Introduction
Clinical trials play a crucial role in advancing medical treatments and interventions. To ensure the safety, integrity, and compliance of these trials, researchers and sponsors must adhere to the regulations outlined in CFR Title 21 Part 812. This article provides a comprehensive overview of the key sections and responsibilities outlined in the regulation, including orphan drug exclusivity, device classifications, informed consent, labeling and distribution, monitoring, reporting and recordkeeping, and compliance with ethical and regulatory standards.
By understanding and implementing these guidelines, researchers and sponsors can conduct clinical trials that uphold the highest standards of research integrity and ultimately contribute to meaningful medical advancements and improved patient outcomes.
Key Sections of CFR 21 Part 812
CFR 21 Part 812 details the important rules for performing experiments, specifically focusing on the complexities of orphan medications and tools. Orphan drug exclusivity is a pivotal aspect, granting seven years of marketing exclusivity to sponsors of designated orphan drugs, provided no previous approval exists for the same indication. This exclusivity incentivizes the development of treatments for rare diseases, affecting subsets of populations where alternative drug use may be inappropriate due to factors like toxicity or mechanism of action. The FDA also delineates the distinctions between small molecule drugs and macromolecules, offering exclusivity unless a subsequent drug proves clinically superior. In the realm of gadgets, the FDA establishes definitions and requirements for limited devices, first importers, and the classification of devices, which includes careful consideration of labeling and advertising practices to uphold gadget integrity and effectiveness. Recent FDA actions, such as the final rule on direct-to-consumer prescription drug advertisements, emphasize the importance of clear and neutral presentation of side effects, underscoring the agency's commitment to safeguarding public health.
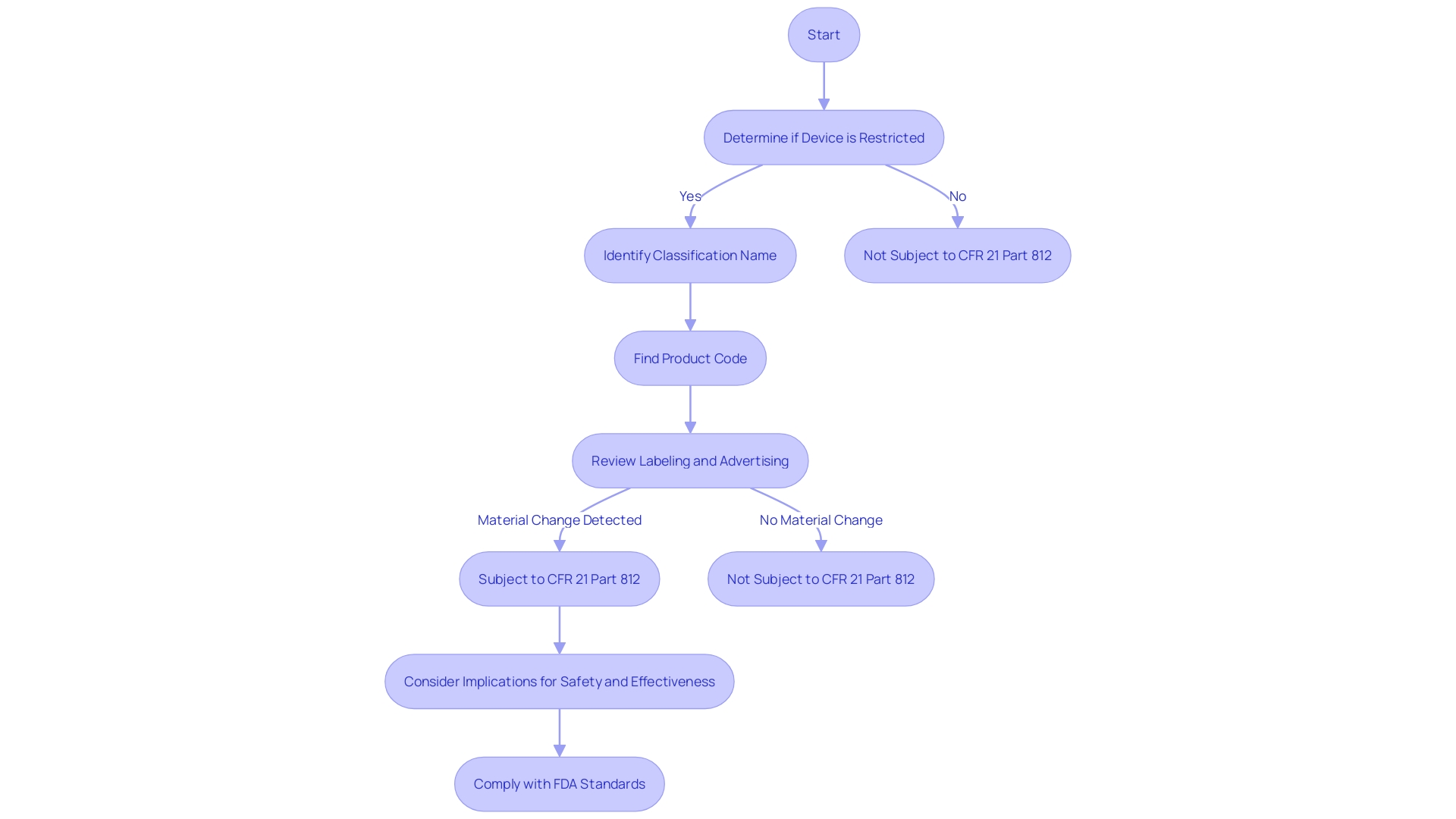
Determining the Applicability of CFR 21 Part 812
