Introduction
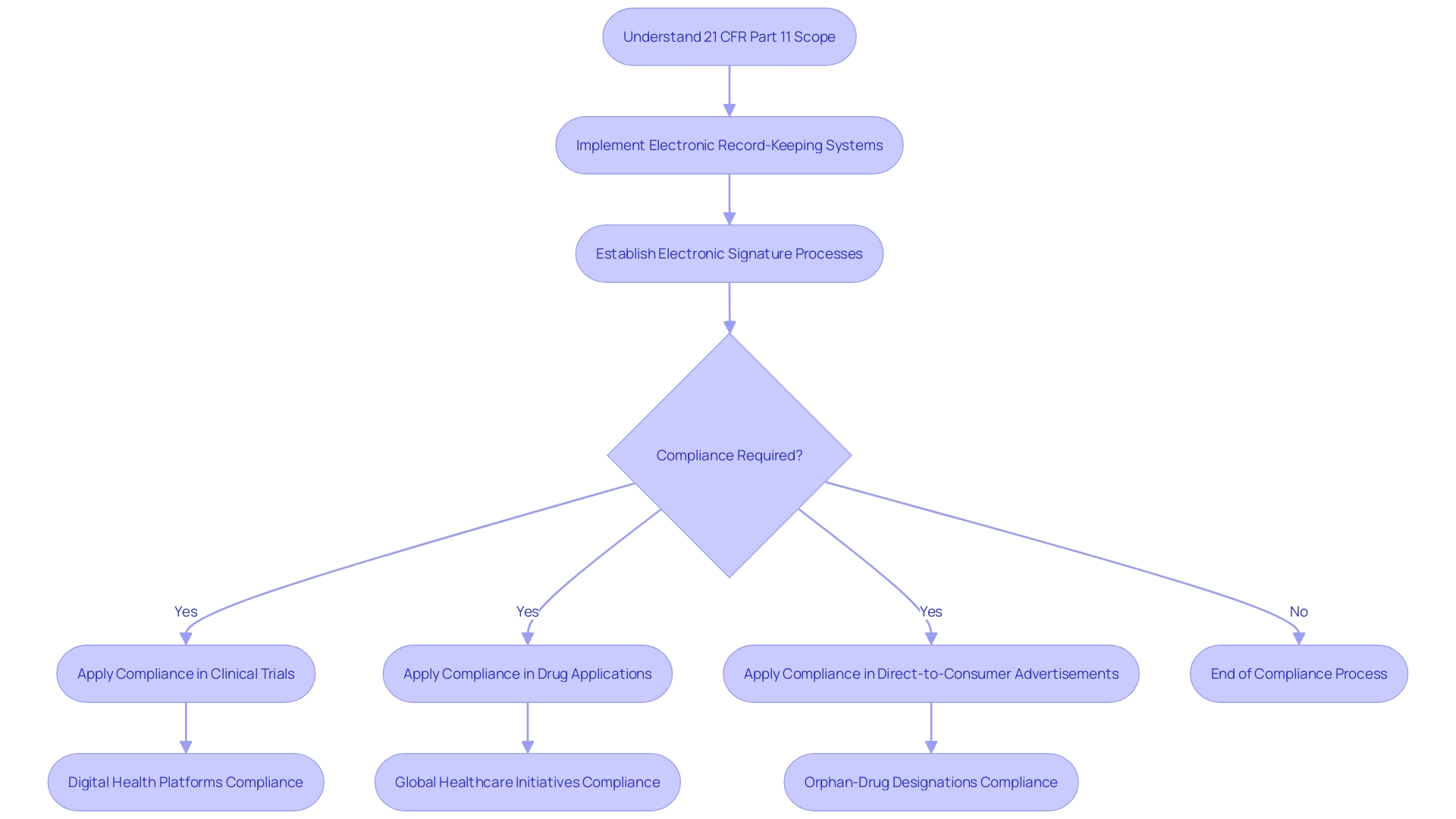
21 CFR Part 11, a critical section of the Code of Federal Regulations, outlines the guidelines set by the United States Food and Drug Administration (FDA) on electronic records and electronic signatures. This regulation has significant implications for entities involved in clinical research and drug development. It covers a wide range of records, including those related to clinical trials and drug applications, and applies to all electronically executed signatures that are intended to be equivalent to handwritten signatures.
