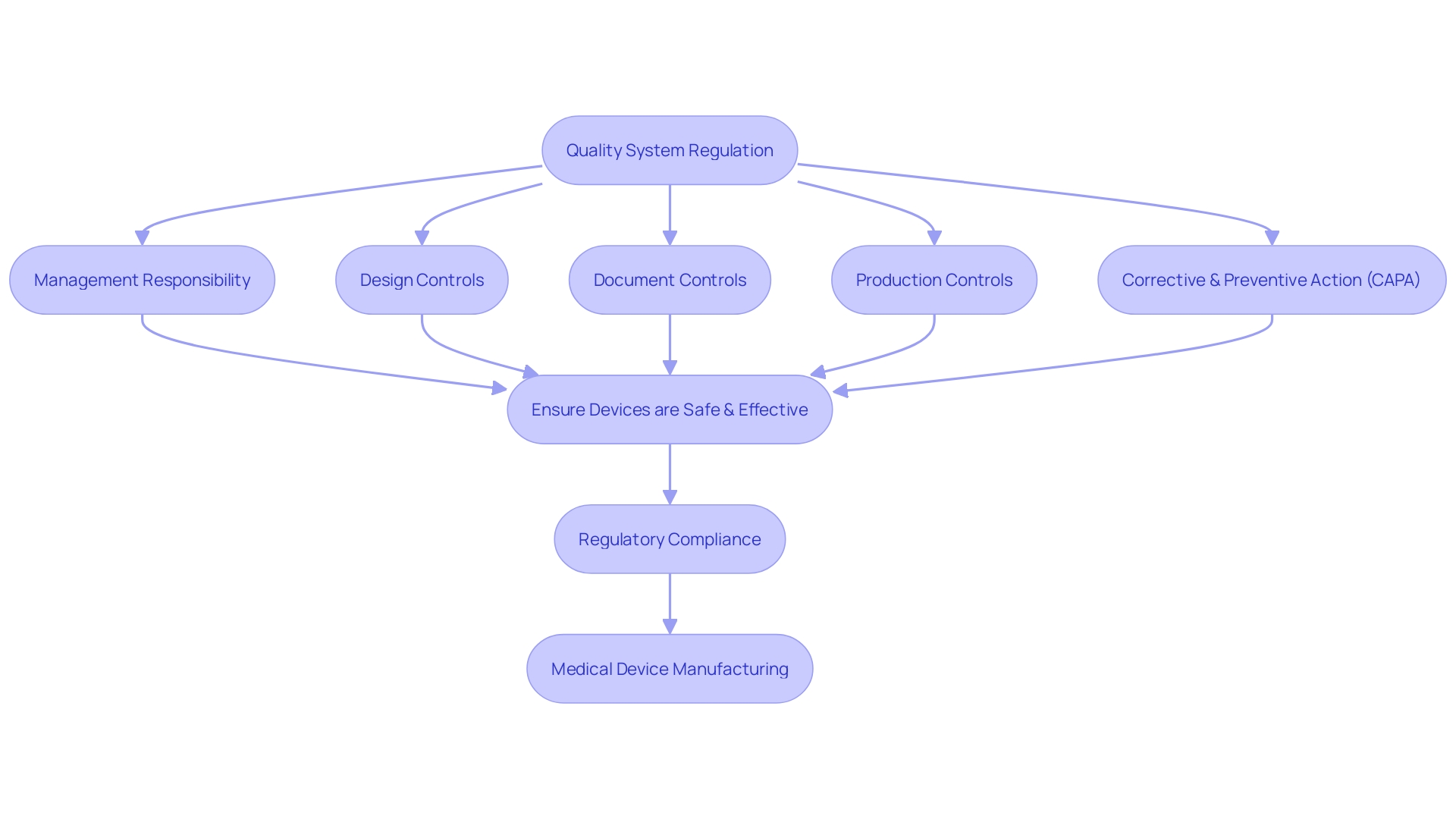
Quality System Requirements
Quality system requirements for medical devices, as detailed in 21 CFR Part 820, form the backbone of device safety and efficacy. These regulations are critical in a field as diverse and intricate as medical device manufacturing, which includes a vast array of products from glasses to advanced MRI machines, and involves disciplines ranging from bioengineering to software technology. The World Health Organization notes the existence of over 10,000 different types of medical devices, highlighting the importance of stringent quality controls.
- Management Responsibility: Ensures that executives uphold a commitment to quality, providing necessary resources and fostering a quality-centric culture within the organization.
