Introduction
The FDA's 510(k) regulatory pathway is a critical avenue for medical device manufacturers to gain market access in the United States. The process involves a meticulous comparison of the new device to a similar one already on the market, known as the predicate device. To ensure safety and effectiveness, the FDA examines the device's intended use and technological characteristics.
Understanding the competitive landscape and presenting significant data with clarity are key factors in obtaining clearance. However, challenges such as revenue strategies and achieving clinical results can arise post-approval. It's important for manufacturers to have a deep understanding of their device and market context.
This article explores the eligibility criteria, types of 510(k) submissions, key components of a submission, content requirements, the submission process, common challenges, and best practices for successful approval. By following these guidelines, manufacturers can navigate the FDA's regulatory framework effectively and contribute to healthcare advancements.
Understanding the 510(k) Regulatory Pathway
The FDA's 510(k) regulatory pathway serves as a critical avenue for medical instrument manufacturers to gain market access in the United States. It necessitates a thorough comparison of the new gadget to one already available in the market, referred to as the predicate device. The process involves a thorough evaluation of both the new product's intended use and its technological characteristics to ensure it is at least as safe and effective as its predecessor.
Comprehending the competitive landscape is crucial, where a thorough examination of research literature, clinical studies, and existing market tools forms the strategic approach. This includes scrutinizing instructions for use, warnings, and cautions that are relevant to users, such as clinicians and patients.
Recent FDA statements emphasize the need for clarity in presenting significant information, whereby medical instrument manufacturers should be transparent in communicating risks and benefits. For instance, a clear, conspicuous, and neutral presentation of a drug’s major side effects is now mandated in direct-to-consumer advertisements, reflecting the FDA's commitment to consumer-friendly language.
Real-world implications of the 510(k) process are evident in instances such as Vivos Therapeutics' experience. Despite the anticipation of positive outcomes post-approval, the company faces challenges like implementing effective revenue strategies and achieving the desired clinical results, highlighting the unpredictable nature of medical instrument commercialization.
Data from the Center for Drug Evaluation and Research (CDER) shows that innovative drugs and medical products continue to be approved each year, contributing to healthcare advancements. However, an analysis of medical AI innovations reveals steady growth rather than acceleration in FDA approvals, suggesting that the approval process itself may be a limiting factor in the speed of medical instrument innovation.
Considering these findings, individuals pursuing FDA 510(k) clearance should strive for a comprehensive understanding of their product and its market context, utilizing all accessible resources, including FDA databases that contain summaries of safety and effectiveness for previously approved devices.
Eligibility Criteria for 510(k) Clearance
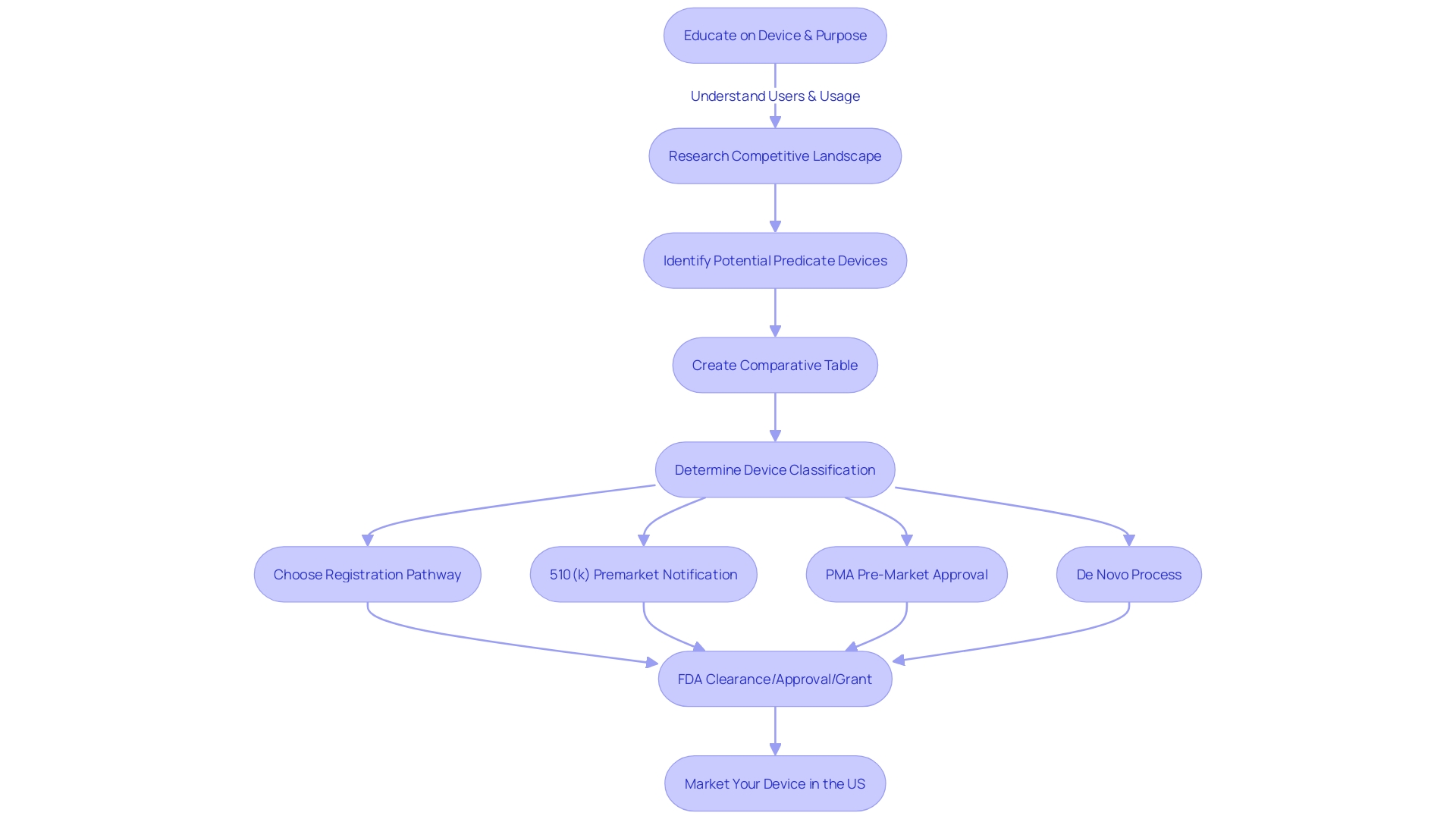
To effectively navigate the FDA 510(k) submission process for medical devices, manufacturers must thoroughly confirm their product's eligibility for clearance. The FDA sets specific criteria that must be met by devices, which depend on demonstrating substantial equivalence to previously cleared devices, referred to as predicate devices. To establish this crucial equivalence, one must delve into the apparatus's intended use and technological characteristics. A comprehensive comparative analysis requires a thorough examination of the competitive landscape, including research literature, clinical studies, and existing marketing materials, to identify an appropriate reference and develop.