Determining whether a clinical trial falls under the purview of CFR 21 Part 812 is crucial for researchers and sponsors in the realm of clinical research. This part of the regulation delineates the circumstances under which a study is considered subject to the FDA's regulations or when it may be exempt. An important aspect of this determination includes comprehending the definitions and terms used within the regulations, such as 'restricted equipment', 'initial importer', and 'classification name', which are vital for compliance. For example, a 'restricted object' refers to an item that has specific sale, distribution, or use restrictions set by FDA regulations or as a condition of premarket approval. In the same way, 'classification name' is the term assigned by the FDA to describe a tool or a group of tools for classification purposes. Furthermore, the 'product code' is a classification technique employed by the FDA to determine the general category of an item. Understanding these terms and how they relate to a study's design and objectives is a foundational step in ensuring adherence to the FDA's standards for safety and effectiveness. Moreover, the nuances of regulations, like the implications of 'material change' in labeling or advertising, which can affect the identity or safety and effectiveness of a device, must be carefully considered to maintain compliance. By meticulously evaluating these criteria, researchers and sponsors can ascertain their study's regulatory status and implement the necessary protocols to align with CFR 21 Part 812.
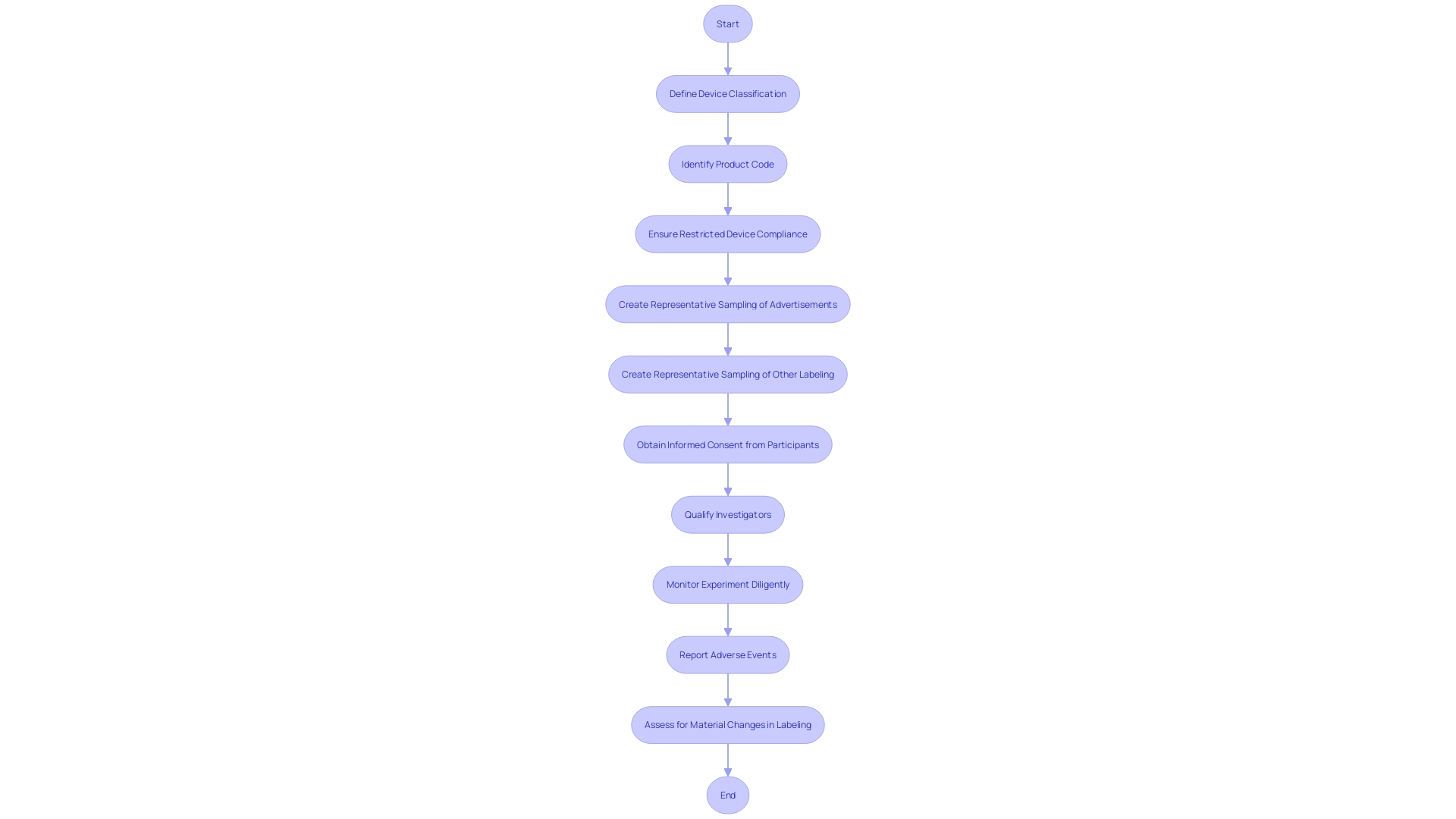
Responsibilities of the Investigator and Sponsor
CFR Title 21 Part 812 outlines essential duties for both researchers and sponsors involved in medical experiments, with a focus on the qualifications of investigators, the procedure of obtaining informed consent, the importance of diligent monitoring, and the strict reporting of adverse events. The credentials of investigators are crucial to ensure that experiments are carried out by individuals with the knowledge and honesty necessary to uphold the safety and rights of participants. Informed consent, a cornerstone ethical requirement, must be obtained from participants who are fully aware of the study's scope and potential risks. Monitoring is essential not only for participant safety but also for the integrity of the data collected. Furthermore, timely reporting of adverse events is crucial in maintaining transparency and enabling prompt action to protect participants. These responsibilities are not mere bureaucratic formalities; they are the foundations that safeguard participant welfare and the credibility of clinical research.