- Design Controls: Design processes must meet predefined requirements, mitigating risks and adhering to safety standards. This is crucial as the design is the starting point for a device's life cycle.
- Document Controls: Proper documentation is key to maintaining regulatory compliance and ensuring traceability throughout the device's life cycle.
- Production Controls: Standardized manufacturing processes help guarantee consistency and reliability in product quality.
- Corrective and Preventive Action (CAPA): An essential system to identify, address, and prevent the recurrence of product and quality issues.
Adherence to these requirements is ensured through current good manufacturing practice (CGMP) regulations, which cover all aspects of production, from design to servicing. Importantly, these requirements are not uniformly applied; they vary depending on the class of the device, with certain class I devices exempt from design controls.
UL Solutions' Mary Joyce emphasizes the booming medical device sector in Michigan, a hub for talent and manufacturing. Meanwhile, the FDA's communication channels remind us of the importance of confidentiality and transparency in the public feedback process for regulatory matters.
Incorporating digital quality systems into existing frameworks is also vital. Strategies include setting clear objectives, developing a phased implementation plan, and building upon the success of previous digital transitions, ensuring a robust foundation for future processes.
Quality assurance is more than a regulatory hurdle; it's about delivering devices that genuinely improve patient outcomes. From concept through post-market surveillance, the full life cycle of a medical device is subject to rigorous quality checks. These checks are paramount, as any lapse can lead to significant harm, as highlighted by the challenges presented in the documentary 'The Bleeding Edge.'
Manufacturers must navigate a complex landscape of stringent regulations, rapid technological advancements, and global supply chain intricacies while maintaining a competitive edge. This multifaceted approach to quality assurance ensures that medical devices consistently meet the highest standards of safety and effectiveness.
Personnel Requirements and Training
Comprehending 21 CFR Part 820 is essential for medical device manufacturers to ensure their products are safe, effective, and compliant with the Federal Food, Drug, and Cosmetic Act. This regulation details the necessary methods, facilities, and controls for the design, manufacture, packaging, labeling, storage, installation, and servicing of medical devices intended for human use. It underscores that only operations relevant to a manufacturer's engagement need to adhere to this part, with design controls being mandatory only for class I devices specified in § 820.30(a)(2).
Personnel qualifications and responsibilities are central to compliance with these regulations. Manufacturers must establish and maintain procedures for identifying training needs and ensure all personnel are trained to adequately perform their duties. This includes understanding device design, production, and distribution, as well as the overarching regulatory requirements.
Training programs are vital, as they equip employees with the necessary expertise to ensure medical devices are developed in line with the highest standards.
Recent updates from the FDA, such as the "Direct-to-Consumer Prescription Drug Advertisements" final rule, emphasize the importance of clear and understandable communication, which extends to labeling and promotional materials for devices. Definitions like 'consignee,' 'correction,' and 'device user facility' have specific meanings under the regulations, clarifying the roles and responsibilities of various stakeholders. This precise language use is also reflected in the training materials, which must be clear and tailored to the needs of health professionals.
Moreover, the FDA's role in protecting public health by ensuring device safety and effectiveness highlights the critical nature of adherence to CGMP requirements. Compliance training is a key component of this, covering a broad spectrum of topics from safety to legal regulations. This training is not just about avoiding legal repercussions; it's about fostering a culture of safety and ensuring psychological safety for employees.
For medical device manufacturers, demonstrating conformity with regulations is not just a matter of legal compliance but also a business imperative. The cost of non-compliance can be significant, with FDA-dependent product development stages accounting for a substantial portion of market entry costs. Real digitalization, which involves understanding important data and how to apply it, is an enabler for compliance and business efficiency.
In summary, 21 CFR Part 820 sets forth the framework for medical device manufacturers to ensure their products meet safety and effectiveness standards. Personnel training is a crucial component of this framework, ensuring that those involved in design, production, and distribution of medical devices possess the necessary knowledge and skills. As the medical device industry continues to evolve, staying updated with FDA regulations and effectively implementing them through comprehensive training programs remains imperative for manufacturers.
Compliance and FDA Inspections
Adherence to 21 CFR Part 820 is not just a regulatory requirement; it's a cornerstone of ensuring medical device safety and effectiveness. This segment delves into the comprehensive measures required for compliance, including rigorous internal and quality audits, as well as meticulous process validation. The significance of these strategies is further exemplified by the Impella Connect System case, where software functions integral to patient monitoring necessitated premarket authorization, underscoring the importance of meeting the FDA's device definition as per section 201(h) of the Act.