Compliance with Part 11 is necessary for using electronic records and signatures instead of traditional paper-based methods. The FDA's recent emphasis on clear communication of drug side effects in direct-to-consumer advertisements highlights the importance of effective electronic information dissemination. As digital health platforms gain traction, exemplified by Rwanda's nationwide digitization strategy, understanding and complying with Part 11 become even more crucial for efficient and interoperable healthcare information systems.
Additionally, Part 11's relevance extends to unique treatment approvals and exclusivity periods, as highlighted by the FDA's orphan-drug designation. Researchers and organizations engaged in clinical trials must be well-versed in Part 11 requirements to ensure full compliance and contribute to advancing medical science and patient outcomes.
Scope and Application of 21 CFR Part 11
21 CFR Part 11 is a critical section within the Code of Federal Regulations that sets forth the United States Food and Drug Administration’s (FDA) guidelines on electronic records and electronic signatures. Understanding its scope and application is essential for any entity engaged in clinical research or drug development. This regulation encompasses a wide range of records, including those related to clinical trials and drug applications, and applies to all signatures that are intended to be the equivalent of handwritten signatures, executed electronically.
Organizations and individuals must comply with Part 11 if they wish to use electronic records in lieu of paper records or electronic signatures in place of traditional handwritten signatures. The FDA's recent publication regarding the clear and conspicuous presentation of drug side effects and contraindications in direct-to-consumer advertisements underscores the agency's commitment to ensuring that electronic information is communicated effectively and responsibly.
The relevance of Part 11 compliance is further amplified by the growing trend of digital health platforms, as seen in Rwanda's nationwide digitization strategy, which includes the implementation of electronic medical records. Such initiatives highlight the global movement towards more efficient and interoperable healthcare information systems, capable of improving patient care and enhancing compliance with treatment protocols.
Moreover, the FDA’s orphan-drug designation emphasizes the importance of unique treatment approvals and exclusivity periods, which are intricately tied to electronic records and applications. The eCFR (Electronic Code of Federal Regulations) further facilitates the understanding of these regulations by providing an organized and user-friendly presentation of the CFR sections.
In light of these considerations, it is imperative for researchers and organizations involved in clinical trials to be well-versed in the requirements of 21 CFR Part 11, ensuring that their electronic record-keeping and signature processes are in full compliance. As clinical trials evolve and international efforts like Rwanda's healthcare initiatives continue to emerge, adherence to Part 11 becomes not just a regulatory mandate but also a strategic component in advancing medical science and patient outcomes.
