Introduction
Navigating the FDA's regulatory framework is a critical step for medical device manufacturers aiming to enter the U.S. market. Understanding the nuances of FDA clearance and approval processes is vital for manufacturers to successfully bring their medical devices to market, while ensuring the highest standards of patient safety and product efficacy. In this article, we will explore the different types of FDA clearances and classifications for medical devices, including the 510(k) clearance process and the determination of device classification.
We will also delve into the requirements for preparing a 510(k) submission, the content needed for a comprehensive submission, and the importance of finding and using predicate devices. Additionally, we will discuss the FDA review and clearance process, as well as post-clearance requirements and maintenance. Finally, we will explore the common types of 510(k) submissions and the resources and guidance documents available to manufacturers.
Join us as we unravel the complexities of FDA clearance and provide you with the essential information you need to navigate this vital process.
Understanding FDA Clearance and 510(k) Clearances for Medical Devices
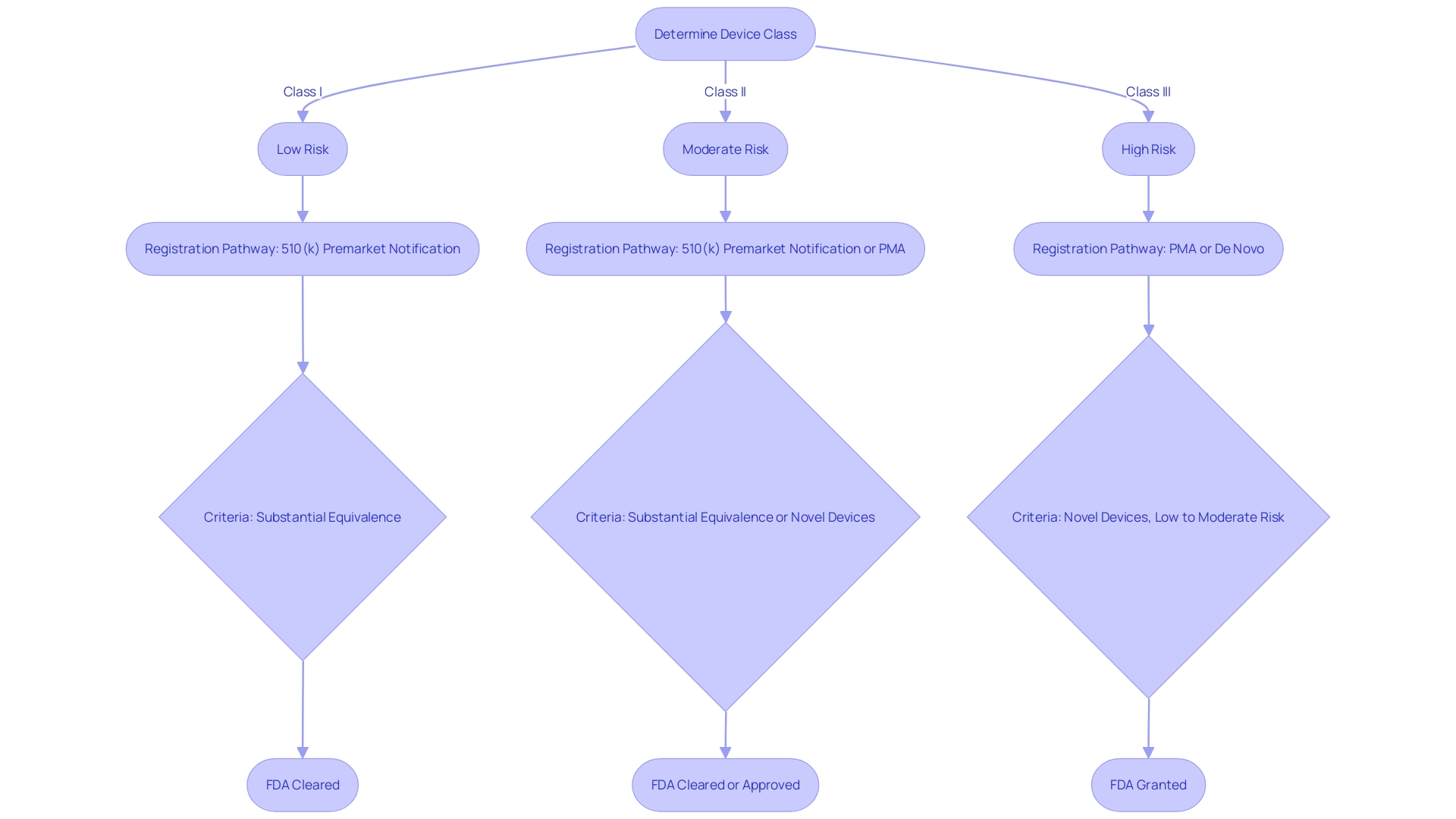
Navigating the FDA's regulatory framework is a critical step for medical device manufacturers aiming to enter the U.S. market. Medical devices are categorized into three classes based on potential risks, with Class I representing low-risk devices and Class III representing high-risk devices. Determining the correct device classification is pivotal, as this dictates the subsequent registration pathway—510(k) Premarket Notification, Pre-Market Approval (PMA), or the De Novo process. A 510(k) clearance, for example, is sought for devices that are substantially equivalent to a legally marketed device. In contrast, PMA is required for novel devices that imply a significant risk to patients, and the De Novo process is for low to moderate risk devices that lack a comparable predicate.
The FDA's standards emphasize the use of consumer-friendly language and terms to ensure information is easily understandable. This is exemplified by the recent FDA rule requiring clear, conspicuous, and neutral presentation of major side effects in direct-to-consumer prescription drug advertisements. Such transparency is equally important in medical device regulation, where understanding the distinctions between 'Registered,' 'Cleared,' 'Approved,' and 'Granted' is essential for compliance and patient safety.
The documentary 'The Bleeding Edge' highlighted concerns about the 510(k) clearance process, revealing that clinical trials are not always mandated, which can lead to expedited use of certain devices, but may also pose risks. This underscores the importance of stringent regulatory oversight to safeguard public health.