Moreover, the FDA's recent enforcement actions against e-cigarette products and the management of misinformation during the Covid-19 pandemic highlight the agency's commitment to safeguarding public health.
The emphasis on continuous quality improvement is evident from the cases where firms were directed to refine complaint coding and quality data analysis. One such instance involved a retrospective review of complaints, procedure revisions, and staff training to address coding issues effectively. Similarly, another firm's misclassification of research software with AI capabilities as non-regulatory highlighted the necessity of software validation under 21 CFR 820.30(g), demonstrating the regulatory oversight for all medical device software.
To maintain compliance, it is crucial to understand the market of the medical device, including where it is sold, imported, and used. The device's lifecycle, complexity, and the environment of use determine the extent of regulatory requirements. For instance, the OECD Conflict Minerals policy assists companies in responsible mineral sourcing, which can be pertinent to certain medical devices.
Additionally, the distinction between 'servicing' and 'remanufacturing' is of regulatory significance, with the latter bearing implications for the entity's responsibilities. The FDA provides guidance to aid in discerning whether activities are considered 'remanufacturing', thus ensuring that serviced devices maintain their quality, safety, and effectiveness.
Manufacturers must establish a quality management system that is traceable and auditable, as highlighted by the quality system regulation. This system encompasses design, manufacture, packaging, labeling, storage, installation, and servicing of devices, tailored to ensure patient safety and compliance with the Federal Food, Drug, and Cosmetic Act. Notably, the documentary 'The Bleeding Edge' scrutinized the FDA's 510(k) clearance process, which revealed that clinical trials are not always requisite for device clearance.
This has led to patient injuries in the past, prompting regulatory scrutiny and device market withdrawals, further emphasizing the critical nature of upholding 21 CFR Part 820 requirements.
Record Keeping and Documentation
Maintaining meticulous records is a cornerstone of adhering to 21 CFR Part 820. Under this regulation, manufacturers must document deviations from current good manufacturing practice, any unexpected events, and all activities that may influence a product's safety, purity, or potency. Such events must be thoroughly reported if they occur within the manufacturer's facility or at a contracted site.
Moreover, a thorough understanding of key definitions is crucial for compliance. For example, 'restricted device' refers to devices with specific sale, distribution, or usage limitations mandated by the FDA, while 'initial importer' denotes the first point of entry for a device into the market without any alterations to packaging or labeling. Each term carries significant implications for how devices are handled and documented.
The eCFR, an electronic resource, has streamlined access to these regulations, presenting them in a user-friendly format. This development is particularly beneficial in an era where the healthcare industry is rapidly transforming, necessitating quick adaptation and the adoption of digital platforms to manage operations effectively.
With the burgeoning demand for adept healthcare operations leaders, it's increasingly important for organizations to ensure their compliance mechanisms are robust. As per expert insights, effective internal controls are not only mandated by regulations such as the FCPA but are also integral to any best practices compliance program. These controls must extend to all areas, including labeling, advertising, and any material changes that could impact device identity and effectiveness.
Furthermore, when considering the lifecycle of a medical device, the CGMP requirements stipulate that manufacturers must adhere to quality system regulations that encompass design, manufacturing, packaging, labeling, storage, installation, and servicing. Compliance with these requirements is intended to guarantee the safety and efficacy of devices, aligning with the Federal Food, Drug, and Cosmetic Act.
In short, the landscape of healthcare compliance is complex and dynamic, underscored by the necessity for precise recordkeeping and a deep understanding of regulatory definitions and criteria. Leaders in the field must navigate these requirements with a clear grasp of the regulations to maintain the integrity and trustworthiness of their operations.
Common Mistakes and Best Practices for Compliance
Navigating the complexities of 21 CFR Part 820 is essential for organizations to ensure that their medical devices are safe, effective, and compliant with the Federal Food, Drug, and Cosmetic Act. This regulation outlines the current good manufacturing practice (CGMP) requirements within the quality system regulation framework. It is critical for manufacturers to understand the specific CGMP requirements that pertain to their operations, as these dictate the methods and controls for design, manufacture, packaging, labeling, storage, installation, and servicing of all medical devices intended for human use.
To ensure medical device compliance, it is first important to know the product market. Understanding where products are sold, imported, and used helps determine which regulations apply. Some companies opt for global regulatory compliance to account for changing market strategies and regulations.
It is equally crucial to understand that for Class I devices, design controls are required only for those devices listed in § 820.30(a)(2), and this regulation serves as guidance for manufacturers of components or parts of finished devices.