The process of testing, as depicted by the arduous experience of individuals with transthyretin-mediated amyloidosis, can be a substantial endeavor for those involved. Participants endure rigorous testing and potential side effects, all while grappling with their health conditions. This emphasizes the significance of careful adherence to the regulations stipulated by the CFR, ensuring that the experiments are not only compliant but also respectful of the sacrifices made by participants.
Furthermore, the ever-changing characteristic of medical investigation, as demonstrated by the regular modifications in project personnel and the intricacy of overseeing multiple examination locations, emphasizes the necessity for an organized approach to examination administration. The CFR's guidelines provide a framework that can help navigate these complexities, ensuring resources are allocated effectively and amendments to protocols are handled efficiently.
The governing framework for medical experiments is not static; it evolves alongside advancements in medical science and ethical considerations. The recent modernization of ClinicalTrials. Gov reflects this progression, aimed at enhancing the clarity and accessibility of study information for all stakeholders. It is imperative for investigators and sponsors to stay abreast of these changes to maintain compliance and uphold the highest standards of research integrity.
Ultimately, the dedication to comprehending and implementing the rules outlined in CFR Title 21 Part 812 is a commitment to excellence in research. It is through such dedication that medical advancements can be achieved and patient outcomes meaningfully improved.
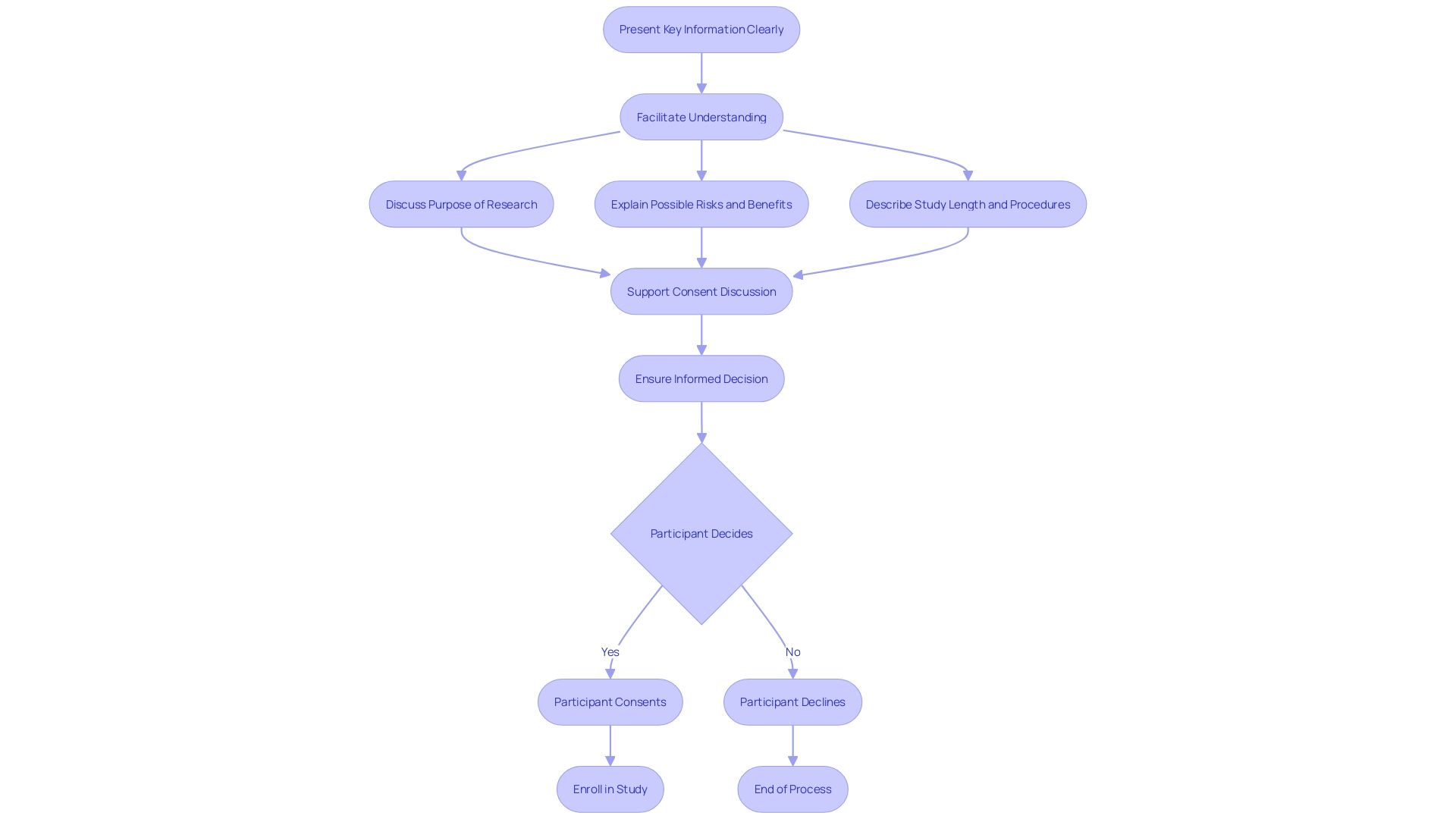
Informed Consent Process
The informed consent process is a crucial element of medical experiments, guaranteeing that participants are adequately informed about the study they may join. CFR 21 Part 812 outlines the requirements for informed consent documents, which are designed to help prospective subjects understand the implications of participating in research and to facilitate