Introduction
The 510(k) FDA approval process is a crucial step for medical device manufacturers seeking to bring their innovations to the U.S. market. This regulatory pathway ensures that a device is safe and effective for consumer use, and it plays a vital role in protecting public health. Understanding the nuances of the 510(k) process, including the identification of suitable predicate devices and the creation of a comparative analysis, is essential for compliance and patient safety.
This article explores the importance of 510(k) FDA approval, the different types of submissions, the role of predicate devices, and best practices for a successful submission. By delving into the details of this process, manufacturers can navigate the regulatory landscape with confidence and contribute to the advancement of healthcare outcomes.
What is 510(k) FDA Approval?
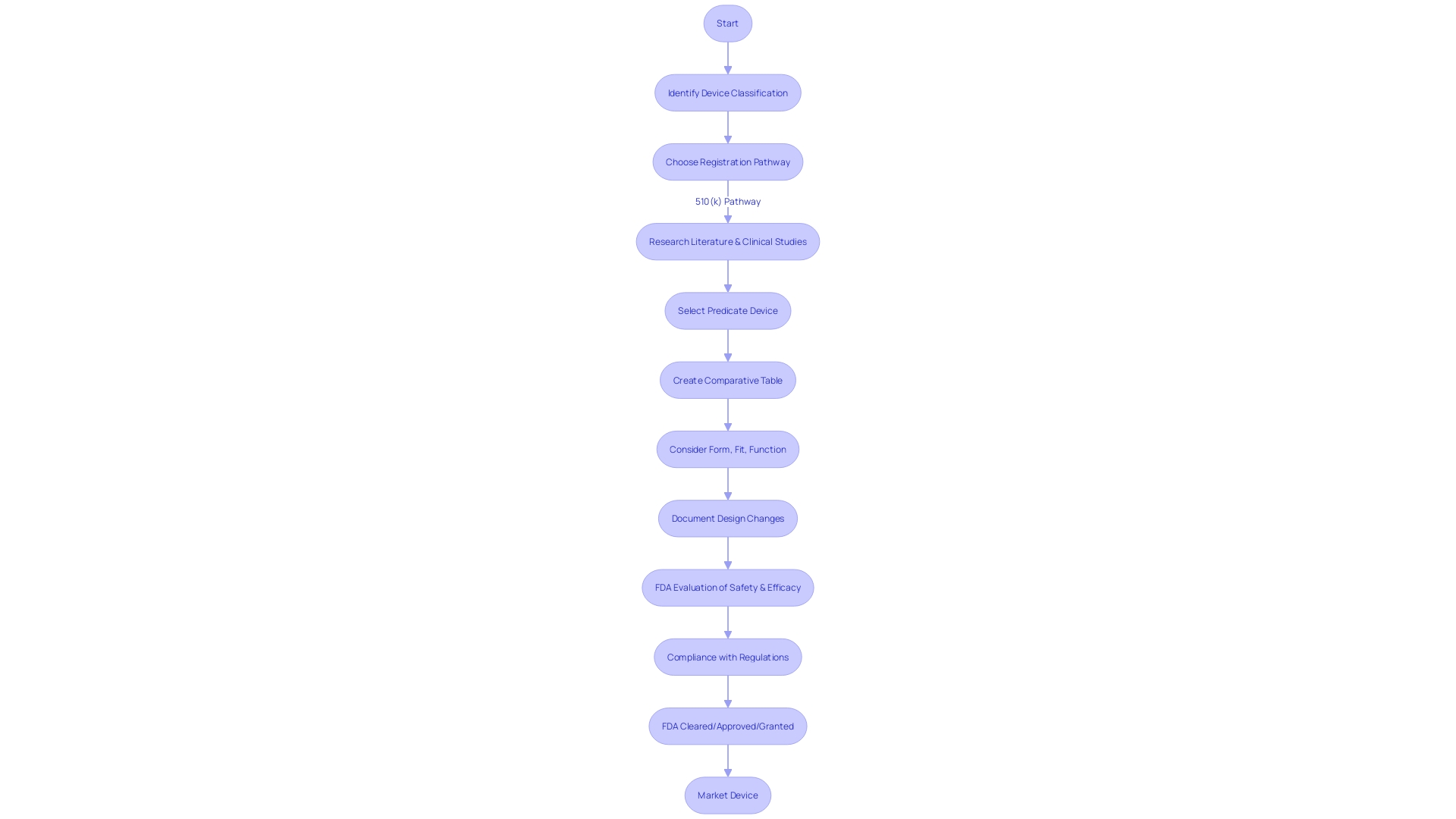
The 510(k) pathway is a critical regulatory process for medical equipment manufacturers aiming to market their innovations in the United States. This premarket submission to the FDA is intended to show that a product is as safe and effective as a legally marketed reference item. Thorough understanding of the equipment, its target users, and the competitive environment is essential. This entails examining research literature, current clinical studies, and marketing materials of other products to discover an appropriate precedent. The creation of a comparative table juxtaposing the new tool with the predicate helps illustrate substantial equivalence. The FDA's role extends beyond just assessing equivalence; it ensures public health by evaluating the safety and security of a vast array of products, from drugs to medical devices. Recent initiatives highlight the FDA's commitment to clarity and public safety, such as the final rule requiring direct-to-consumer prescription drug ads to clearly and neutrally present major side effects. In the same way, the FDA's systematic assessment for post-market safety of food chemicals showcases its overarching responsibility to safeguard public health. Understanding the nuances of the 510(k) process, such as when clinical trials may be bypassed, is imperative for compliance and patient safety. Continuous education on regulations and a thorough comparison of the new equipment against existing ones are vital steps in preparing a successful 510(k) submission.
Why is 510(k) FDA Approval Important?
For medical product manufacturers, securing 510(k) clearance from the FDA is a pivotal step in bringing their products to the U.S. market. This mechanism, which guarantees that an apparatus is secure and efficient for consumer utilization, is a crucial element of the FDA's responsibility in safeguarding public health. The significance of this clearance cannot be overstated; without it, companies are barred from commercial distribution within the country, which could severely disrupt their business and financial stability.
The 510(k) process, named after the section of the legislation that describes it, is a pathway to market for medical instruments that are substantially equivalent to a legally marketed product — referred to as a predicate item. Manufacturers must prove that their new product is as safe and effective as an established item that has already obtained FDA clearance. This comparison is vital for the FDA's assessment, but it's important to note that for many equipment, especially those considered to be of lower risk, clinical trials may not be required, as highlighted by the 2018 documentary 'The Bleeding Edge.'