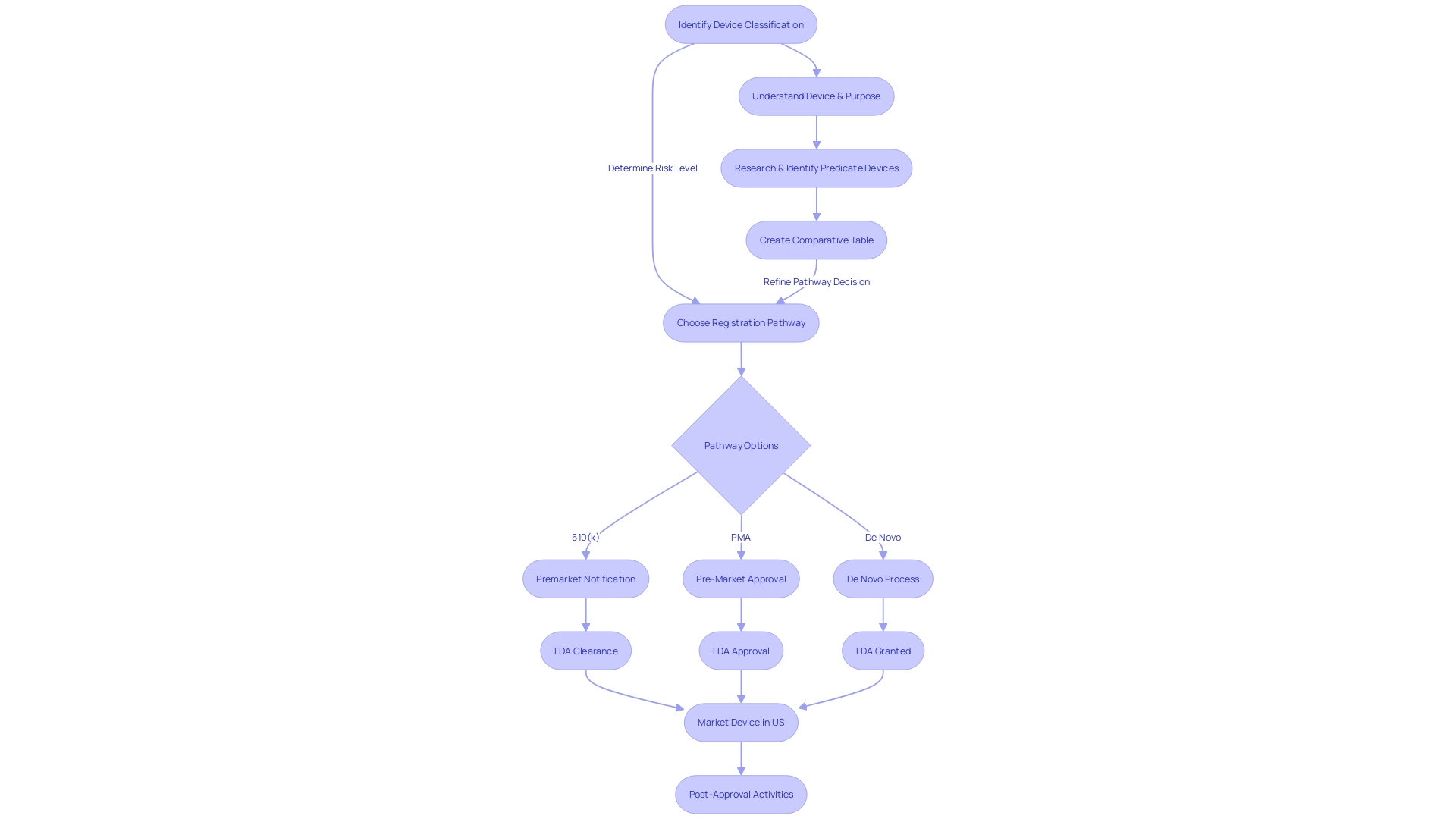
Understanding the classification of the equipment is also critical since the FDA categorizes medical devices into three risk-based classes, each dictating a distinct regulatory pathway—Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo classification process. Only with FDA clearance, approval, or a grant under the De Novo process can an item be legally marketed in the U.S. It's essential to comprehend and adhere to the definitions of terms such as Registered, Cleared, Approved, and Granted, which are often used interchangeably but have distinct regulatory implications.