The MedTech industry, being one of the most regulated sectors, faces the constant evolution of regulatory requirements. Companies must develop tailored policies and procedures that address the specific regulatory needs of each market. For instance, the FDA has recently published standards for direct-to-consumer prescription drug ads, emphasizing the need for clear, conspicuous, and neutral presentation of a drug's major side effects and contraindications.
These standards highlight the necessity for companies to incorporate consumer-friendly language and ensure that information is easily understandable.
Moreover, insights from industry reports suggest that professionals in the medical device sector are seeking new strategies to navigate the increasing regulatory demands efficiently. These strategies include streamlining document preparation processes to minimize delays and errors, and understanding the impact of regulatory initiatives on the industry. For example, the documentary 'The Bleeding Edge' shed light on the FDA's 510(k) clearance process and the absence of clinical trials for many devices, raising awareness about the importance of stringent regulatory compliance.
The lessons learned from companies like Lex Machina, which faced challenges in managing their database infrastructure, emphasize the value of smart digitalization. By understanding what data is crucial and how to organize work product accordingly, organizations can enhance their compliance status and business operations.
In summary, maintaining high standards of quality and minimizing the risk of non-compliance involves a comprehensive understanding of 21 CFR Part 820, developing market-specific compliance strategies, and embracing digitalization to support regulatory adherence.
Harmonization with ISO 13485 and Other Standards
Understanding the intricacies of regulatory frameworks is crucial for organizations involved in clinical trials. The Code of Federal Regulations (CFR) Title 21 Part 820 and the International Organization for Standardization's ISO 13485 both set forth requirements for quality management systems in the medical device industry. These standards are integral to ensuring that devices are consistently produced and controlled according to quality standards, safeguarding public health.
ISO 13485 is harmonized with 21 CFR Part 820, which means that manufacturers following ISO standards will find significant overlap with FDA regulations. However, there are nuances and specific requirements within each that must be carefully navigated. For instance, 21 CFR Part 820 defines key terms such as 'restricted device,' 'classification name,' and 'product code,' which are essential for compliance with FDA's regulatory framework.
Understanding these definitions helps in aligning the quality management system with FDA expectations.
Moreover, the FDA encourages stakeholders to automate and streamline validation processes. The transition from manual, paper-based systems to automated, computer-based systems can enhance compliance and efficiency. This shift is reflected in the FDA's support for modernized data management strategies in clinical research, emphasizing the importance of defining a data strategy before protocol design to leverage insights while maintaining patient safety and data quality.
In practice, adhering to these standards has real-world implications. For example, the case of Balwani, who faced significant legal repercussions for fraudulent activities, underscores the gravity of compliance. Additionally, the Summit held in Washington, DC, provides a platform for the Medtech community to discuss process development, highlighting the industry's focus on regulation and standardization.
It's also imperative for organizations to understand the public nature of regulatory submissions and comments. Comments submitted electronically become part of the public record, underscoring the need to avoid including confidential or sensitive information. This is a key consideration for companies as they navigate the regulatory landscape, ensuring transparency while protecting proprietary data.
Adapting to these regulations is not just about compliance; it's about contributing to a culture of quality and safety in the development of medical devices. By aligning with international standards like ISO 13485 while adhering to 21 CFR Part 820, organizations can effectively manage their quality systems, address global market needs, and maintain the trust of stakeholders and the public alike.
Conclusion
In conclusion, 21 CFR Part 820, the Quality System Regulation (QSR) established by the FDA, is vital for ensuring the safety, effectiveness, and compliance of medical devices in the industry. Adhering to these regulations is crucial for manufacturers to maintain the integrity and trustworthiness of their products.
The article has covered key aspects of 21 CFR Part 820, including personnel requirements and training. Personnel qualifications and comprehensive training programs are essential in meeting the highest standards of device design, production, and distribution.
Common mistakes and best practices for compliance have been discussed, emphasizing the importance of understanding the product market, developing tailored strategies, and embracing digitalization to support regulatory adherence.
The harmonization of 21 CFR Part 820 with ISO 13485 and other standards has also been highlighted. Manufacturers following ISO standards will find significant overlap with FDA regulations, but careful navigation of specific requirements is necessary.
In summary, adherence to 21 CFR Part 820 is crucial for medical device manufacturers to ensure the safety, effectiveness, and compliance of their products. By understanding and implementing these regulations, manufacturers can continue to deliver devices that improve patient outcomes while maintaining the highest standards of quality and compliance.