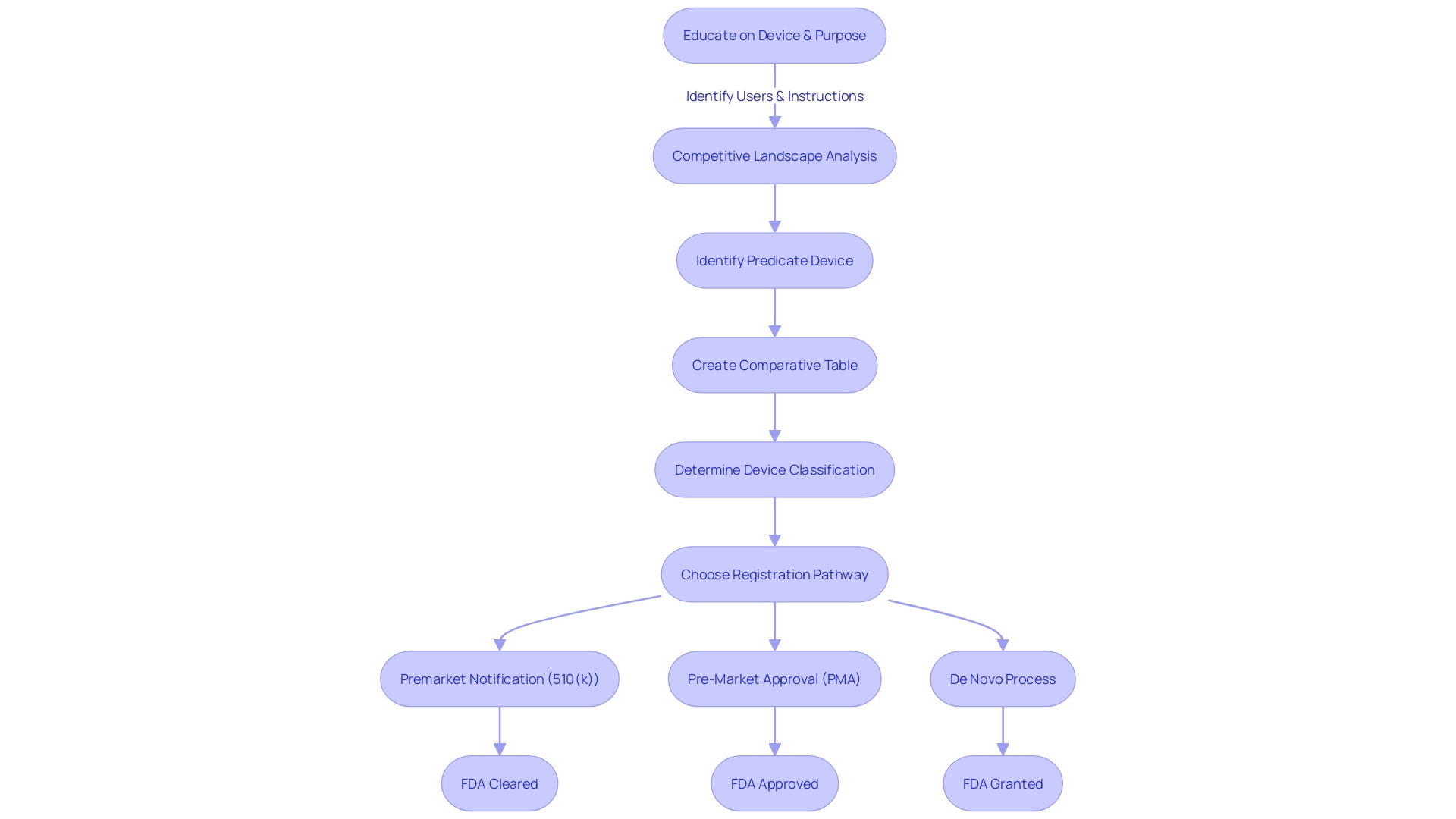
Comprehending the precise categorization of a medical instrument is crucial as it determines the extent of regulatory oversight required to guarantee safety and effectiveness. The classification of objects is based on the risk they pose to individuals, with Class I items posing the least risk and Class III the most. Based on the categorization, a product may undergo the 510(k) procedure, Pre-Market Approval (PMA), or De Novo approach. For example, the FDA commented on a situation where a company's software for an apparatus required validation under 21 CFR 820.30(g), highlighting the thoroughness of the FDA's review process.
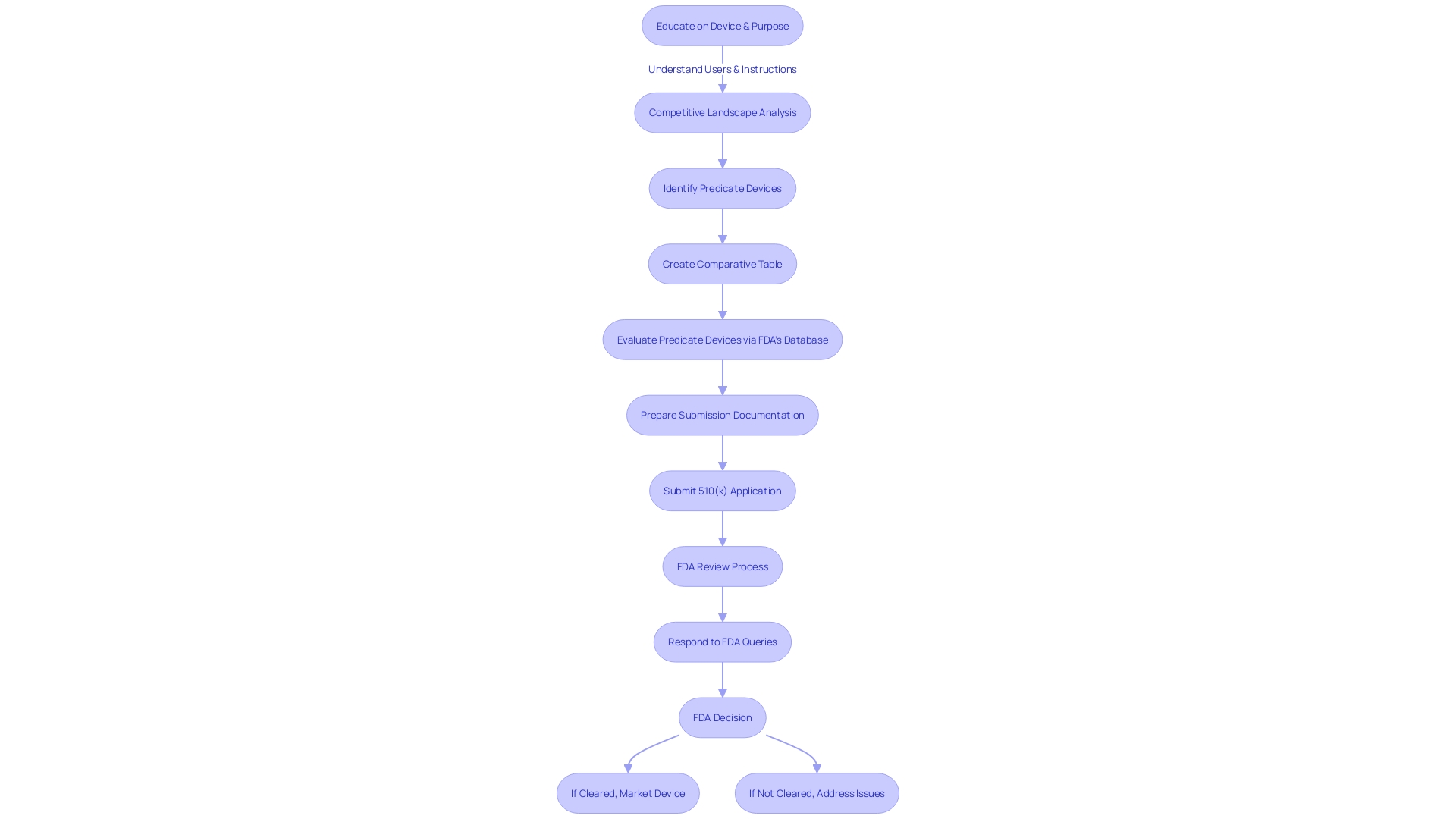
The path to clearance involves a profound comprehension of the apparatus, its users, and the competitive landscape. Regulatory professionals are recommended to identify potential predicate products and analyze their safety and effectiveness summaries in the FDA's 510(k) database. This groundwork is crucial for a successful submission.