In the realm of regulatory compliance, the role of voluntary consensus standards is invaluable. These standards, developed by Standards Development Organizations (SDOs) following principles of transparency, openness, and due process, underpin regulatory quality and foster innovation by setting rules and guidelines for common use. Manufacturers seeking recognition for a standard must provide detailed information, including the standard's reference number, designation, and a proposed list of relevant equipment.
The FDA's recent ruling on direct-to-consumer prescription drug advertisements emphasizes the significance of presenting information in a clear, conspicuous, and neutral manner, applying principles that are equally important for medical apparatus labeling and advertisements. Such regulatory measures ensure that the safety, effectiveness, and security of medical apparatus are communicated effectively to the public, reflecting the FDA's overarching mission to protect public health.
Types of 510(k)s: Traditional, Abbreviated, and Special
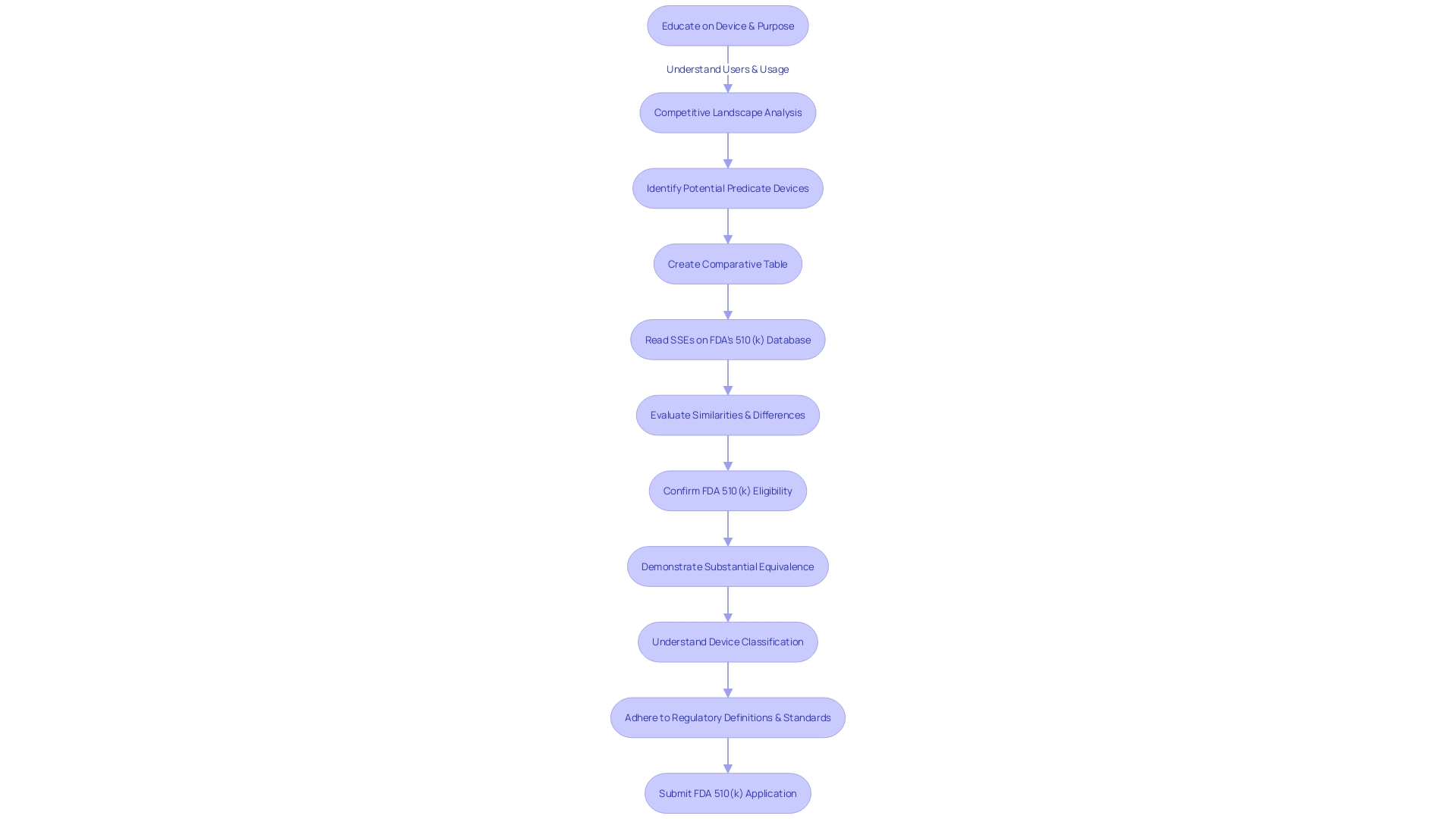
Comprehending the different kinds of FDA 510(k) submissions is vital for medical professionals in the field of equipment. The primary categories include traditional, abbreviated, and special 510(k) processes. Each category serves a unique purpose and comes with specific requirements and review procedures.
The traditional 510(k) is the most common pathway and is appropriate when an apparatus is significantly similar to a legally marketed predicate. Manufacturers should conduct a comprehensive study on the intended use, technology, and safety of the product by reviewing clinical studies, literature, and existing product information to identify a suitable predicate.
An abbreviated 510(k) allows for a streamlined review process by using guidance documents, special controls, and recognized standards. This pathway is suitable when a manufacturer can demonstrate conformity to these aspects, potentially accelerating the review timeline.