While the FDA's mission to protect public health by ensuring safety and efficacy extends to a wide range of products, its role in medical device regulation is particularly crucial. The agency's efforts to streamline regulatory pathways and foster collaboration are ongoing, aiming to reduce approval times for devices addressing urgent medical needs, especially in innovative fields like digital health.
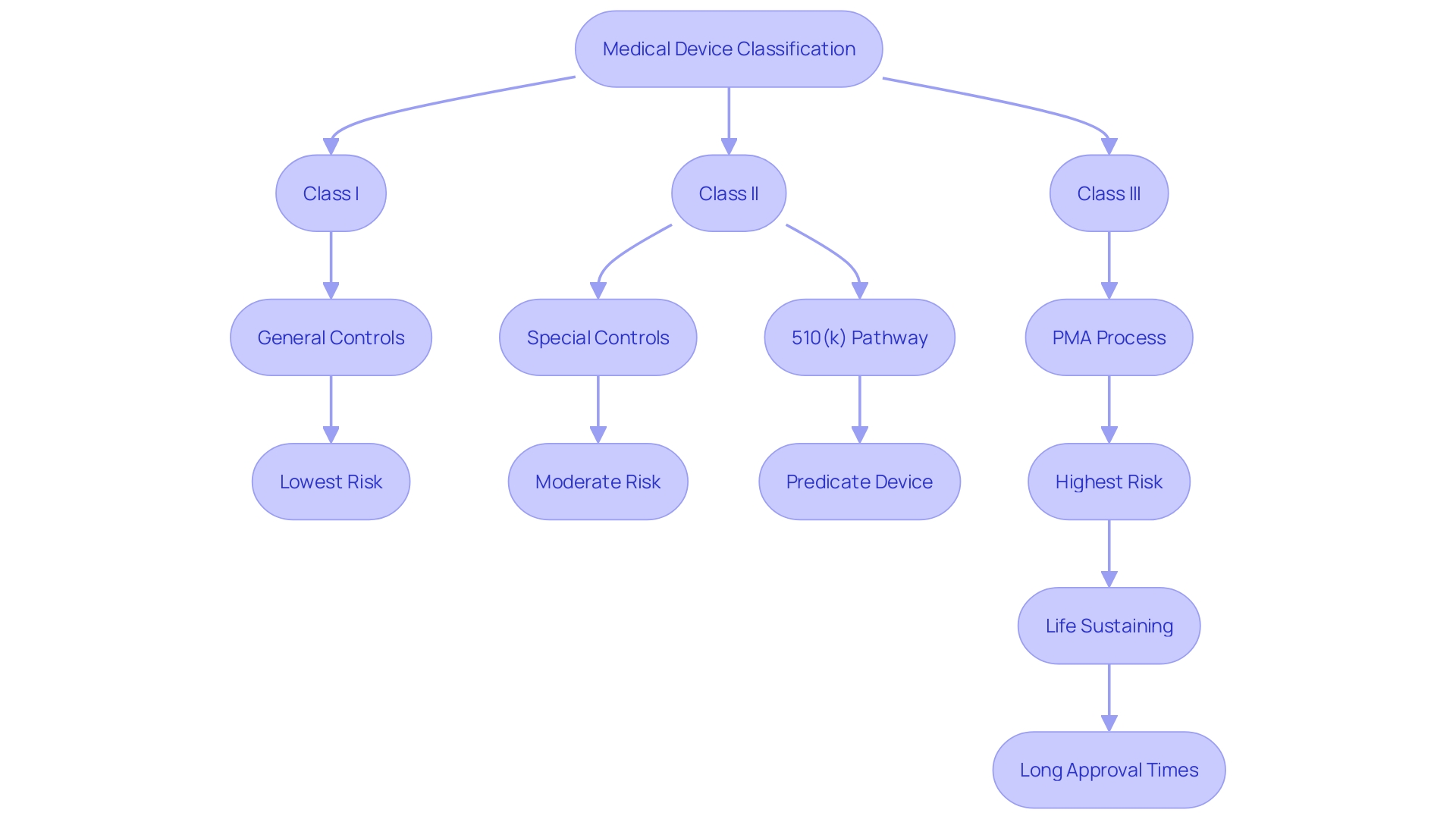
Class III devices, which include life-sustaining implants like pacemakers, undergo the most rigorous review process due to their critical nature. These high-risk devices represent a small percentage of FDA-regulated medical devices but require a comprehensive regulatory approach to ensure their safety and effectiveness.
Overall, understanding the nuances of FDA clearance and approval processes is vital for manufacturers to successfully bring their medical devices to market, while ensuring the highest standards of patient safety and product efficacy.