While the 510(k) pathway is one of several routes to market, it is a common route for many manufacturers. The FDA's Center for Drug Evaluation and Research (CDER) emphasizes the significance of clear communication and comprehensive data in the application for a new instrument, whether it's a novel entity or related to an existing product. As medical technology advances, companies like Medtronic are continuously delivering innovative healthcare solutions, emphasizing the impact of FDA clearance on the ability to provide new treatments and improve patient care.
The FDA clearance is not just a regulatory hurdle; it is a testament to a company's commitment to safety and quality. It is a journey that requires meticulous preparation, an in-depth understanding of regulatory requirements, and a focus on the ultimate goal of enhancing healthcare outcomes. As the medical equipment industry expands and develops, the significance of navigating the FDA's approval procedure remains crucial for companies aiming to have a significant influence on global health.
Understanding Medical Device Classes and the 510(k) Process
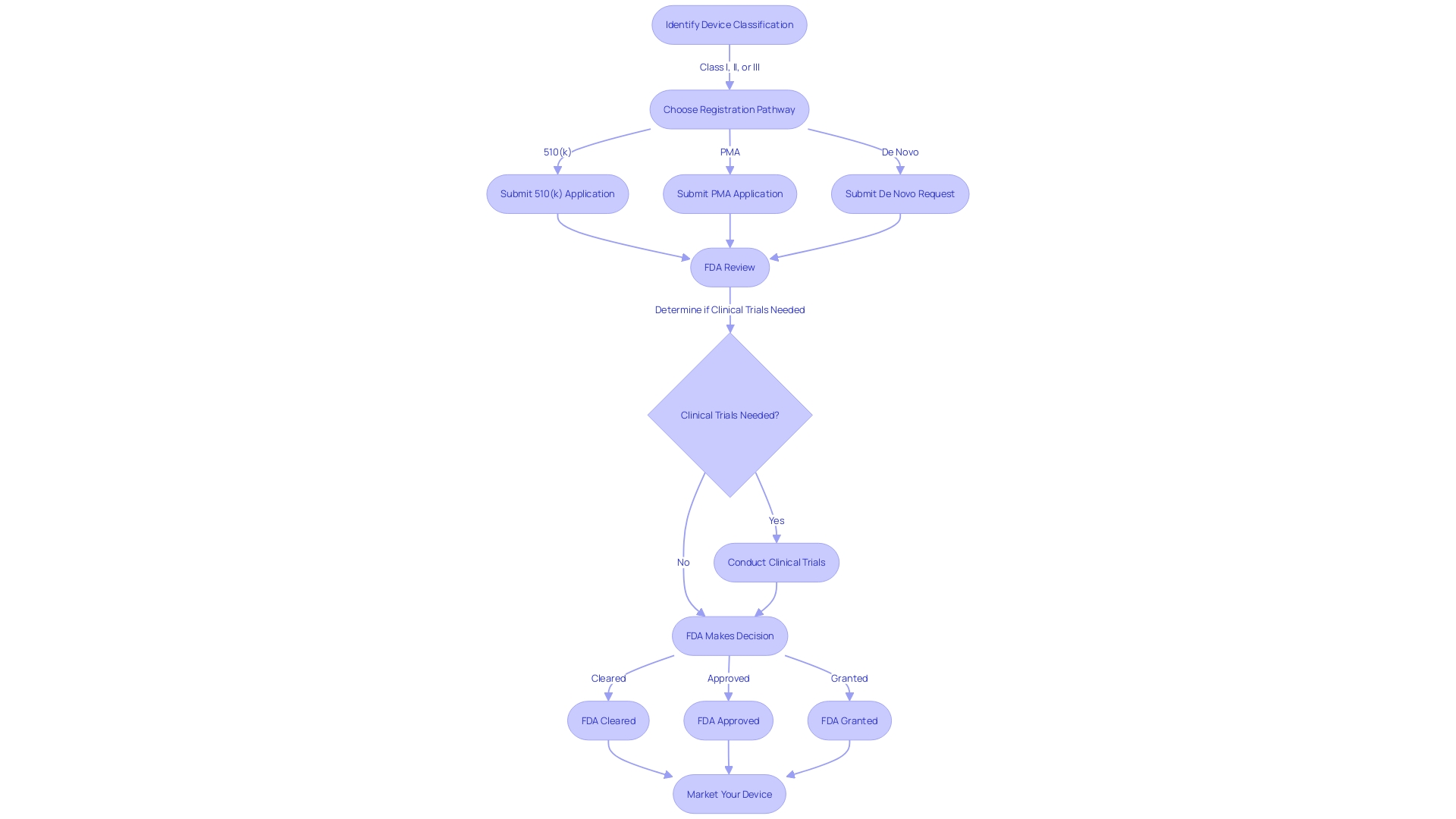
The FDA classifies medical equipment into three primary categories based on the level of risk they pose and the regulatory measures necessary to ensure their safety and effectiveness. Class I products are considered low-risk and are subject to the least regulatory oversight, while Class III products represent the highest-risk category and are subject to the most stringent controls, often requiring a rigorous premarket approval process. Class II products fall in the middle, representing moderate risk. They are usually subject to the 510(k) process—an application submitted before marketing that shows the product to be marketed is at least as safe and effective as a legally marketed item (predicate item) that is not subject to premarket approval.
Determining the accurate categorization for a medical product is crucial and can be accomplished by referring to the relevant description in the Code of Federal Regulations (CFR), particularly 21 CFR Parts 862-892, which classifies more than 1,700 unique varieties of products by medical specialization. In another way, the FDA's classification database allows searches by name to determine risk class. For example, Software as a Medical Device (SaMD) that meets the FDA's criteria can be acknowledged as a Class III product through such research.
The importance of the 510(k) pathway in Class II approvals is noteworthy, as it allows for the clearance of products that demonstrate substantial equivalence to an existing reference item. 'Nevertheless, the procedure has undergone examination, as emphasized in the 2018 film 'The Bleeding Edge,' which exposed how certain instruments were expedited without medical tests, resulting in unfavorable incidents such as hemorrhaging, organ perforation, and cobalt intoxication.'. In response to such concerns, the FDA continues to enhance its regulatory procedures to guarantee patient safety, as demonstrated by their significant oversight that has been on the High Risk List since 2009. The FDA's commitment to transparency and rigorous assessment is further exemplified by the recent establishment of clear standards for the presentation of major side effects in direct-to-consumer prescription drug advertisements.
As medical instruments continue to advance, it is crucial for stakeholders to acquire a comprehensive understanding of the instruments, including user instructions, warnings, cautions, and the competitive landscape. In-depth understanding of predicate instruments, their intended application, technological features, and safety effectiveness summaries accessible on the FDA's 510(k) database are priceless in navigating the approval procedure. Furthermore, the agreement norms established by acknowledged Standards Development Organizations (SDOs) support the regulatory structure, highlighting the significance of openness, fair representation, and proper procedure in the creation and application of medical products.
Overall, the 510(k) clearance process plays a crucial role in the regulatory landscape for Class II equipment, balancing the requirement for innovation with the imperative of patient safety.