Special 510(k) applications are for modifications to an apparatus that already has 510(k) authorization. This category is perfect for alterations impacting the safety and efficiency of the equipment, where the modifications do not justify a conventional evaluation.
When compiling a document, it is crucial to create a comparative chart outlining the resemblances and distinctions between the new apparatus and the precedent. This includes understanding the users, usage instructions, warnings, and competitive landscape. It's also advisable to consult the Summaries of Safety and Effectiveness Data (SSEs) available on the FDA's database.
In the context of sharing feedback or comments on regulatory matters, remember that any information submitted electronically becomes public. Therefore, it's necessary to avoid including any confidential details unless properly submitted through designated confidential channels.
As the healthcare industry evolves, with an estimated 14,000 independent practices being acquired in 2021 alone, the adoption of digital tools and technologies becomes increasingly important. These advancements not only assist in streamlining management in private practices but also contribute to the regulatory processes, as observed with the utilization of online platforms like Pay.gov for secure payment filing related to 510(k) applications.
A 510(k) filing is a display of significant similarity to a lawfully marketed product, guaranteeing the new instrument is secure and efficient. While the preferred payment method for entries is online, it's important to note that only full payments are accepted, emphasizing the need for precise and complete financial handling during the process of submitting. By comprehending these types of applications and prerequisites, experts in the medical industry can navigate the regulatory structure of the FDA more efficiently.
Key Components of a 510(k) Submission
Creating a thorough 510(k) application is crucial in maneuvering through the regulatory terrain for medical instruments. The Food and Drug Administration (FDA), safeguarding public health, requires thorough documentation to determine the safety, effectiveness, and security of medical instruments. A thoroughly prepared 510(k) application must include a comprehensive description of the apparatus, a clearly defined intended purpose, reliable performance data, and comprehensive labeling information.
A profound understanding of the key to a successful submission includes its operational context and user base, which ranges from healthcare professionals to patients. It is vital to absorb the instructions for use, considering all warnings and precautions. Working closely with marketing teams can provide insights into the competitive field, allowing the identification of similar products, referred to as predicate devices, which act as benchmarks for comparison.
A comparative analysis is crucial, involving an examination of research literature, clinical studies, and other relevant materials to differentiate your product from existing ones. This analysis should manifest into a comparative table that meticulously contrasts your apparatus against the selected predicate devices. Delving into the Summaries of Safety and Effectiveness Data (SSED) available in the FDA’s 510(k) database is a critical step in this process, as it provides valuable information regarding the similarities and differences between devices.