Electronic Records and Electronic Signatures
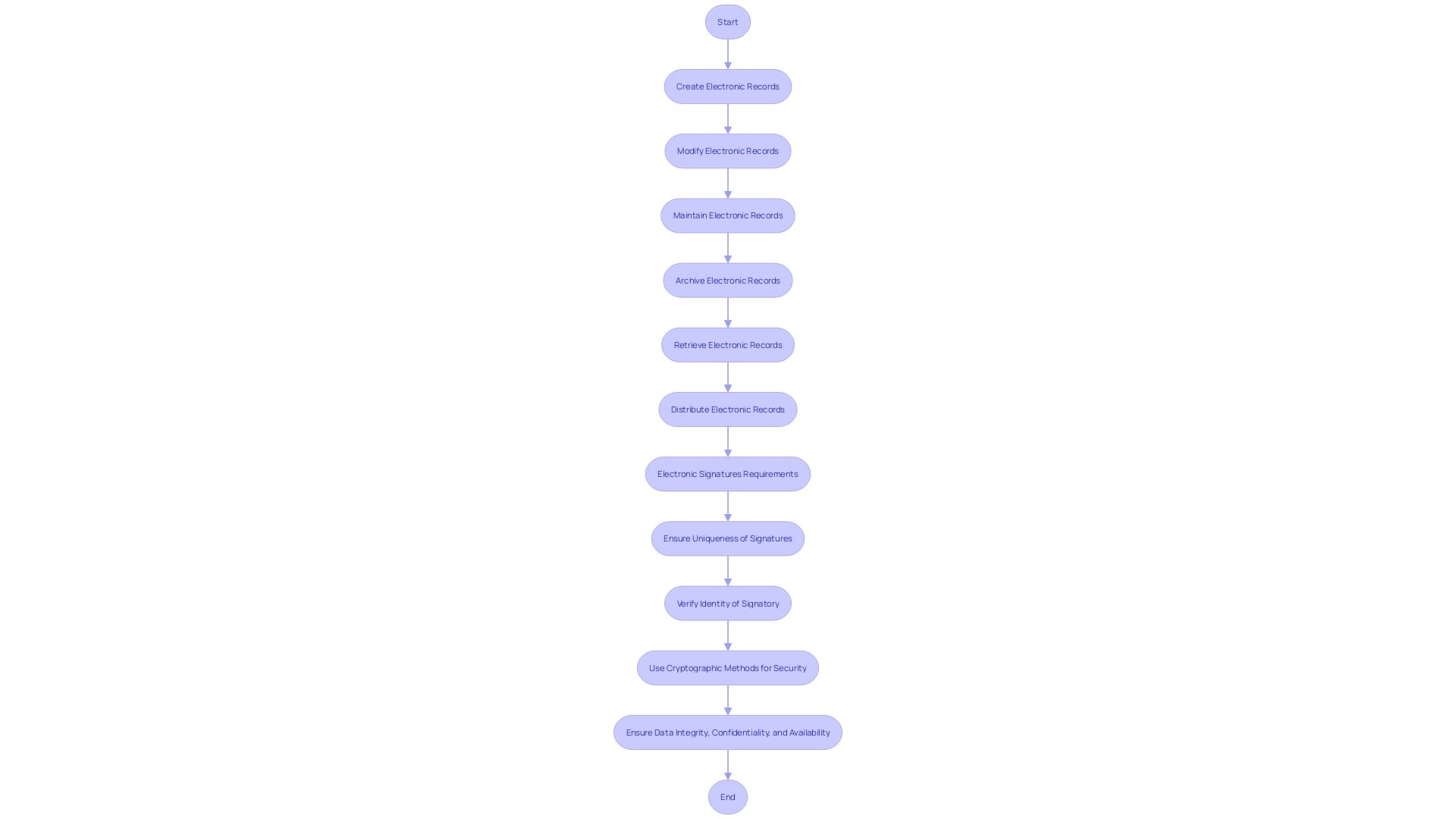
21 CFR Part 11 sets the standard for electronic records and electronic signatures in the realm of clinical trials, asserting the conditions that make them as reliable and authentic as their paper counterparts. This part defines electronic records as any combination of text, graphics, data, audio, pictorial, or other information representation in digital form that is created, modified, maintained, archived, retrieved, or distributed by a computer system. For these records to be deemed credible, they must be created and preserved in a manner that ensures their integrity, confidentiality, and availability over time.
Regarding electronic signatures, they must be uniquely linked to the signatory, capable of verification, and be under the sole control of the person using it. The use of electronic signatures is also governed by cryptographic methods to ensure security and prevent unauthorized access or alterations. These regulations are vital for upholding the integrity of data in clinical research, where accurate and reliable data are paramount for patient safety and effective treatments.
The FDA invites public comments on these regulations to understand their impact and to refine their practical utility, ensuring they align with the dynamic nature of technological advancements and their application in clinical research. As clinical trials increasingly incorporate digital tools such as electronic health records (EHRs) and digital therapeutics, regulatory frameworks like 21 CFR Part 11 become even more critical. They provide the necessary guidelines to balance innovation with patient safety and data integrity in an ever-evolving digital health landscape.
Controls for Closed Systems
Closed systems are essential in ensuring the confidentiality, integrity, and availability of electronic records and signatures, as mandated by 21 CFR Part 11. These systems must be equipped with stringent access controls to prevent unauthorized entry and manipulation of sensitive data. User authentication protocols must be robust, often involving multiple factors to verify the identity of each user.
Additionally, data encryption serves as a critical barrier, safeguarding information as it is stored and transmitted, thereby maintaining its integrity and ensuring it remains tamper-proof.
For example, adherence to guidelines like those from the National Institute of Standards and Technology's Guide to Industrial Control Systems Security can be instrumental in implementing a closed system. Such standards provide a framework for assessing and mitigating risks associated with various components, including sensors and controllers, that communicate with critical building hardware.
These measures are not just theoretical; real-world applications at agencies like the U.S. Environmental Protection Agency (EPA) demonstrate the practicality of securing building automation components against cyber threats. The importance of such security measures is further emphasized by historical events, such as the delay in the federal government's response following President Reagan's death, which underscored the vulnerability of systems when regular protocols are disrupted.