Key Components of a 510(k) Submission
Crafting a 510(k) submission demands meticulous attention to detail and a strong comprehension of the medical instrument in question. A comprehensive description of the equipment is the beginning, outlining the specifications, materials, and operating principles. The intended use follows, clarifying the medical conditions the tool aims to diagnose, treat, or prevent, and the context in which it will be used. Technological characteristics, including design features, components, and the underlying technology, must be compared to a predicate device—this comparison forms the crux of the submission, demonstrating substantial equivalence. Performance data, encompassing clinical or non-clinical studies, validates the device's safety and efficacy, ensuring it meets the necessary standards for FDA clearance.
In the pursuit of completeness, manufacturers can look to recent FDA guidelines, which emphasize clarity and neutrality in presenting data. For example, the FDA's recent regulation on prescription drug ads mandates that information should be communicated in simple terms, with audio and text elements coordinated for improved understanding—principles that are relevant to the 510(k) submission procedure.
Adequate documentation and supporting evidence are pivotal. Manufacturers should emulate the exhaustive detail found in regulatory submissions, such as those compiled by life sciences companies, which integrate data from various sources, including experiments, meetings, and presentations. By adopting a similar level of thoroughness, manufacturers can streamline the FDA's review process.
A practical approach to comprehending the apparatus and its environment involves exploring research literature, clinical studies, and competitor analysis. Engaging with marketing teams and utilizing resources like FDA's 510(k) database can significantly enhance the comparative analysis required for identifying an appropriate predicate item. Such preliminary work, influenced by the experiences of entrepreneurs like those from Artos, can significantly diminish the intricacy and length of the 510(k) submission procedure.
Finally, manufacturers must remain vigilant about the confidentiality of sensitive information. The FDA's instructions for submitting comments stress the importance of safeguarding personal and proprietary data—a consideration that extends to all aspects of a 510(k) submission.
The 510(k) Submission Process
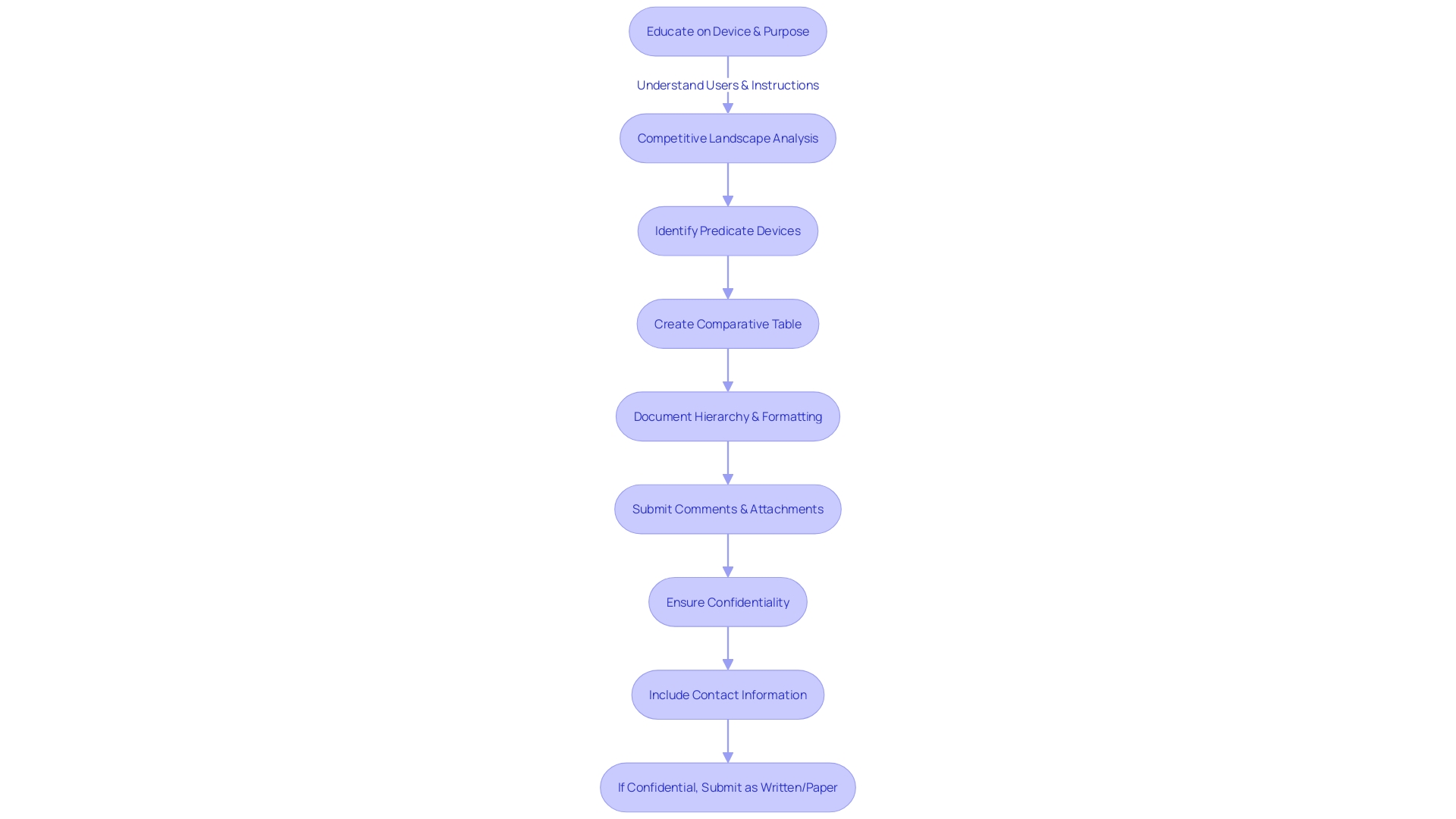
Navigating the 510(k) submission process is a critical step for manufacturers aiming to gain FDA clearance for their medical products. It is a multifaceted journey that starts with a comprehensive understanding of the object in question, including its intended users and usage instructions, along with an awareness of any potential risks. Manufacturers must delve into market research and competitive analysis, examining similar products to establish an appropriate predicate and create a comparative analysis.
The submission itself must be meticulously prepared, following the FDA's structured hierarchy of documentation to ensure clarity and compliance. This includes a comprehensive package that addresses all aspects of the device's design, testing, and intended use. Public comments play a part in this procedure, offering a platform for feedback that the Health and Human Services Department considers during their review.
Once submitted, the FDA's Center for Drug Evaluation and Research (CDER) evaluates the application. Their decision-making method is informed by extensive knowledge in science, medicine, and manufacturing, which is crucial for the assessment of new medical products. CDER's annual approval of a broad spectrum of new drugs and biological products underscores their commitment to innovation and public health.
It is significant to acknowledge that the FDA's review is thorough and relies on a comprehensive comprehension of the product and its context. Forward-looking statements by manufacturers about the expected benefits of FDA 510(k) approval must be tempered with an understanding that there are risks and unknowns that could impact the outcome. Factors such as market dynamics, clinical results, regulatory scrutiny, and financial considerations can all influence the success of a new medical equipment entering the market.