Understanding the 510(k) Program
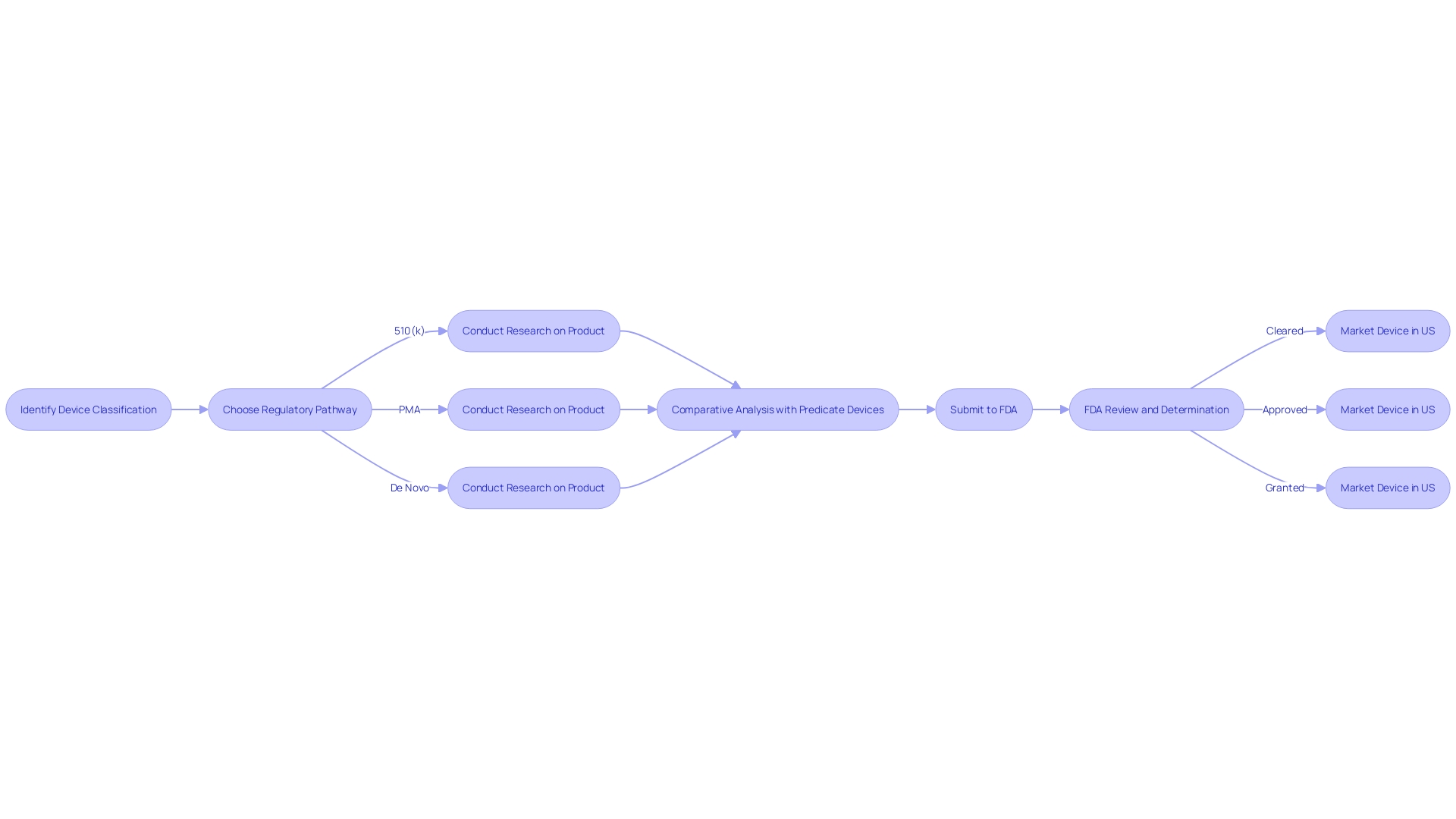
The 510(k) clearance, a pivotal component of the FDA's regulatory framework, plays a critical role in ensuring medical devices are safe and effective before they reach the market. It obliges manufacturers to establish that their new device is 'substantially equivalent' to an existing device, known as a predicate, that already has market clearance. Substantial equivalence signifies that the new device is at least as safe and effective as the predicate.
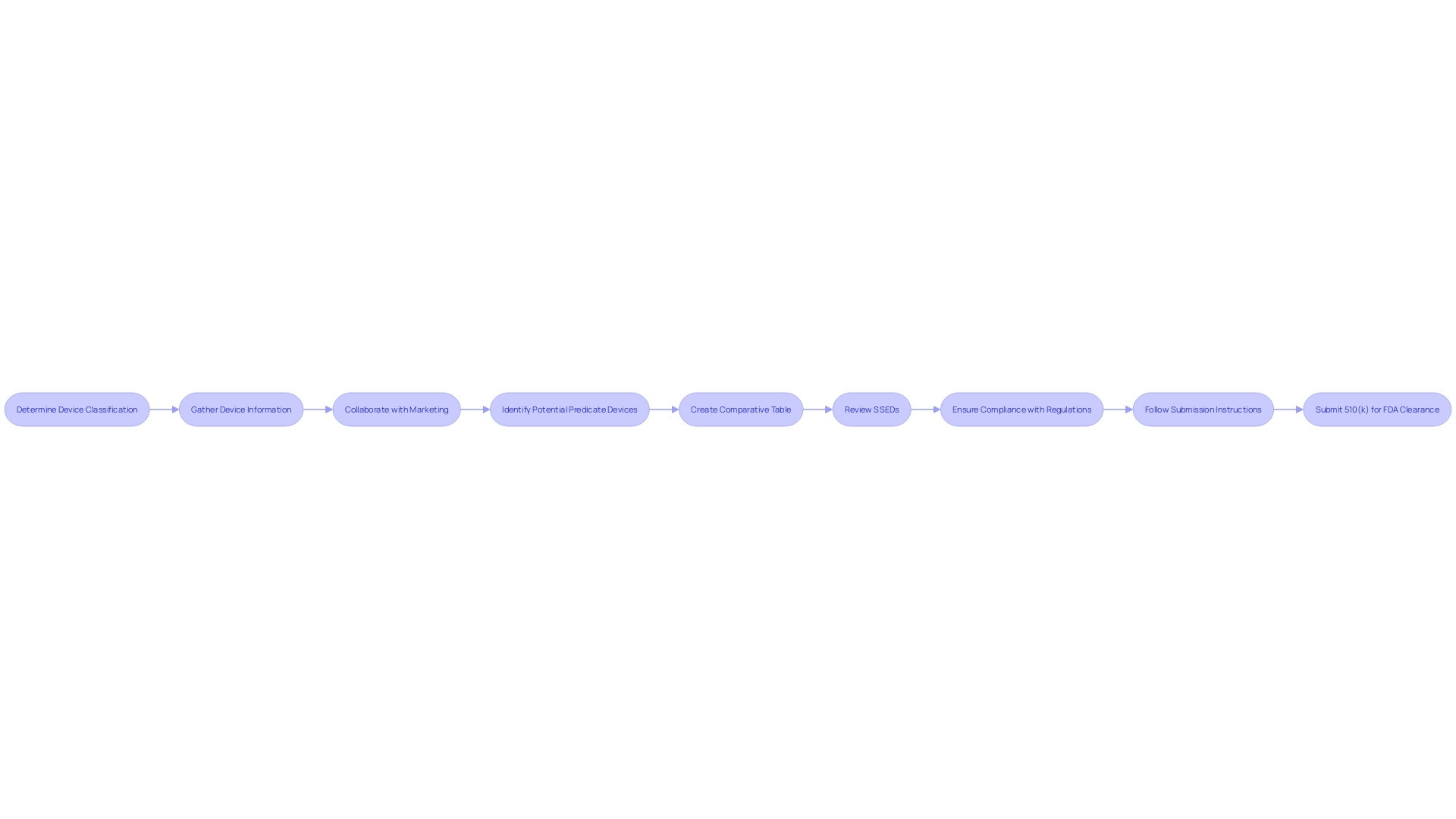
When preparing for a 510(k) submission, thoroughness is key. Stakeholders, including clinicians, physicians, and patients, must be well-informed about the device, particularly concerning its usage, associated warnings, and precautions. Additionally, a comprehensive analysis of the competitive landscape is crucial, often necessitating collaboration with marketing teams to scrutinize competitor devices. This involves an in-depth review of research literature, clinical studies, and promotional materials to pinpoint potential predicate devices that share the intended use and technological characteristics of the new device.