informed decision-making. These documents should not simply list isolated facts; instead, they should present information in a comprehensive yet comprehensible manner. Despite efforts to comply with evolving regulations, informed consent documents have grown in length and complexity, sometimes causing confusion rather than clarity. Over the past twenty years, the average length of these documents has ballooned from a few pages to over twenty. This increase in size and legal terminology has created a difficulty for the typical U.S. adult to comprehend, resulting in an obstacle in enrollment for medical studies, especially among marginalized minority populations.
It is crucial for researchers to tackle the fundamental inquiries that participants may have about a study, such as its objective, duration, necessary procedures, potential risks, and benefits, as well as how it will impact their healthcare and any associated costs. Draft guidance from the FDA encourages starting the informed consent document with this key information, presented in a clear and concise way to support participants' understanding. This approach aligns with the fundamental principles of respect for individuals, beneficence, and justice as outlined in the Declaration of Helsinki and the Belmont Report. Furthermore, results from a Research!America and ACRO survey in October 2023 show that while the public firmly backs the incorporation of medical research into healthcare, there is a notable disparity in conversations about experiments between healthcare providers and patients. To enhance this, 77% of participants express a preference for obtaining information about experiments from their doctors or healthcare providers.
In conclusion, informed consent is more than a regulatory requirement; it is a process that must acknowledge and respect each individual's right to make fully informed health care decisions without undue influence. The aim is not only to comply with the law but to genuinely aid participants in understanding the nature, purpose, risks, and benefits of both medical and non-medical procedures and treatments.