When you put together the required documents, it is crucial to support each section with data and evidence, making sure that all supporting materials are systematically referenced. This comprehensive approach not only aids the FDA's review process but also positions your request for a favorable outcome, aligning with industry standards for clarity and precision, as exemplified by recent FDA guidelines for direct-to-consumer advertising.
To summarize, a 510(k) requires thorough focus on specifics and a methodical approach to documentation, guided by a comprehensive understanding of the device's application and the regulatory structure governing medical devices. The objective is to present a compelling narrative that satisfies the FDA's stringent criteria for market entry, thereby advancing the availability of innovative and safe medical technologies.
Content Requirements for a 510(k) Submission
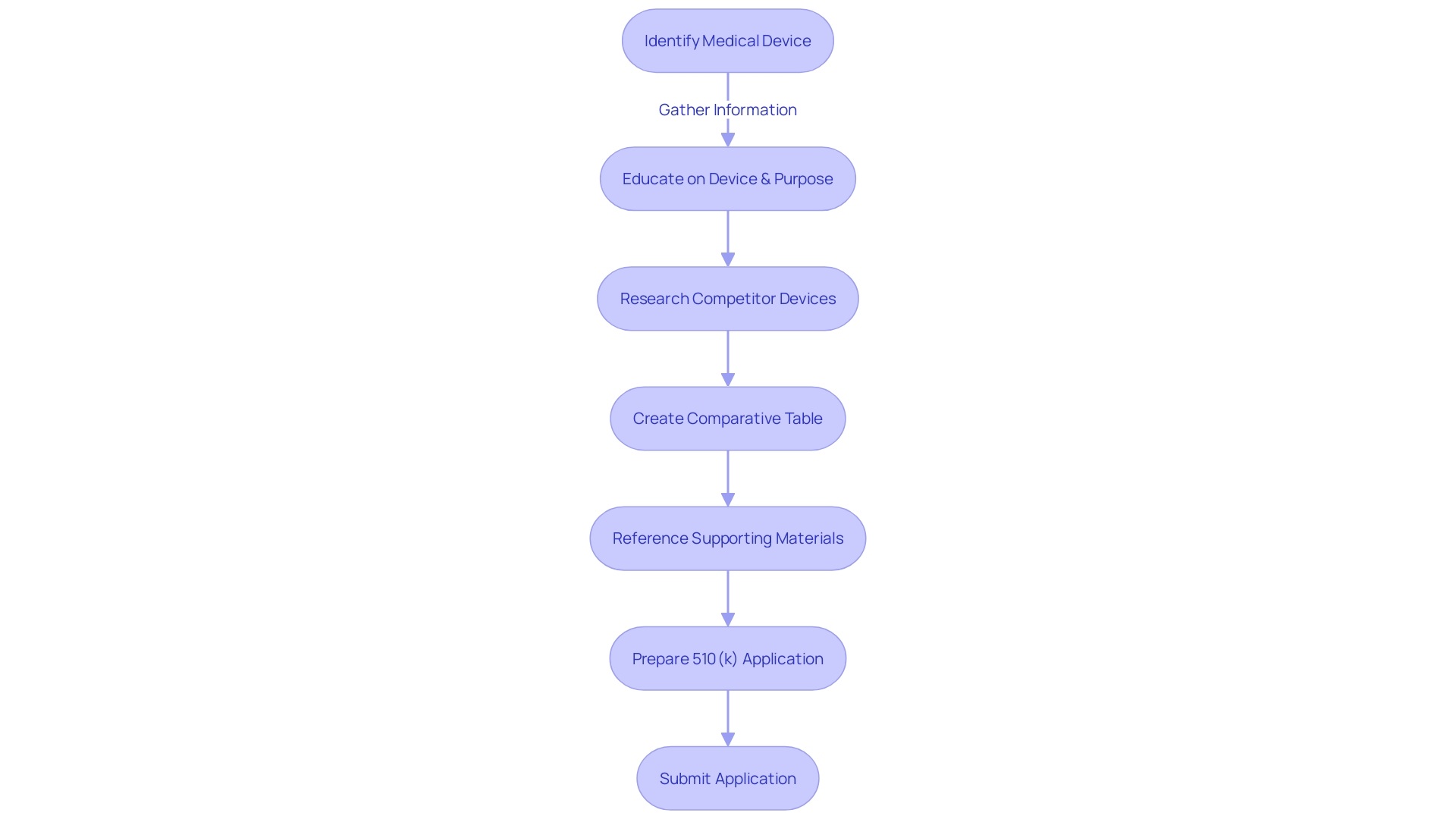
For a successful FDA 510(k) submission, a thorough understanding of the equipment in question is crucial. This includes being knowledgeable about the features and application of the equipment, which covers the individuals it is intended for, such as healthcare professionals and individuals, and the guidelines for its utilization, along with any related alerts and precautions. It's important to explore research literature, clinical studies, and competitive analysis to discover predicate instruments that have the same intended use and technological characteristics as your product.
Creating a comparative analysis table is a crucial part of showing how your product compares to identified predecessors, a necessary component in the 510(k) process. This process is not only about pinpointing technological similarities but also involves reading through the Summaries of Safety and Effectiveness Data (SSEs) available in the FDA's 510(k) database to discern any significant differences.
When putting together the 510(k) application, it's crucial to comply with the FDA's content requirements, which require specific documentation and a structured format. This documentation must include a patent certification or statement for any patents that claim the drug substance, product, or method of use, associated with the device. A comprehensive examination of the applicable FDA regulations, standards, and good manufacturing practices is essential to guarantee that the document reflects adherence to all relevant requirements.
Moreover, staying informed about the latest FDA regulations and updates, such as the new rules for direct-to-consumer prescription drug advertisements, can provide insights into the current regulatory environment. This knowledge can assist in preparing a document that fulfills the FDA's expectations for clarity, conciseness, and neutrality, particularly in presenting safety and efficacy information.
To summarize, the content prerequisites for an FDA 510(k) application are meticulous and require thorough understanding of the product, the competitive environment, and current FDA regulations and protocols. By adhering to these criteria, manufacturers can assist in guaranteeing that their applications are thorough and in line with the FDA's goal of protecting public health by ensuring the safety and efficacy of medical equipment.