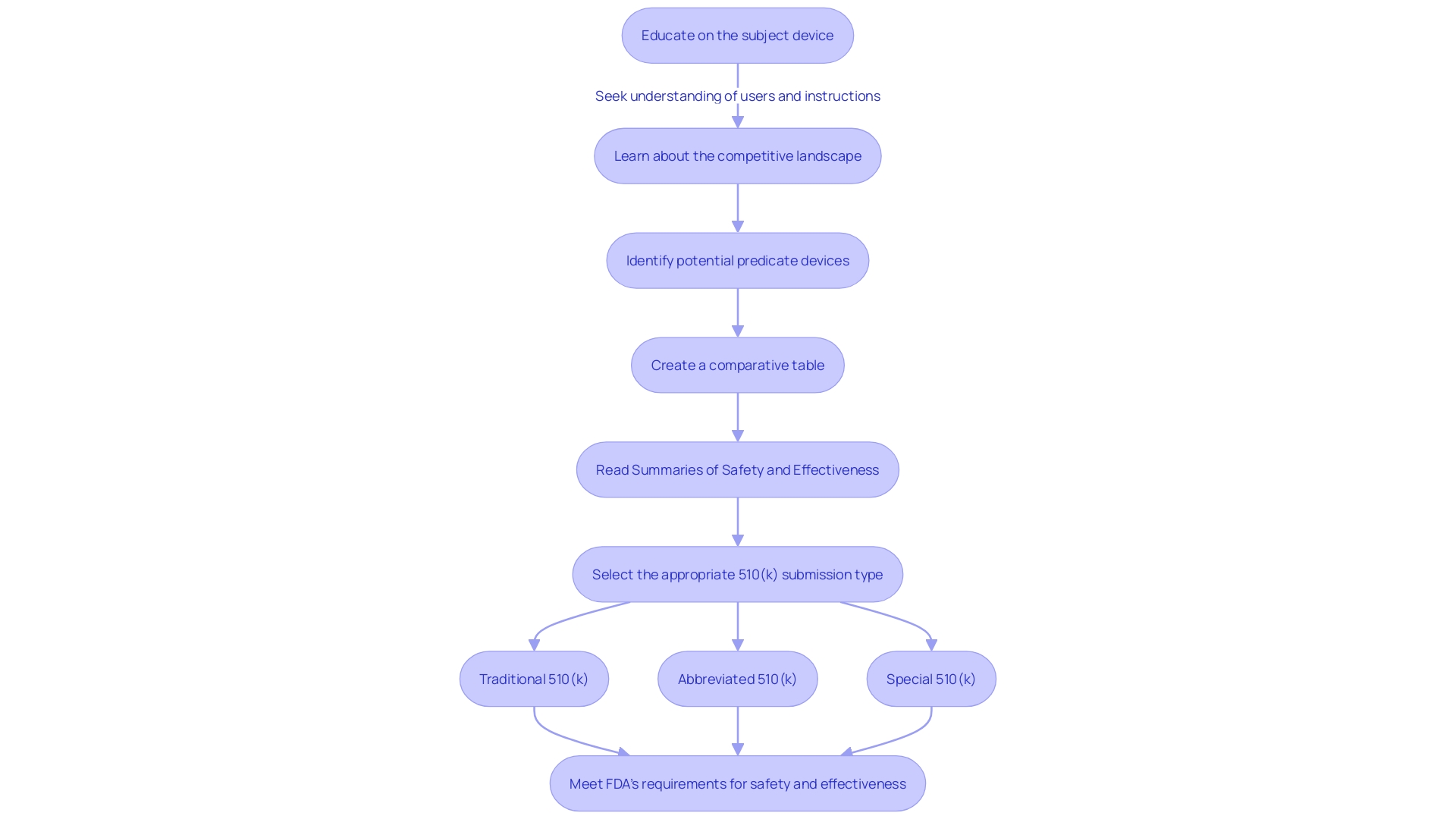
Types of 510(k) Submissions
Understanding the various types available, such as the traditional, abbreviated, and special 510(k), is essential for navigating the 510(k) submission. A traditional 510(k) is the standard route for clearance and involves a thorough review of non-clinical and, when applicable, clinical data to demonstrate that a new product is substantially equivalent to a reference item. In scenarios where the indications for use or technological characteristics differ from the predicate, or if non-clinical testing isn't sufficient to prove substantial equivalence, a traditional 510(k) submission is necessary.
For an abbreviated 510(k), the focus is on leveraging guidance documents, special controls, and recognized standards to demonstrate substantial equivalence. It's a streamlined process that can expedite the review by aligning with FDA's current thinking.
Meanwhile, a special 510(k) is suitable when a manufacturer makes modifications to its own cleared product. This pathway can be utilized if the modifications do not impact the intended use or alter the fundamental scientific technology of the apparatus.
When selecting the suitable 510(k) submission type, manufacturers must thoroughly comprehend the product, its users, intended use, and the competitive landscape. This includes reviewing research literature, clinical studies, and competitor products to identify appropriate predicates and generate a comparative analysis.
It's also important for manufacturers to be aware of the FDA's current list of AI/ML-enabled products, which demonstrates the evolving regulatory landscape and helps determine the most appropriate submission pathway.
In summary, choosing the appropriate 510(k) submission type relies on a thorough understanding of the item in question, the current market, and current regulatory guidance, ensuring that manufacturers meet the FDA's strict requirements for safety and effectiveness.