Moreover, manufacturers are advised to meticulously create a comparative table, contrasting their device with the identified predicate(s), and consult the FDA's 510(k) database for summaries of safety and effectiveness that might highlight pertinent similarities and differences.
Statistical data underscores the importance of the 510(k) process, where Class I and II devices, deemed low to moderate risk, typically gain clearance through this pathway. Although clinical trials may not always be mandated for these devices, the process remains a rigorous assessment to ensure patient safety and device efficacy. For instance, Class III devices, which are critical to life-sustaining or supporting functions, constitute roughly 10% of the FDA-regulated devices and necessitate a more comprehensive review.
The regulatory landscape continues to evolve, with recent pushes for more streamlined pathways, especially for devices that meet urgent medical needs. These initiatives are a response to the burgeoning fields of digital health and personalized medicine, aiming to bring innovative solutions to the market promptly, without compromising safety or effectiveness.
Determining Device Classification
Navigating the classification of medical devices is a pivotal step for manufacturers aiming to enter the U.S. market. The Food and Drug Administration (FDA) assigns devices to one of three classes—Class I, II, or III—based on the level of risk they present to patients and the regulatory controls necessary to ensure safety and efficacy. Class I devices pose the least risk and are subject to the least regulatory control, while Class III devices are for more serious conditions and require the highest level of regulation, including approval through the rigorous Pre-Market Approval (PMA) process.
Understanding the correct classification is essential because it influences the registration pathway manufacturers must follow. Most Class I and some Class II devices can often be marketed after submitting a Pre-market Notification, commonly referred to as 510(k), provided they are substantially equivalent to a legally marketed predicate device. Alternatively, if no such predicate exists, the De Novo pathway offers a route to classify novel devices. Class III devices, which carry the highest risk, usually require PMA, demonstrating safety and effectiveness through extensive data, including clinical trials.
Identifying a predicate device—a previously cleared device with similar technology and intended use—is a critical aspect of the 510(k) process. Manufacturers should conduct thorough research, including reviewing clinical studies and marketing materials of existing devices, to establish a comparative analysis. This comparison not only helps in determining device equivalence but also in understanding the competitive landscape. The FDA's database provides Summaries of Safety and Effectiveness that can be instrumental in this evaluation.
It's worth noting that while the 510(k) clearance process has faced scrutiny for not always requiring clinical trials, it remains a widely used pathway for medical devices to reach the market. Manufacturers are responsible for ensuring compliance with all FDA guidelines and for continuously monitoring the safety and performance of their devices post-market to protect public health.
Preparing a 510(k) Submission
Determining the classification of a medical device is the initial step in the journey towards FDA clearance. For manufacturers, the next crucial phase involves the meticulous preparation of the 510(k) submission. This comprehensive submission must encompass a detailed description of the device, its intended uses, performance data, and labeling information, which are key to a successful review by the FDA.
Understanding the device fully is paramount. This means grasping the intended user base—whether clinicians, dentists, or patients—and the device's instructions for use, which should include any warnings or precautions. Collaborating with the Marketing department can provide insights into the competitive landscape, which is invaluable when determining potential predicate devices that share the same intended use and technological characteristics. These predicate devices, once identified, can be compared using a well-structured comparative table.
Moreover, it is advisable to review the Summaries of Safety and Effectiveness Data (SSEDs) from the FDA's 510(k) database, which can offer a clear perspective on the similarities and differences between your device and existing predicates. This information will be instrumental in substantiating the safety and effectiveness of the new device.
An organized submission is key, as highlighted by the scrutiny of the FDA's clearance process in the 2018 documentary 'The Bleeding Edge'. The film revealed that not all devices require clinical trials, which underscores the importance of a well-structured 510(k) submission that can demonstrate a device's safety without trial data. This can be particularly critical as some fast-tracked devices have been associated with patient injuries.
In the context of regulations, it is also essential to be mindful of labeling requirements. For instance, when considering color additives in devices, the FDA requires compliance with specific regulations to ensure safety. Every component of the submission, including color additives, must adhere to the defined conditions of safe use to avoid complications during the review process.
Furthermore, when submitting comments or documentation to the FDA, it is crucial to follow the detailed instructions provided. Submitters must ensure that no confidential information is inadvertently made public, which includes personal medical information or proprietary business details.
In conclusion, a comprehensive and well-organized 510(k) submission is not just about compliance—it's about demonstrating a deep understanding of the device, its users, and the market. This approach not only facilitates the FDA review process but also contributes to the safety and efficacy of medical devices entering the market.
Content Requirements for a 510(k) Submission
The 510(k) submission process is a critical step in bringing a medical device to market, requiring a detailed demonstration of the device's safety and effectiveness. To ensure a complete submission, manufacturers must provide comprehensive documentation. This includes performance testing data that reflects how the device functions under expected conditions of use, and sterilization information which outlines the processes used to maintain device sterility and ensure patient safety. Biocompatibility data is also paramount, as it assesses the potential for an adverse reaction due to direct contact with the body.
Furthermore, clinical data may be necessary when there are differences in indications for use or technological characteristics compared to a predicate device, when non-clinical testing cannot establish substantial equivalence, or when a new or increased risk is identified. These scenarios, along with the FDA's draft guidance, illuminate the circumstances in which manufacturers must go beyond non-clinical data to prove substantial equivalence. It's essential to have a deep understanding of the device, including its users, intended uses, and the competitive landscape, as well as a comparative analysis of potential predicate devices.