The 510(k) Submission Process: eSTAR and eCopy
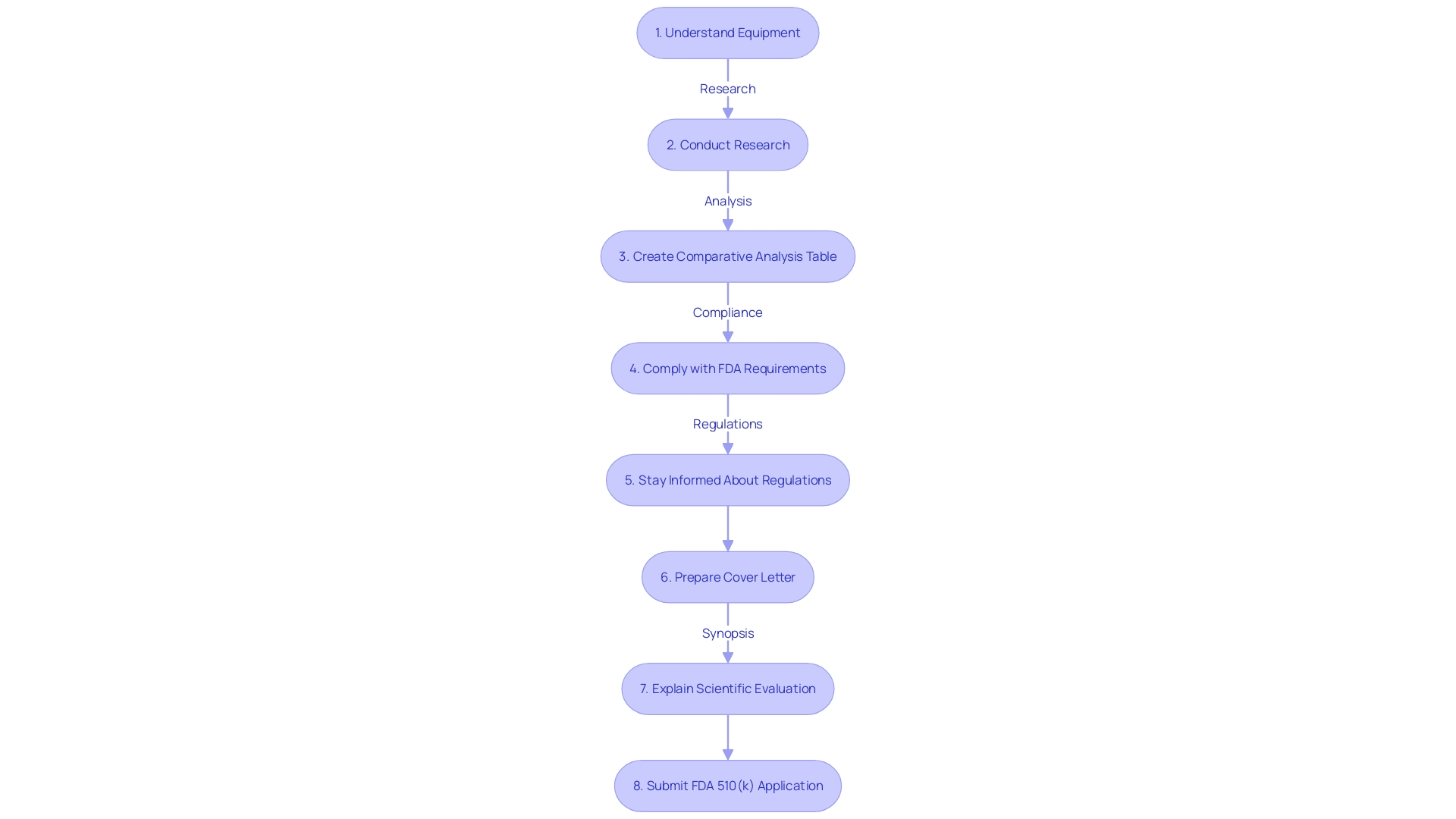
As part of the FDA 510(k) submission process, a comprehensive understanding of the subject equipment is crucial. It necessitates a meticulous approach commencing with an in-depth exploration of the intricacies of the tool's use, encompassing an understanding of user profiles such as clinicians and patients, and a comprehensive examination of the warnings and instructions for use. This fundamental understanding is enhanced by an examination of the competitive environment, identifying previous instruments that have similar intended purposes and technological characteristics. It is important to create a comparative table, drawing from a variety of sources such as research literature, clinical studies, and marketing materials. Moreover, reviewing the Summaries of Safety and Effectiveness (SSEs) accessible through the FDA’s 510(k) database is a key step in evaluating the similarities and differences between the subject device and its potential predicates.
Once equipped with this extensive background information, submitting the 510(k) application becomes a more structured task. The electronic Submission Template and Resource (Estar) and the electronic copy (eCopy) are the primary tools provided by the FDA for sending documents. These platforms facilitate a streamlined electronic process, ensuring that all relevant details are provided in an organized manner. It's essential to follow the FDA guidelines meticulously when preparing your documentation to avoid the inclusion of confidential information inadvertently. All entries become part of the public domain, so it's crucial to ensure that private data, like medical or Social Security numbers, are not included unless intended for public disclosure.
In today’s digital age, the FDA emphasizes the importance of clear and accessible communication, as evidenced by their recent guidelines on prescription drug advertisements. These standards emphasize the necessity for user-friendly terminology and the presentation of information in both modes, principles that are also relevant to 510(k) filings. Submitters must ensure that their documents comply with these high standards of clarity and comprehensibility to successfully navigate the review process.
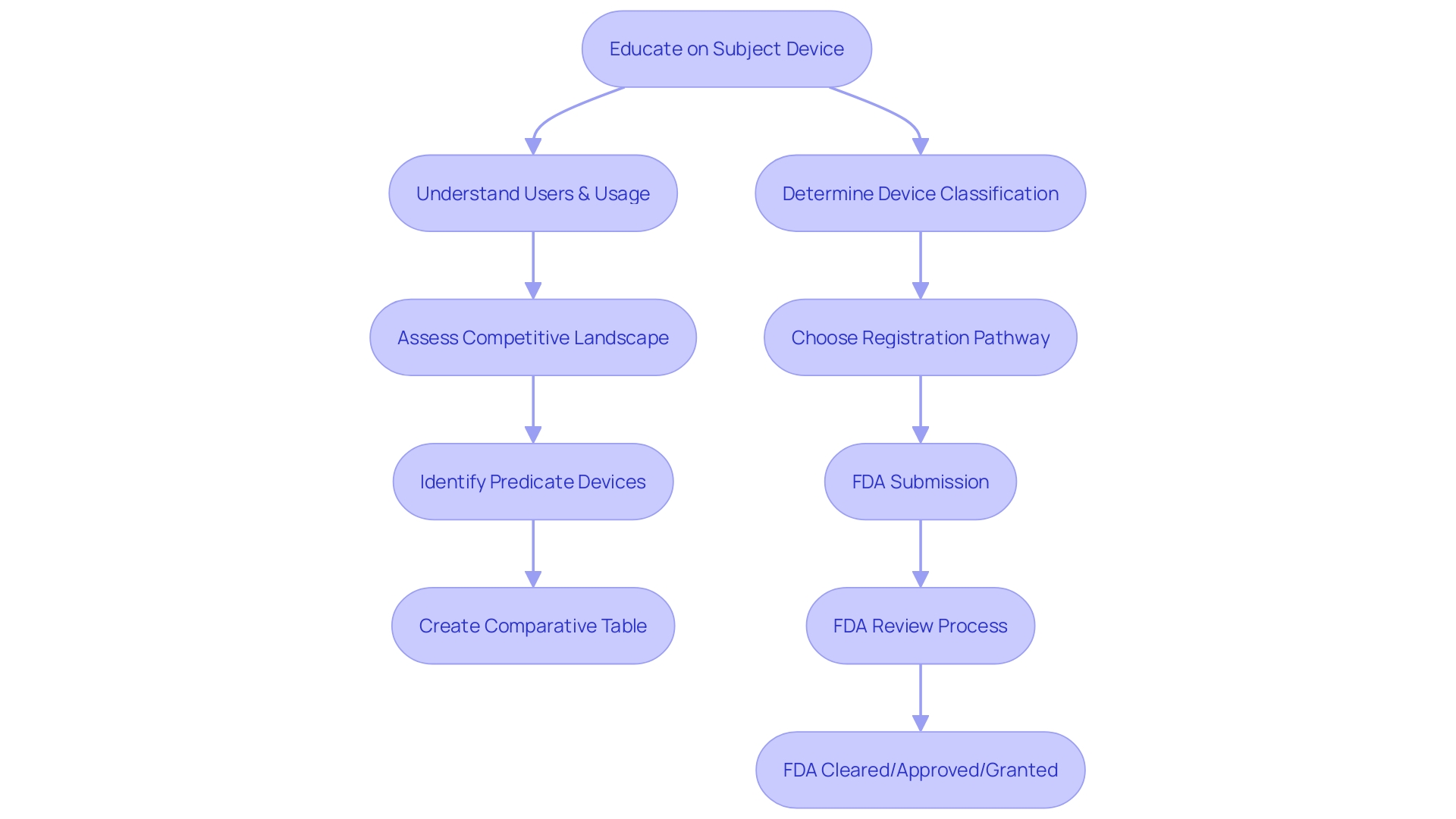
Acceptance Review and Substantive Review Process
Upon receipt by the FDA, a 510(k) application undergoes a meticulous two-phase review process: the acceptance review and the substantive review. The acceptance review serves as a preliminary check to ensure that all essential documentation is complete and in order. This stage is vital as unfinished or insufficient entries can result in delays or a rejection to acknowledge the application for further assessment. After a successful acceptance review, the submission enters the substantive review phase, where the FDA conducts a thorough evaluation of the medical apparatus's safety and effectiveness. Throughout this stage, the FDA evaluates the proposed instrument against a legally marketed precedent to establish substantial similarity.