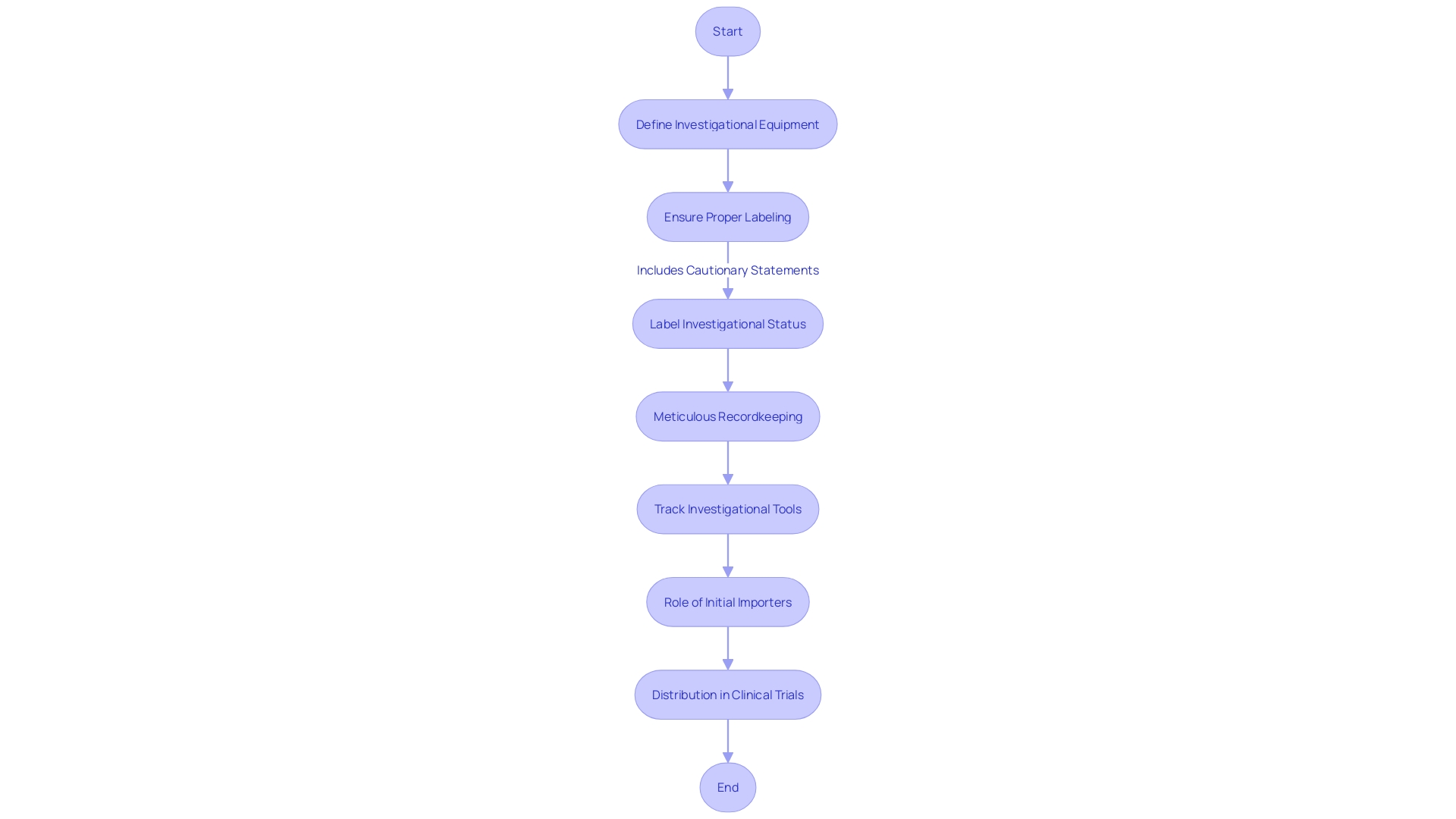
Labeling and Distribution of Investigational Devices
CFR 21 Part 812 establishes the guidelines for labeling and distribution of investigative equipment, a crucial element to the integrity of clinical trials. Appropriate labeling is not just obligatory but also a protective measure for participants; it must clearly indicate the investigational status of the equipment with labels such as 'Investigational Use Only' along with necessary cautionary statements. These requirements are crucial for maintaining transparency and patient safety, as evidenced by the Impella Connect System case, where the system’s software functions for remote monitoring were deemed to require premarket authorization due to their direct impact on patient care.
Furthermore, the distribution process demands meticulous recordkeeping and tracking to ensure that investigational tools are managed correctly. The importance of these regulations is highlighted by a case where a firm had to undergo retrospective complaint reviews and revise procedures due to inadequacies in complaint coding and risk analysis, as mandated by 21 CFR 820.30(g).
The FDA defines a 'restricted product' as one that has specific limitations on sale, distribution, or use, as established by regulation or order. Every equipment is given a 'classification name' and 'product code' for identification and regulatory purposes. Advertisements and labeling materials, excluding labels and package inserts, must not promote the product beyond its approved use, and any material change in these can significantly affect the item's identity and its safety and effectiveness.
The distribution chain also involves 'initial importers' who further the marketing of products from foreign manufacturers to the end consumer, without altering the packaging or labeling. Following these guidelines is crucial for upholding the trust and safety of all stakeholders in the trial process. This is part of the FDA’s broader role in protecting public health by ensuring the safety and efficacy of medical devices, among other responsibilities.