The documentation should also include detailed device specifications and engineering drawings, a comprehensive list of components, manufacturing details, and relevant FDA reference numbers for legally marketed accessories or components intended for use with the device. It is advisable to consult the latest FDA guidelines and review the summaries of safety and effectiveness for predicate devices to ensure an informed and robust submission.
Manufacturers must remain aware of the public nature of comments submitted electronically to the FDA and take care to exclude confidential information. All submissions, including those with confidential data, should adhere to the FDA's instructions for written or paper submissions. By following these guidelines meticulously, manufacturers can ensure a thorough evaluation of their submission and move towards a successful clearance for their medical device.
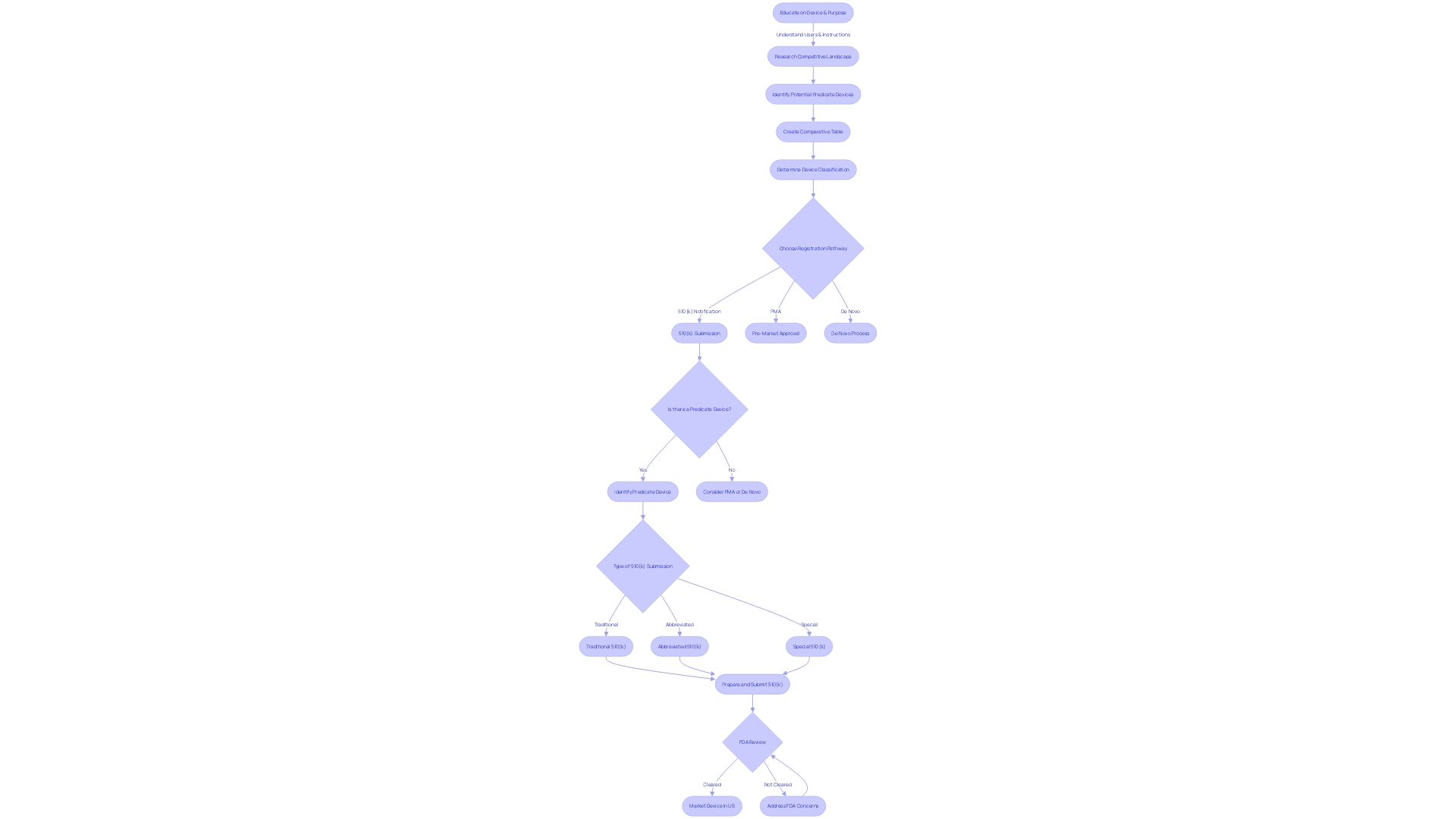
Finding and Using Predicate Devices
To navigate the 510(k) clearance process effectively, identifying a predicate device is paramount. This entails a meticulous evaluation of devices currently on the market to establish a benchmark—termed substantial equivalence—to your new medical device. This comparison is not a mere formality; it is a detailed assessment of intended use, technological characteristics, and safety and effectiveness parameters.
The FDA's classification system divides medical devices into three categories based on risk, each requiring a corresponding regulatory pathway. For devices falling under the 510(k) spectrum, it's critical to determine not just any predicate, but one that aligns closely with your device's intended use. Moreover, your chosen predicate should not introduce new questions of safety or effectiveness due to differing technological characteristics.
Embarking on this journey requires a comprehensive understanding of the device landscape. Engage with marketing teams to delve into competitive analysis, scrutinizing research literature, clinical studies, and existing product documentation. These insights are instrumental in creating a comparative table that juxtaposes your device with a potential predicate, highlighting similarities and differences.
An illustrative case is the recent modernization initiative by the FDA, which emphasizes the strategic selection of predicates. Public feedback and subsequent FDA guidance now illuminate best practices for predicate selection, including the utilization of established devices that bring the advantage of historical safety data to the table.
Furthermore, the agency's ongoing commitment to public health safety, as evidenced by its broad oversight responsibilities, underscores the importance of a rigorous 510(k) process. As the FDA continues to advance the efficiency of its review mechanisms, manufacturers must remain vigilant and informed to successfully demonstrate substantial equivalence and secure clearance for their medical devices.