Throughout the review process, the FDA emphasizes the importance of understanding the subject apparatus, its users, and instructions for use, which includes potential warnings and cautions. This comprehension is additionally enhanced by a comparative analysis with competitor products, echoing Medtronic's approach that combines extensive expertise with a commitment to tackle demanding health problems. This thorough evaluation can result in various outcomes: the product may be approved for sale, further details may be required, or in certain instances, the application may be declined.
The FDA's dedication to ensuring the safety, quality, and efficacy of medical tools is paralleled by its efforts to streamline processes, as seen in the broader healthcare context where technology and digital platforms are increasingly integral to medical tasks. This drive for efficiency and clarity is also evident in the FDA's guidance documents, which outline the procedures for industry engagement and public comment submissions, ensuring transparency and public involvement in the regulatory process. The dynamic nature of healthcare technology and the FDA's role in regulating it underscore the importance of these review processes in safeguarding public health while fostering innovation.
Common Challenges in 510(k) Submissions
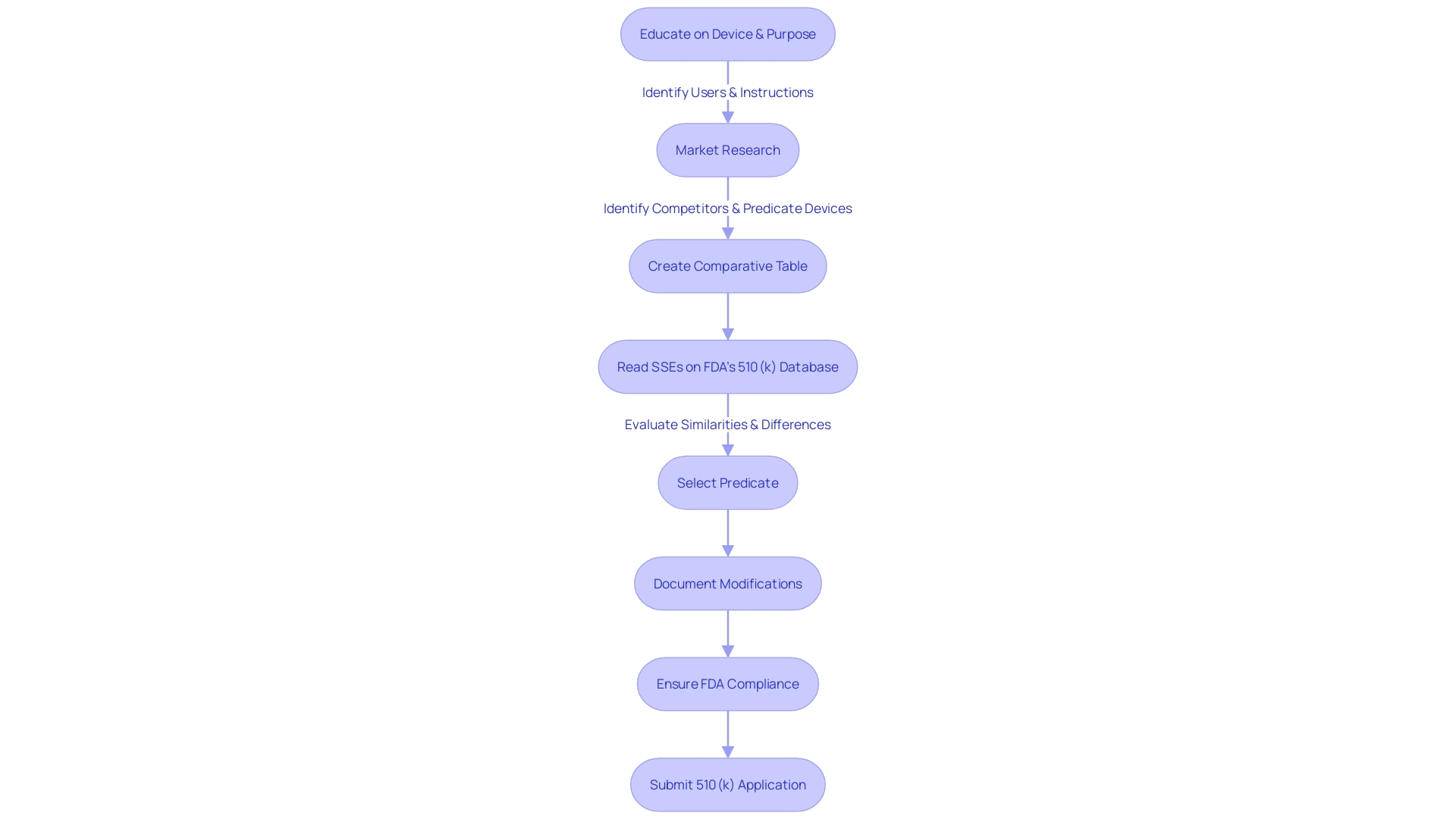
The 510(k) application process for medical instruments is intricate and demands a solid grasp of the subject instrument, its users, and the competitive landscape. Manufacturers must meticulously collate supporting data, ensuring that the product demonstrates substantial equivalence to a legally marketed predicate. This involves creating a comparative table to evaluate similarities and differences in technological characteristics and intended use, as informed by the FDA's 510(k) database and guidance documents.
The FDA's modernization initiative has enhanced the efficiency of the review process, recognizing the benefits of using older predicates, such as the accumulation of long-term safety data. Manufacturers are encouraged to follow best practices for selecting a predicate, which are detailed in the FDA's recently released draft guidance document.
Modifications to an apparatus, whether caused by client input, components, or production procedures, demand meticulous record-keeping and may demand a fresh filing. Consideration of the 'three F's'—form, fit, and function—is crucial for identifying the impact of any modifications.
Industry experts emphasize the importance of adapting to the evolving regulatory landscape to ensure compliance and efficiency in document preparation. Acknowledging the diversity of both human and the wide range of medical equipment available in the market is crucial when making regulatory considerations.
In light of this, the FDA also sets standards for clear communication, as seen in the recent final rule on direct-to-consumer prescription drug advertisements, ensuring that major side effects and contraindications are presented clearly and understandably.
Ultimately, thorough preparation, informed by FDA guidelines and industry best practices, is essential for a successful 510(k) submission and to navigate the complexities of the regulatory environment.
Best Practices for Successful 510(k) Approval