Monitoring of Clinical Investigations
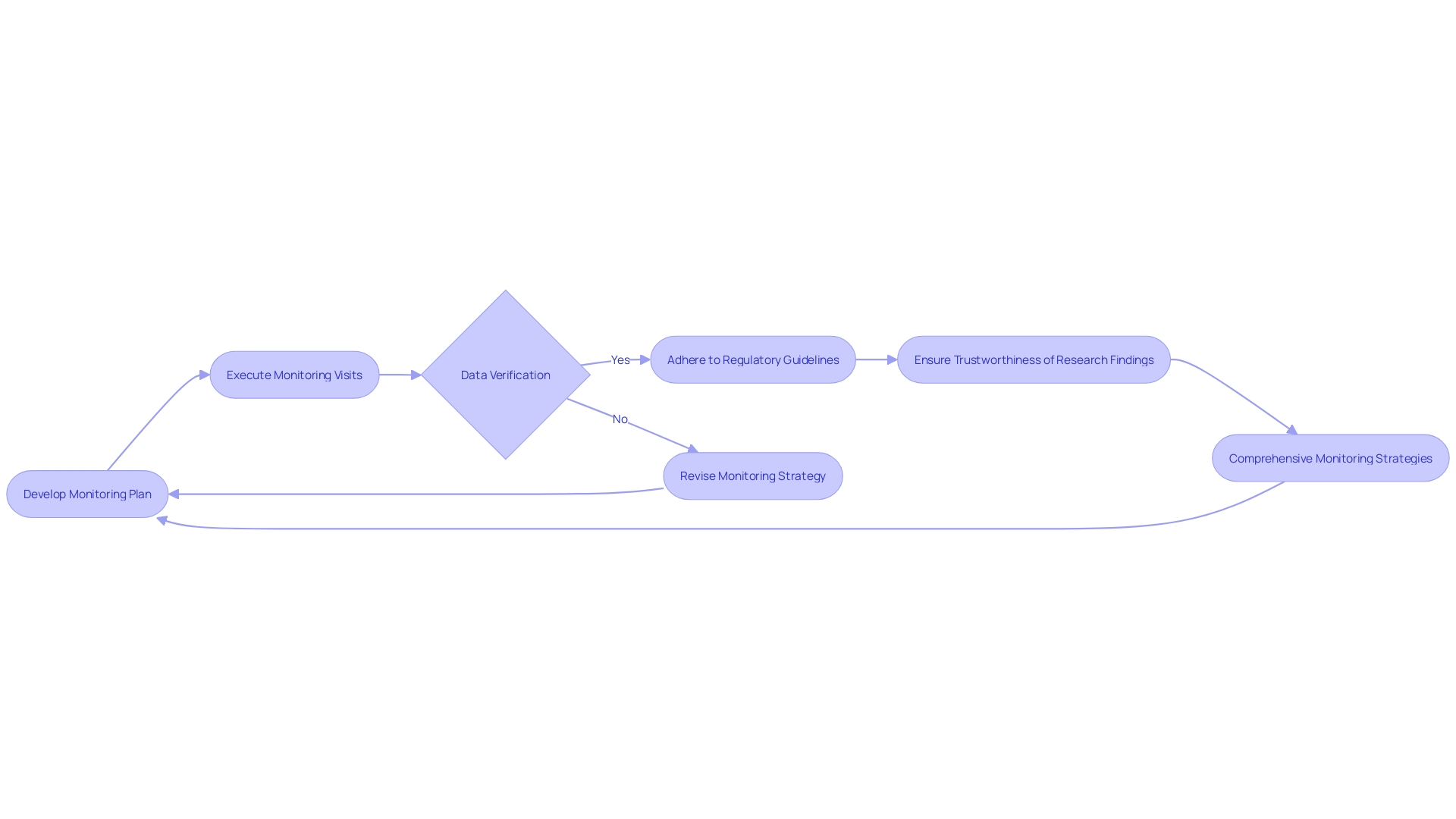
Monitoring the quality and integrity of investigations is a cornerstone of conducting effective research. CFR 21 Part 812 delineates clear guidelines for this process, emphasizing the development of comprehensive monitoring plans and the execution of monitoring visits. Moreover, it underscores the criticality of data verification to uphold the trustworthiness of research findings. By adhering to these guidelines, researchers and sponsors can forge robust monitoring strategies that not only align with compliance mandates but also bolster the dependability of the data collected. Furthermore, comprehending these regulatory requirements is crucial in promoting collaboration and knowledge sharing across medical sites, as demonstrated in the instance of the 30-month Articulate Pro project that integrated an AI-driven decision support software in prostate cancer diagnosis. The project's phased approach and emphasis on advanced training reflect the value of methodical planning and continuous education in clinical research. Furthermore, with the FDA's commitment to public health and safety, as exemplified by the new standards for direct-to-consumer prescription drug advertisements, the clarity and conciseness in communication of trial information become increasingly important. The explanation of terms like 'limited equipment' and 'first importer', along with instructions for promoting and marking, emphasize the FDA's methodical approach to guaranteeing that medical instruments are precisely portrayed and supervised after being released. Such meticulous attention to regulatory details facilitates a more efficient drug approval process and ultimately serves to improve the landscape of drug therapy and medical device utilization.