Submission Process and User Fees
To expedite the FDA clearance process for medical devices, it's crucial to have a thorough understanding of the submission protocol and the associated user fees. First and foremost, identifying the correct classification of your medical device is essential, as the FDA categorizes devices into three levels based on patient risk. The classification determines which registration pathway to follow—be it a Premarket Notification (510(k)), Pre-Market Approval (PMA), or the De Novo process. Each pathway has its unique requirements and nuances.
The submission process involves meticulous preparation of the required documentation, which includes a comprehensive understanding of the device's users and its competitive landscape. This preparation is augmented by the creation of a comparative table to highlight the similarities and differences with potential predicate devices.
Regarding user fees, it's important to remember that the FDA, an agency dedicated to public health assurance, requires these fees to process submissions. These are typically updated annually and vary depending on the registration pathway chosen.
The electronic submission system is designed to facilitate an efficient application process. However, manufacturers must be cautious not to include confidential information, such as medical data or Social Security numbers, in their public comments. If confidential information must be submitted, it should be done through a written/paper submission as detailed by the FDA's guidelines.
Understanding the distinctions between terms like 'Registered', 'Cleared', 'Approved', and 'Granted' is also vital, as these reflect different levels of FDA assessment and endorsement. For instance, 'Cleared' implies that a device is deemed safe and effective for its intended use based on substantial equivalence to a legally marketed device.
Moreover, staying informed about the latest FDA news, such as recent final rules on direct-to-consumer prescription drug advertisements, can provide insights into the agency's current focus and standards, which may impact submission strategies.
Lastly, it's beneficial to consider the broader context of medical device innovation, like the expanding use of 3D printing in manufacturing, which the FDA closely monitors to ensure the continued safety and efficacy of new products entering the market.
FDA Review and Clearance Process
The review process for a 510(k) submission is a meticulous and multi-stage procedure that ensures medical devices meet the necessary safety and effectiveness standards before they can be released to the market. The submission starts with an initial acceptance review, where the FDA assesses whether the submission is complete and meets the minimum threshold for substantive review. If the 510(k) submission passes this stage, it moves on to the substantive review phase. During this critical phase, the FDA rigorously examines the device's intended use, technological characteristics, and compares it to one or more legally marketed predicate devices. This comparison ensures that the new device is at least as safe and effective as the existing one.
For instance, the Impella Connect System underwent such a review process. As a device composed of both hardware and software elements, it provides crucial support for heart function in a clinical setting, while allowing health care providers to remotely monitor and receive important notifications related to patient status, a function deemed essential by the FDA under section 201(h) of the Act.
It's essential for manufacturers to deeply understand the device they're submitting, including its users, usage instructions, associated risks, and competitive landscape. This knowledge forms the basis of a comprehensive comparative analysis against potential predicate devices. The FDA's classification system plays a pivotal role here, as each class of medical device carries different patient risk levels and subsequent regulatory pathways—be it a Premarket Notification (510(k)), Premarket Approval (PMA), or De Novo process. The terminology—Registered, Cleared, Approved, and Granted—reflects the various stages and outcomes of this process, each with distinct implications for the device's market journey.
The final decision by the FDA can result in clearance, meaning the device can be marketed in the U.S., or a request for additional information, which must be satisfactorily addressed before moving forward. In some cases, the FDA may determine that the device is not substantially equivalent to any existing devices, which could require a more extensive review process or a different regulatory submission.
In the current landscape, the FDA continues to adapt and refine its processes to ensure clarity and efficiency in its regulatory oversight. For example, recent updates to the FDA's guidelines for direct-to-consumer prescription drug advertisements underscore the agency's commitment to clear and understandable communication, a principle that is mirrored in the review process for medical devices to ensure the safety and effectiveness information is easily accessible to all stakeholders.
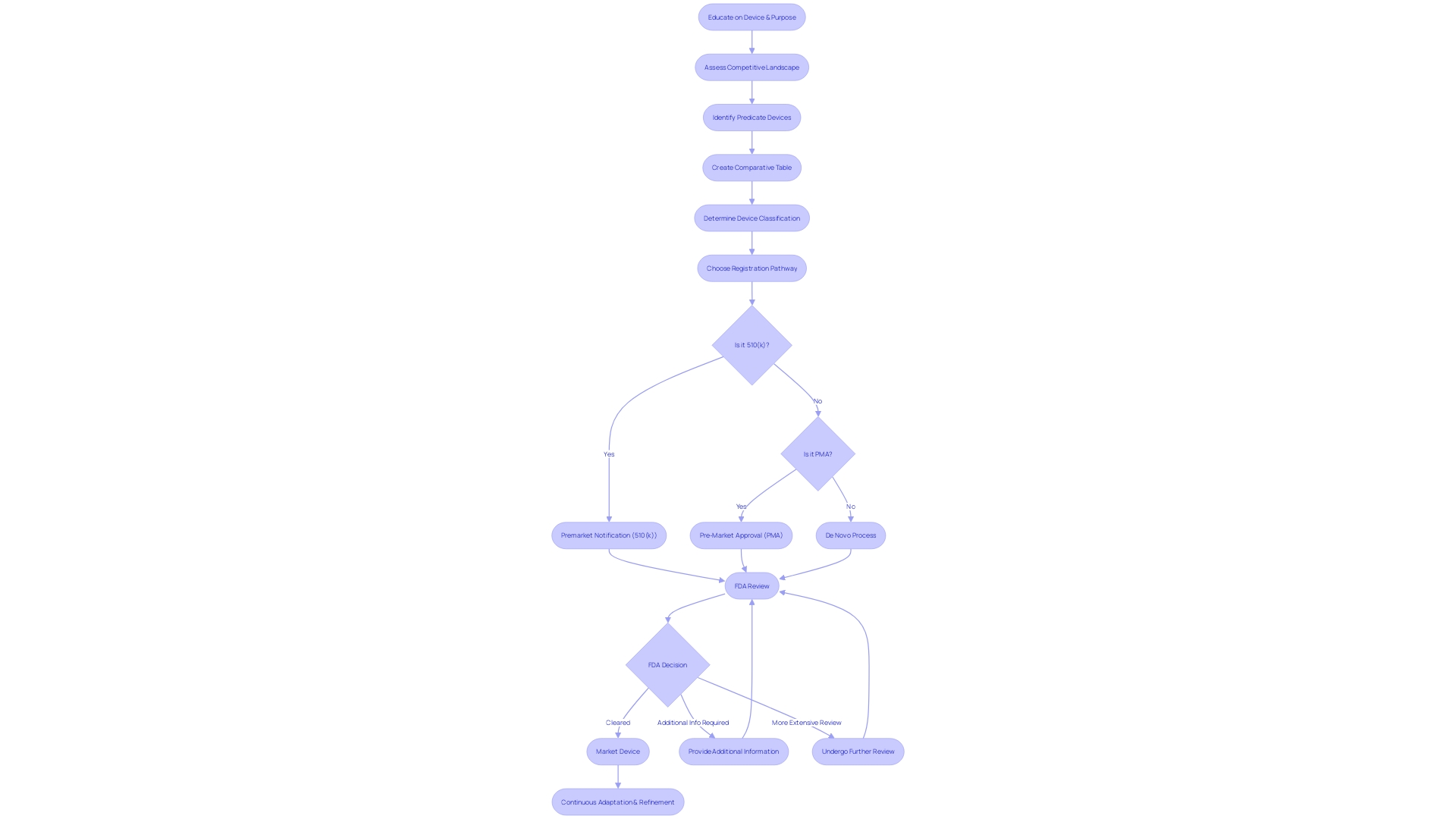
Post-Clearance Requirements and Maintenance
Securing FDA clearance is merely the beginning for medical device manufacturers, who must navigate a complex landscape of ongoing responsibilities to maintain compliance. It's imperative to understand the FDA's three-tier classification system, which assesses medical devices based on potential risk to patients. Once classified, the path forward may involve 510(k) clearance, Pre-Market Approval (PMA), or the De Novo process, each with distinct implications for the device's market journey.