Securing FDA 510(k) approval is often a strategic milestone for medical equipment manufacturers. It is a rigorous process that requires meticulous attention to detail and adherence to specific regulatory standards. To navigate this process successfully, manufacturers should engage in thorough preparation, which includes developing a comprehensive understanding of the product's users, usage instructions, and the competitive landscape. By utilizing research literature, clinical studies, and the FDA’s 510(k) database to identify potential precursor instruments with similar intended uses and technological characteristics, manufacturers can create a comparative table that will be crucial in illustrating substantial equivalence.
In addition to preparation, proactive communication with the FDA is paramount. Recent updates from the FDA underscore the importance of presenting information in a clear, conspicuous, and neutral manner. This approach should be reflected in all communications with the agency, ensuring that the major statements regarding the medical instrument are easily understandable and transparent. Furthermore, conducting pre-submission meetings can provide valuable insights into the FDA's current thinking and expectations, which can be instrumental in avoiding common pitfalls and streamlining the review process.
It's important to note that while securing FDA approval is a significant achievement, it is not the end of the journey for a medical equipment company. As per the experiences shared by industry veterans, successful medical device companies often engage in strategic post-approval activities such as forming alliances, pursuing acquisitions, or entering into licensing agreements to maximize the product's commercial success. However, the path to approval and beyond is fraught with challenges, including regulatory scrutiny and the need for continuous innovation and adaptability to market needs. Companies should be prepared for the possibility that actual results may differ from initial expectations, underscoring the need for a robust and flexible business strategy.
Conclusion
In conclusion, the FDA's 510(k) regulatory pathway is critical for medical device manufacturers to gain market access in the United States. Manufacturers must meet eligibility criteria and submit a meticulous application that demonstrates substantial equivalence to a legally marketed predicate device. Understanding the competitive landscape, preparing key components of the submission, and adhering to content requirements are crucial for success.
The 510(k) submission process involves an acceptance review and a substantive review, where the FDA evaluates the device's safety and effectiveness. Manufacturers must be prepared for challenges such as selecting appropriate predicate devices, documenting changes, and adapting to the evolving regulatory landscape.
To increase the chances of approval, manufacturers should engage in thorough preparation, proactive communication with the FDA, and consider post-approval strategies. While FDA approval is significant, it is important to remember the journey doesn't end there. Continuous innovation, adaptability, and a robust business strategy are necessary for long-term success in the market.
By following these best practices and navigating the FDA's regulatory framework effectively, manufacturers can contribute to healthcare advancements and bring innovative and safe medical technologies to market.