The Role of Predicate Devices in 510(k) Submissions
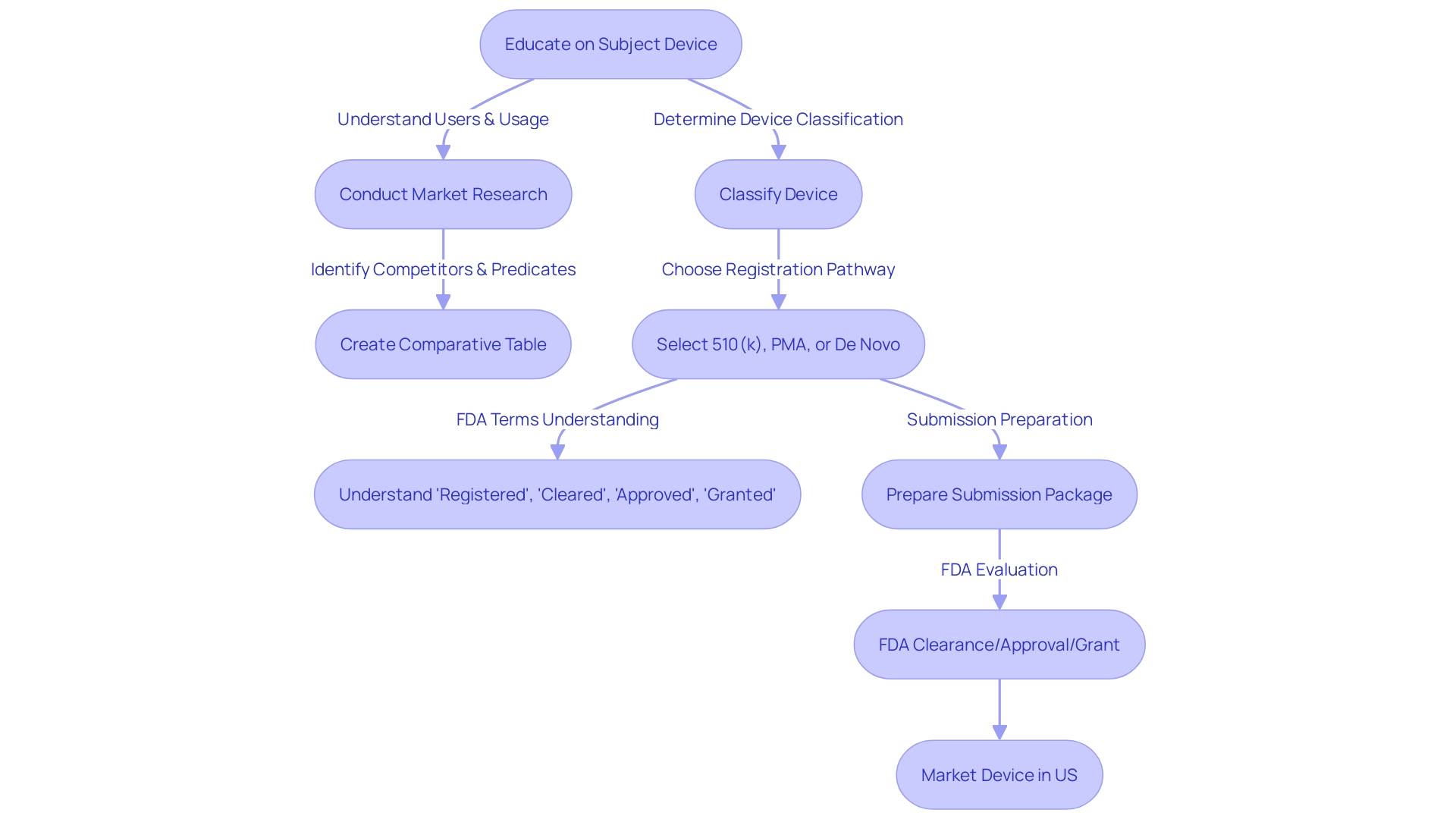
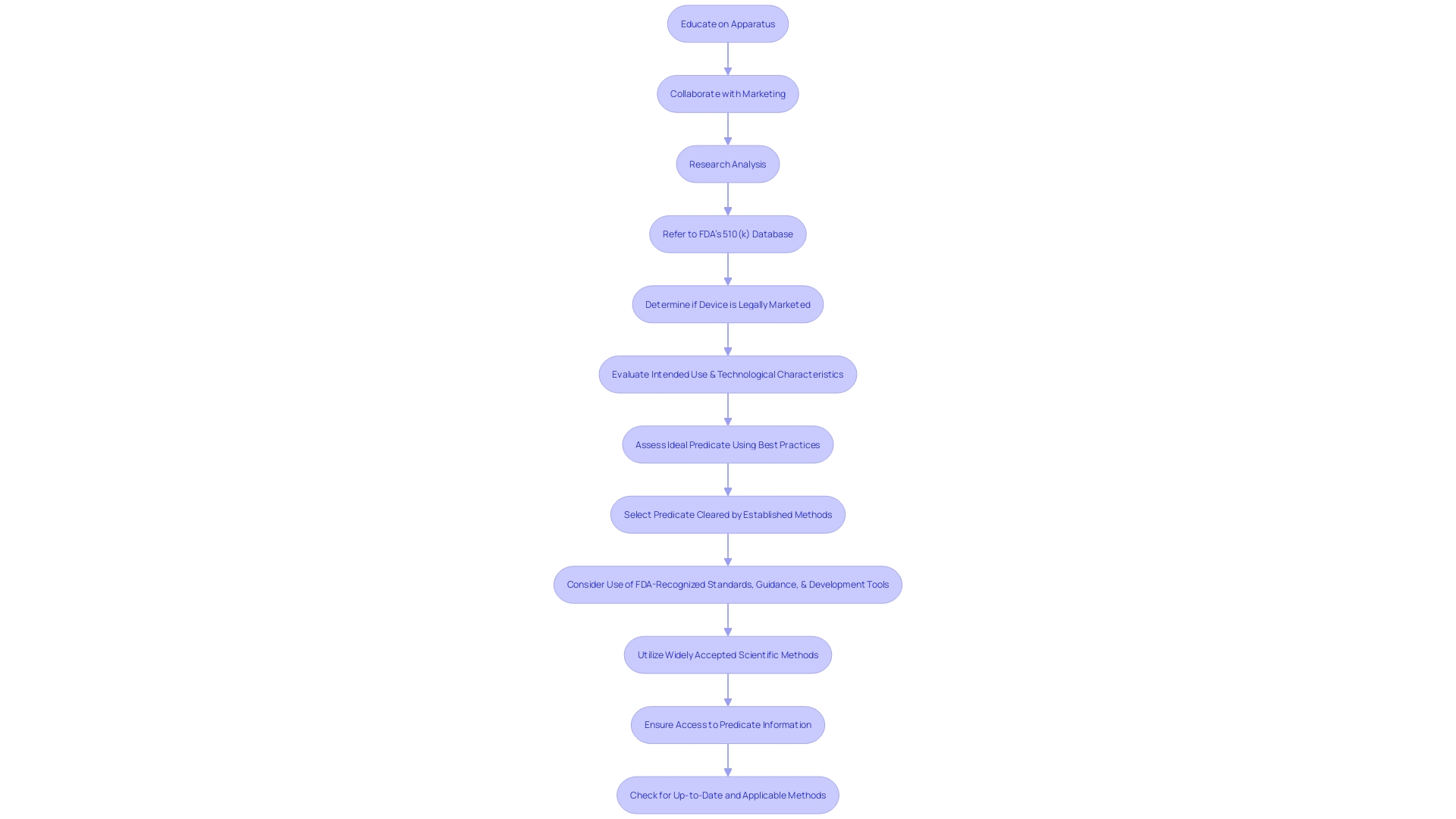
Comprehending and choosing a suitable predicate instrument is a crucial stage in the 510(k) submission procedure for FDA authorization. A predicate product refers to a legally marketed item that is similar to the one being proposed, which is used to demonstrate substantial equivalence. The equivalency is based on intended use and technological characteristics. The procedure starts with a comprehensive education on the apparatus in question, encompassing its function, users, and instructions for utilization, along with any warnings and cautions linked to it.
To determine the right predicate, it is beneficial to collaborate with the marketing department to analyze the competitive environment, review research literature, clinical studies, and competitors' marketing materials such as websites, brochures, and labels. This research helps in identifying objects with the same intended use and similar technological characteristics. Creating a comparative table may aid in this analysis. Moreover, referring to the Summaries of Safety and Effectiveness Data (SSED) accessible on the FDA's 510(k) database offers valuable insights into the similarities and distinctions among products.
This comprehensive comprehension is not only vital for selecting the appropriate predicate apparatus but also for ensuring the safety and efficacy of the new apparatus, as the FDA's primary duty is to safeguard public health. In recent times, standards for clear and neutral presentation of information in direct-to-consumer advertisements have been established, reflecting the FDA's commitment to transparent communication. These measures are a component of the FDA's wider responsibility to protect human health, including the control of medical instruments.