Post-clearance, the obligations are multifaceted, encompassing vigilant post-market surveillance to ensure patient safety, timely reporting of adverse events, and regular updates to device labeling. Quality system regulations (QSR) also play a critical role, dictating a framework for manufacturers to adhere to, ensuring devices consistently meet FDA standards for quality and safety.
Manufacturers must be attuned to the FDA's expectations for quality systems, which are intentionally broad to accommodate the diversity of medical devices and their production processes. The non-delegable responsibility of compliance with these quality requirements emphasizes the FDA's commitment to safeguarding public health.
Navigating this regulatory environment is a dynamic challenge, with global market strategies and evolving regulations demanding a proactive and informed approach. The FDA's vigilance is underscored by statements from officials, reiterating their resolve to protect patients and enforce compliance rigorously. Understanding and fulfilling these post-clearance duties is not just a legal necessity but a commitment to patient well-being and the integrity of the medical device industry.
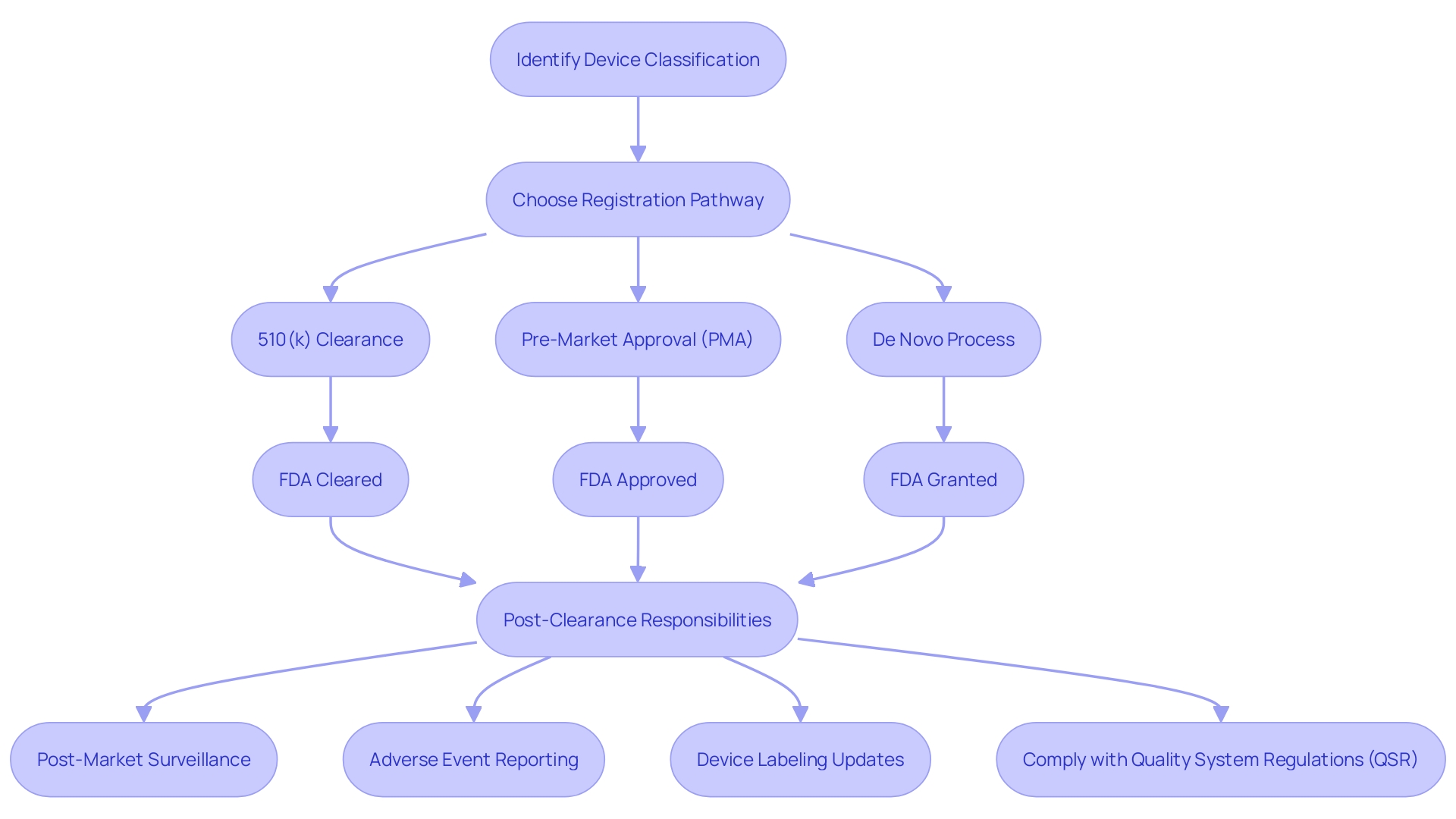
Common Types of 510(k) Submissions: Traditional, Special, and Abbreviated
Navigating the 510(k) clearance pathway for medical devices requires an in-depth understanding of the different submission types available. There are three primary types of 510(k) submissions: traditional, special, and abbreviated. Each of these has its own criteria and is suited to particular circumstances surrounding a device's entry to the market.