Reporting and Recordkeeping
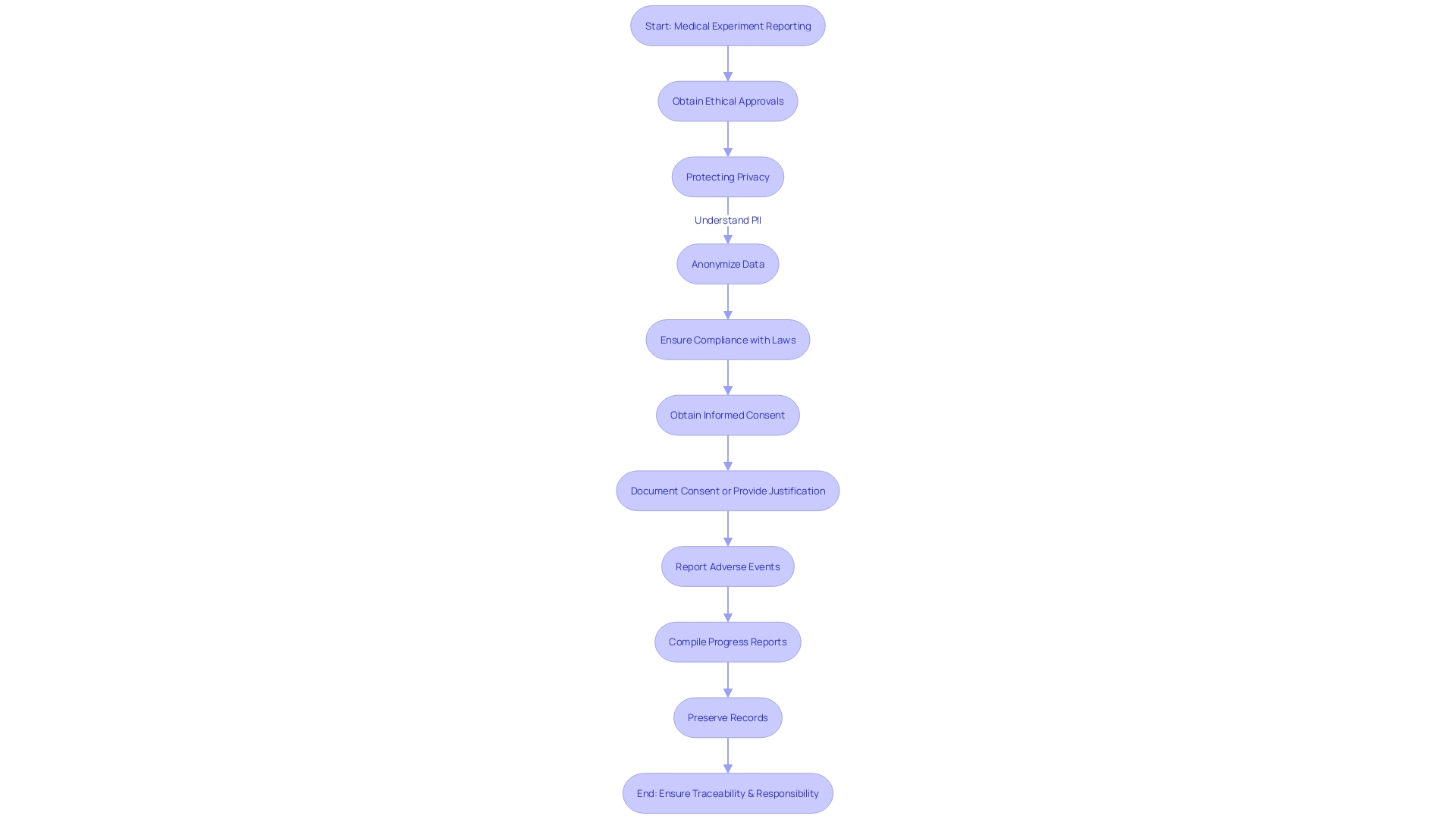
Keeping detailed records and comprehensive reporting is essential in the field of medical experiments, where openness and responsibility are the foundations of ethical research. CFR Title 21 Part 812 delineates the specific requirements for reporting and recordkeeping, which are crucial for documenting adverse events, compiling progress reports, and preserving records. These regulations serve as a backbone for ensuring that all aspects of a clinical trial are traceable and well-documented.
Under this framework, researchers must report any event that deviates from current good manufacturing practice or unexpected events that may affect the safety, purity, or potency of a product. This includes incidents occurring not only within their own facilities but also at any contract-associated facilities. The meaning of a 'limited apparatus' and the consequences for its sale, distribution, or use are also clearly defined, as are the terms 'first importer,' 'classification title,' and 'product code,' which are essential in comprehending the regulatory atmosphere.
The Food and Drug Administration (FDA), responsible for safeguarding public health, has established these standards to ensure that medical devices and pharmaceuticals are safe and effective. The agency's recent "Direct-to-Consumer Prescription Drug Advertisements" rule exemplifies the commitment to clarity and transparency in healthcare communications, mandating that advertisements for prescription drugs on television and radio present major side effects and contraindications in a clear, conspicuous, and neutral manner.
Clinical experiments, as mentioned by specialists in the domain, establish the basis for progressing medical treatments and interventions. With patient care and outcomes relying on the integrity of these experiments, it is crucial that they adhere to rigorous standards. These experiments progress through several stages, each intended to thoroughly assess the safety and effectiveness of new therapies. The individual investment of participants in the experiment, frequently undergoing a multitude of invasive tests and facing potential side effects, highlights the significant responsibility of researchers to uphold the utmost standards of experiment conduct and reporting.
The FDA's definitions and regulations are not merely bureaucratic stipulations; they reflect the agency's commitment to protecting individuals who bravely contribute to medical research. While researchers and sponsors navigate the intricacies of managing tests, compliance with the CFR's reporting and recordkeeping demands guarantees that the efforts made by test participants are respected through the integrity of the research process.