Demonstrating Substantial Equivalence
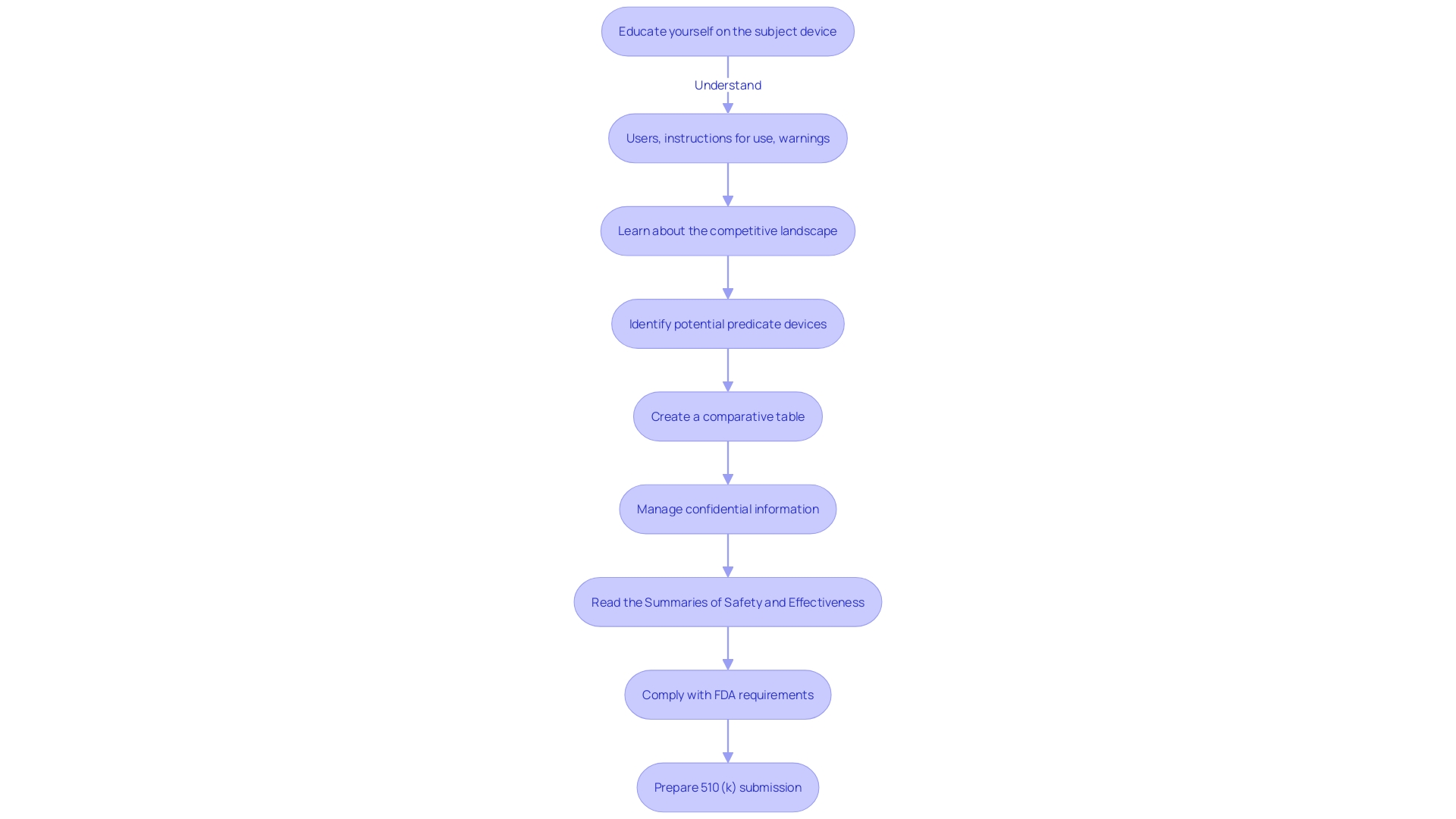
Achieving substantial equivalence is crucial when navigating the 510(k) submission process for FDA approval. Substantial equivalence means that a new medical instrument is at least as safe and effective as a legally marketed prototype. Demonstrating this involves a detailed comparison, including performance data and other relevant evidence, to confirm that any differences from the predicate do not adversely affect safety and efficacy.
When preparing a 510(k) submission, it's crucial to thoroughly research the subject product and understand its intended use, as well as the users it's designed for, such as clinicians, physicians, dentists, or patients. This comprehension should expand to the instructions for use of the apparatus, encompassing any warnings and cautions. With assistance from marketing teams and careful examination of the competitive landscape, a manufacturer can identify potential reference products that have the same intended use and possess similar technological characteristics.
Creating a comparative table becomes a foundational step in this process. By analyzing research literature, clinical studies, and other available resources, like FDA’s 510(k) database summaries of safety and effectiveness, manufacturers can evaluate the similarities and differences between the new product and its predicate. This thorough assessment is vital, as the FDA yearly examines a wide range of new drugs and medical equipment, some of which are completely novel to clinical practice, while others are comparable to current products.
As part of the submission, it's also important to manage the confidential business information judiciously. Any public comments or documentation submitted to the FDA should be carefully examined to ensure no confidential or sensitive information is inadvertently disclosed. Following guidelines for written submissions and marking confidential business information can protect proprietary details while complying with FDA's public transparency requirements.
Common Challenges and Mistakes in 510(k) Submissions
Navigating the 510(k) submission process can be intricate, often fraught with potential missteps such as insufficient testing, lacking documentation, and neglecting to properly address FDA feedback. To reduce these risks, it is essential for manufacturers to acquire a comprehensive understanding of the subject apparatus, including its users (clinicians, physicians, dentists, patients, etc.) and the comprehensive instructions for use, particularly warnings and cautions. Gathering information about the competitive landscape with the assistance of Marketing departments can uncover insights into potential predicate items that have the same intended use and similar technological characteristics. Manufacturers are advised to consult research literature, clinical studies, websites, brochures, and labeling to construct a comparative table that can serve as a vital tool in aligning with FDA's stringent requirements.
Moreover, the importance of adhering to the FDA's guidance documents is underscored by the revised formats, which now present information in a question-and-answer layout, clarifying the scope and expectations. It is imperative to understand that introducing a medical product into interstate commerce without complying with premarket requirements or for an unapproved use is prohibited under the Federal Food, Drug, and Cosmetic Act and the Public Health Service Act. The distribution of such products can compromise critical government interests.
Public comments and feedback play a significant role in shaping regulatory frameworks and ensuring the safety and efficacy of medical devices. Stakeholders must actively participate in the FDA's systematic procedures for safety reviews, as demonstrated by recent public meetings on post-market assessments of food chemicals, highlighting the FDA's dedication to transparency and stakeholder involvement. Such participation helps inform and refine regulatory pathways, of which the 510(k) procedure is a component.
Educational resources, such as clinical investigator training courses, are available to professionals involved in the development of medical products. These courses provide practical knowledge on safety concerns and regulatory compliance, catering to a broad audience including healthcare workers and individuals in biomedical research. Recognizing the crucial role of safety in product development is a cornerstone of 510(k) submissions, ensuring that both the industry and regulatory bodies maintain the highest standards in public health protections.
Best Practices for a Successful 510(k) Submission
A comprehensive understanding of the FDA’s regulatory landscape is vital when preparing for a 510(k) submission, which is the process required to demonstrate that a medical instrument is safe and effective for its intended use. It is crucial to become knowledgeable about the FDA’s regulations and guidance documents, especially the investigational instrument exemption (IDE), which is required for instruments not yet approved by the FDA but intended for use in clinical studies.
The complexities of 510(k) submissions, as experienced by the founders of Artos, highlight the importance of compiling and organizing vast amounts of data, including experiment reports, meeting notes, and more. This meticulous documentation is essential for FDA’s assessment of the product's safety and effectiveness.