In light of the Federal Register's recent call for comments, it is clear that maintaining the security of electronic records is a priority. The FDA's invitation for public commentary on the collection of information underscores the need for practical utility and the minimization of burdens on respondents. This initiative aligns with the three core goals of information security—confidentiality, integrity, and availability—as these principles are fundamental to the trust and reliability of electronic records within closed systems.
The Common Criteria for Information Technology Security Evaluation further supports these principles by offering a structured process for evaluating the security properties of IT products. With mutual recognition of secure IT products across signatories of the Common Criteria Recognition Arrangement (CCRA), the standardization of security controls becomes even more critical.
In conclusion, the successful implementation of these controls within closed systems is not only a regulatory requirement but also a business imperative. It ensures the preservation of the high standards of security and trust essential for the progress and reputation of any research organization.
Controls for Open Systems
Navigating the complexities of 21 CFR Part 11, which governs the use of electronic records and electronic signatures, is crucial for ensuring the integrity of clinical trial data. Open systems, unlike their closed counterparts, require additional vigilance due to their increased susceptibility to security breaches and reliability issues. To this end, specific controls and measures are mandated to preserve the accuracy, reliability, and consistent accessibility of electronic data.
This includes implementing robust audit trails, which provide a secure, computer-generated, time-stamped electronic record that allows the reconstruction of the course of events relating to the creation, modification, and deletion of an electronic record. System documentation is equally important, ensuring that all operations, modifications, and maintenance activities are clearly recorded and justified. Rigorous data integrity checks are also essential, protecting against unauthorized data alterations and ensuring that the data is complete, consistent, and accurate throughout its lifecycle.
The importance of these measures is underscored by reports indicating the challenges and potential pitfalls in data sharing for research, clinical care, and during public health emergencies. Notably, inadequate control groups in clinical trials, such as the comparison of imaging abnormalities between disparate patient populations, highlight the critical need for stringent controls in data handling and analysis. As clinical trials evolve and adapt to technological advancements, regulatory frameworks like 21 CFR Part 11 provide the necessary guidance to maintain the trustworthiness of electronic records.
For instance, a case crossover study design, which compares exposure during different time windows within the same individual, exemplifies the innovative approaches in clinical research that necessitate reliable electronic systems. Moreover, the implementation of the EMA Computerized systems and Electronic Data Guidance by organizations such as ICON demonstrates the industry's ongoing efforts to meet regulatory expectations and maintain high standards of data integrity.
In the context of medical devices, understanding terminology such as 'restricted device,' 'classification name,' and 'product code' is vital for compliance with FDA regulations. Each term provides a layer of specificity and control, from the level of sale and distribution restrictions to the classification and identification of a device's generic category. Transparency in advertising and labeling is further emphasized, requiring a representative sampling that accurately reflects the promotional claims made for the device.
Overall, adherence to 21 CFR Part 11 is not just a regulatory requirement but a cornerstone of clinical research integrity. It ensures that the electronic records and signatures used in clinical trials are as trustworthy as their paper counterparts, ultimately safeguarding the well-being of trial participants and the validity of research outcomes.
Conclusion
Compliance with 21 CFR Part 11 is crucial for entities involved in clinical research and drug development. This regulation sets guidelines for electronic records and signatures, allowing their use in place of traditional paper-based methods. Understanding and adhering to Part 11 become even more important as digital health platforms gain traction and healthcare systems become more interoperable.
Part 11 ensures the reliability and authenticity of electronic records and signatures. Compliance is vital for upholding data integrity in clinical research, where accurate information is essential for patient safety and effective treatments.
Closed systems with stringent access controls and data encryption are essential for maintaining the confidentiality, integrity, and availability of electronic records. Adherence to guidelines, such as those from the National Institute of Standards and Technology, is crucial for implementing a closed system effectively.
Open systems require additional vigilance to protect the integrity of electronic data. Specific controls, such as robust audit trails and data integrity checks, are mandated to ensure the accuracy and reliability of electronic records.
In conclusion, compliance with 21 CFR Part 11 is essential for entities involved in clinical research and drug development. Adhering to the regulations ensures the integrity of electronic records and signatures, contributing to the advancement of medical science, improved patient outcomes, and the highest standards of data integrity.