Compliance with Ethical and Regulatory Standards
Compliance with CFR Title 21 Part 812 is crucial in preserving the ethical and regulatory integrity of clinical trials involving investigational apparatus. This part of the regulations outlines strict prohibitions against the promotion of such products before they have received FDA approval. Moreover, it delineates specific compliance requirements to ensure that research adheres to established safety and efficacy standards. Violations of these regulations can result in significant penalties, highlighting the gravity of non-compliance.
An illustrative case study underscores the ethical quandaries and regulatory challenges intrinsic to the governance of emerging technologies in the healthcare sector. It explores scenarios through vignettes, such as the hypothetical promotion of an investigational device, to emphasize the ethical implications and potential market pressures that could lead to regulatory infractions. These case studies serve as cautionary tales, demonstrating the importance of rigorous adherence to regulations to safeguard patient welfare and the credibility of medical research.
Statistical data further reveals that while 27,133 clinical experiments were conducted in high-income countries in 2022, only 24,791 were in low- and middle-income countries, indicating a disparity that may affect the equitable development and application of medical interventions. Ethical considerations, including informed consent and the respectful treatment of participants, are paramount. Clinical experiments must not only meet scientific standards but also uphold ethical principles that resonate with those guiding the treatment of other public servants, like firefighters, who are appropriately compensated for their contributions to public welfare.
In the context of medical experiments, the assessment of a product's efficacy is not only scientific but also regulatory, influenced by the experiment's structure which must adhere to stringent protocols to guarantee accurate and dependable outcomes. This regulatory framework is essential for ensuring that the development of medical interventions is both ethical and compliant with federal standards, ultimately guiding the responsible advancement of healthcare technologies.
Quality Management Systems in Clinical Investigations
The Code of Federal Regulations (CFR) Title 21 Part 812 provides a framework for the implementation of a Quality Management System (QMS) which is crucial for the integrity and success of trials. A robust QMS ensures that every phase of a clinical investigation adheres to the highest standards, safeguarding both the reliability of the data and the welfare of study participants. Essential aspects of a QMS include rigorous document control to maintain accurate records, comprehensive training programs to ensure that all personnel have the necessary expertise, and the implementation of corrective and preventive actions to address any issues that arise promptly.
For example, the classification name, product code, and representative sampling of advertisements and labeling are essential to the FDA's classification under Section 513 of the Act. Moreover, each change in labeling or advertisements that could impact the device's identity or its safety and effectiveness is deemed a material change, necessitating meticulous record-keeping and management within the QMS.
A case in point is the utilization of Point-of-Care Ultrasound (POCUS) technology, which became embedded in the medical curriculum following a strategic deployment that included assessing the needs across specialty groups and integrating existing systems. Such advancements exemplify the significance of maintaining a QMS that is adaptable and responsive to technological integration and the evolving landscape of medical research.
The medical community is continuously encouraged to adopt more automated and sophisticated QMS practices. For example, the recent push towards computer system validation over manual, paper-based processes indicates a shift towards more efficient, compliant operations. Regulatory bodies, such as the FDA, advocate for such progress, recognizing the potential for improved quality and safety in healthcare delivery.
The significance of a well-implemented QMS extends beyond regulatory compliance; it impacts patient safety and the overall quality of healthcare. As highlighted in various articles, the development and application of quality standards in healthcare is an ongoing endeavor, with the expectation that such measures will lead to better quality care. Additionally, the Joint Commission Journal on Quality and Patient Safety emphasizes the value of sharing knowledge on quality improvement interventions, thereby contributing to the collective effort of enhancing patient care.
In summary, adopting a comprehensive QMS in line with CFR 21 Part 812 is not only a regulatory requirement but a critical component in the pursuit of excellence in clinical research and patient care.
Conclusion
In conclusion, adhering to the regulations outlined in CFR Title 21 Part 812 is crucial for conducting clinical trials that uphold the highest standards of research integrity. The key sections of the regulation, such as orphan drug exclusivity and device classifications, provide guidance on critical aspects of clinical trials. Understanding the applicability of CFR 21 Part 812 and the definitions and terms used within the regulation is foundational for compliance.
The responsibilities of investigators and sponsors outlined in the regulation, including obtaining informed consent, vigilant monitoring, and reporting adverse events, are vital for participant safety and the credibility of clinical research. The commitment to these responsibilities not only ensures compliance but also respects the sacrifices made by trial participants.
The informed consent process is a pivotal component of clinical trials, and it must be approached with clarity and respect for participants' rights. Proper labeling and distribution of investigational devices are essential for maintaining transparency and patient safety. Monitoring and reporting of clinical investigations, along with meticulous recordkeeping, are imperative for upholding the integrity of research findings.
Compliance with ethical and regulatory standards is paramount in clinical trials, and violations can result in significant penalties. Implementing a Quality Management System (QMS) is crucial for the success and integrity of clinical investigations, ensuring adherence to the highest standards and safeguarding participant welfare.
By understanding and implementing the regulations outlined in CFR Title 21 Part 812, researchers and sponsors can conduct clinical trials that contribute to meaningful medical advancements and improved patient outcomes. The commitment to excellence in clinical research is a commitment to the highest standards of integrity and participant welfare.