Furthermore, familiarizing oneself with the subject apparatus and its wider context is of utmost significance. This includes a deep dive into understanding the users, the competitive landscape, and identifying potential predicate objects with the same intended use and technological characteristics. Evaluating similarities and differences with predicate products is crucial when analyzing summaries of safety and effectiveness data (SEts) from the FDA’s 510(k) database.
According to experienced healthcare professionals, the success of medical equipment companies is not only attributed to the tenacity and confidence of their leaders but also hinges on comprehensive knowledge of the apparatus and the market it enters. This includes a strategic approach to submissions that can lead to successful outcomes such as acquisitions, IPOs, or strategic alliances.
Recent FDA publications, like the final rule on direct-to-consumer prescription drug advertisements, emphasize the importance of clear, conspicuous, and neutral presentation of information, which is also relevant when considering the transparency and comprehensibility of submission documents.
To sum up, the 510(k) submission requires a thorough comprehension of FDA regulations, a thorough evaluation of the equipment, and strategic paperwork, all with the goal of guaranteeing a successful assessment by the FDA.
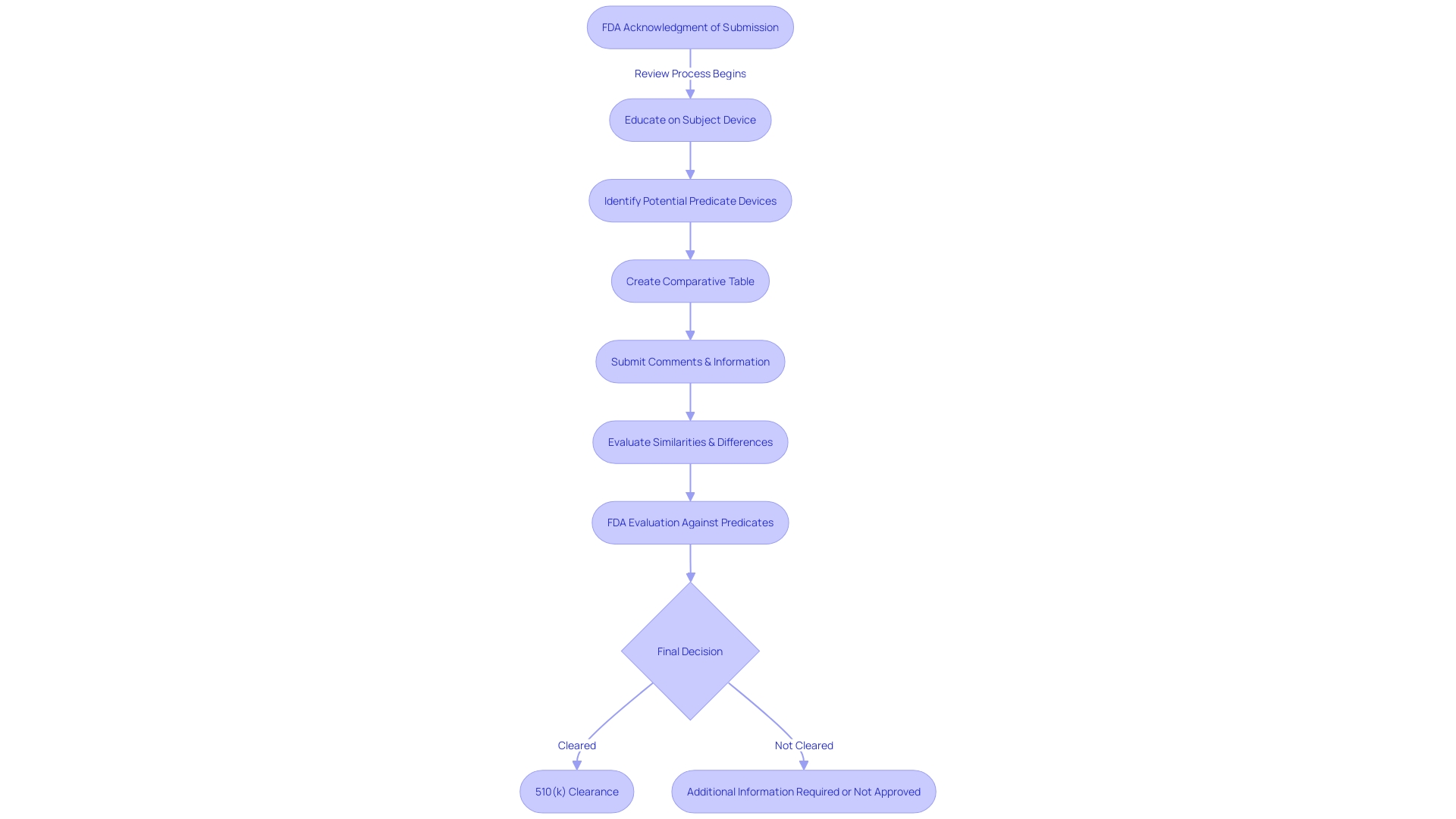
Timeline and Milestones in the 510(k) Review Process
Navigating the 510(k) review pathway is a crucial route for medical apparatus makers to bring their products to market. The timeline of this pathway features distinct stages, beginning with the FDA's acknowledgment of a submission. This first stage is followed by a thorough evaluation where the FDA assesses the product against similar existing devices, referred to as predicates, to ascertain its safety and effectiveness. Manufacturers must thoroughly prepare for this stage by thoroughly comprehending their equipment's intended purpose, technological features, and the competitive environment, often creating a comparative chart to assist in this endeavor. The final decision stage concludes the review. Throughout these phases, open communication with the FDA is paramount, as illustrated by the recent 'Direct-to-Consumer Prescription Drug Advertisements' final rule, which emphasizes the importance of clarity and transparency in communications. This rule serves as a reminder for manufacturers to present information in a consumer-friendly manner, a principle that also applies to the 510(k) submission process. With an informed approach incorporating a comprehensive review of research literature, clinical studies, and competitor analysis, manufacturers can more effectively manage expectations and timelines, ultimately contributing to public health by ensuring the safety, effectiveness, and security of medical devices.
Conclusion
In conclusion, the 510(k) FDA approval process is crucial for medical device manufacturers seeking to bring their innovations to the U.S. market. It ensures the safety and effectiveness of devices and plays a vital role in protecting public health. Understanding the process, including identifying suitable predicate devices and creating a comparative analysis, is essential for compliance and patient safety.
Securing 510(k) clearance is pivotal for manufacturers as it allows them to commercialize their products in the U.S. Without this clearance, companies may face disruptions to their business and financial stability. The process requires demonstrating substantial equivalence to a legally marketed predicate device, with the level of risk determining the regulatory control needed.
Successful submissions demand meticulous attention to detail and a comprehensive understanding of the device. Thorough device descriptions, clarification of intended use, comparison of technological characteristics, and validation of performance data are key components. Adherence to FDA guidelines and a deep understanding of the competitive landscape are crucial.
Navigating the process can be complex, with challenges to avoid such as insufficient testing and lacking documentation. Safeguarding sensitive information is also essential. Best practices include understanding FDA regulations, comprehensive knowledge of the device and market, strategic documentation, and adherence to transparency principles.
In summary, the 510(k) FDA approval process is critical for medical device manufacturers. Navigating it successfully requires a deep understanding of regulatory requirements, a comprehensive assessment of the device, and strategic documentation. By following best practices and adhering to FDA guidelines, manufacturers can contribute to healthcare outcomes while ensuring the safety and effectiveness of their devices.