The traditional 510(k) submission is the standard route for most devices. It necessitates a comprehensive comparison of the new device to one or more predicate devices already on the market, ensuring they are at least as safe and effective. To make an informed traditional submission, thorough research into the use, warnings, and instructions of the device is vital, as is a keen understanding of the competitive landscape and potential predicate devices.
Special 510(k) submissions are for modifications to a device that has already received clearance and where the manufacturer can declare that the changes do not affect the safety and effectiveness of the device. This type of submission can often be expedited if the modifications are well-documented and limited in scope.
Lastly, abbreviated 510(k) submissions are an option when a guidance document exists, a special control has been established, or the FDA has recognized a consensus standard relevant to the device. The submission should reference the information in these recognized materials to support the argument for clearance.
When preparing any type of 510(k) submission, it's essential to protect sensitive information. As comments and submissions to the FDA become public, manufacturers must carefully exclude confidential data, like medical or Social Security numbers, from their documents unless they opt for a written/paper submission with specific confidentiality measures.
To solidify the submission, manufacturers should review the FDA's 510(k) database for Summaries of Safety and Effectiveness to understand how similar devices have been evaluated, ensuring a robust comparative analysis. The goal is to navigate the 510(k) process with the utmost precision, leveraging existing data and clear documentation to facilitate a smooth and compliant journey towards device clearance.
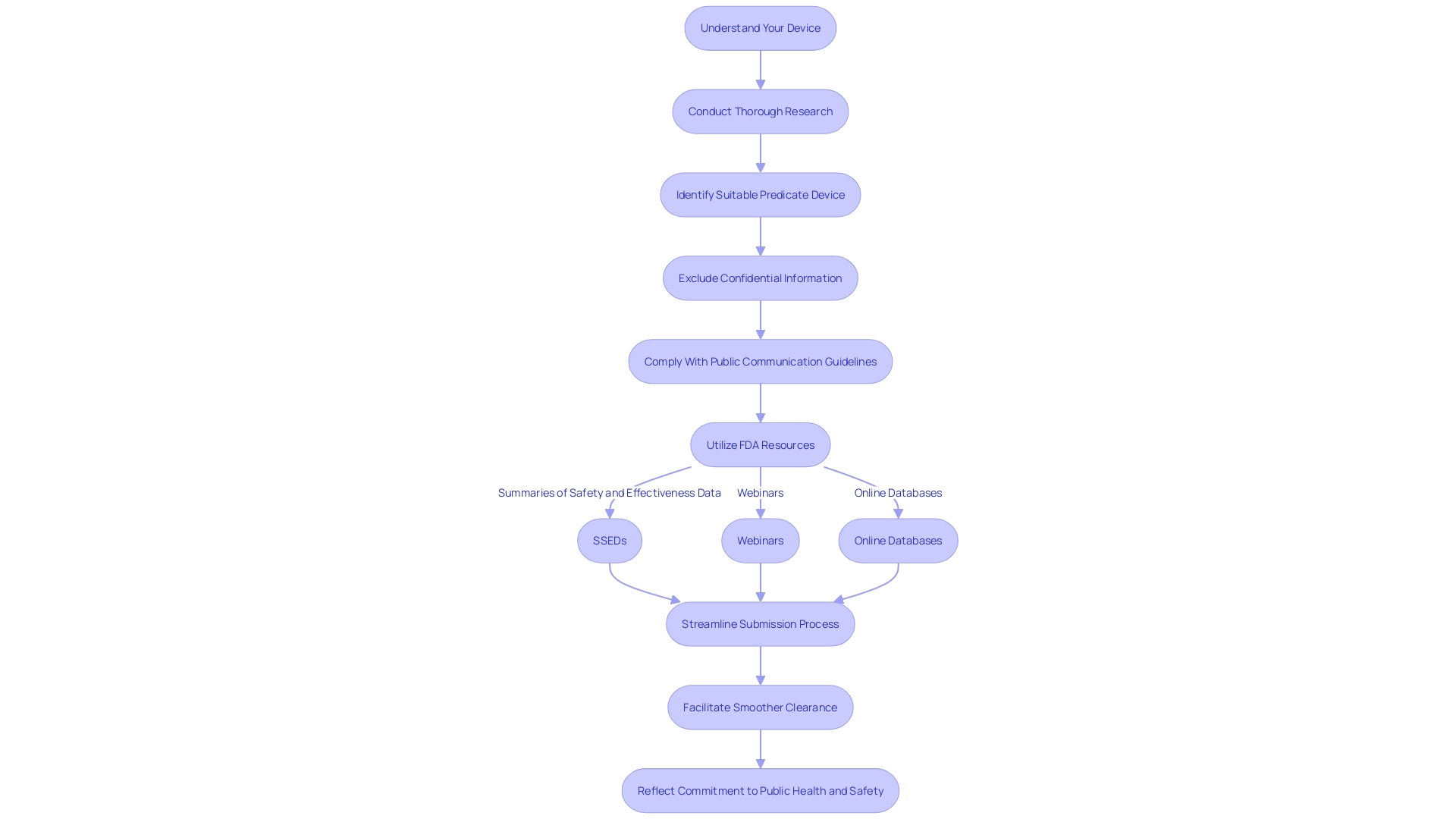
Resources and Guidance Documents for 510(k) Submissions
To streamline the 510(k) submission process, the FDA offers a suite of resources and guidance tailored to the needs of manufacturers. Understanding the device in question, including its intended users and usage instructions, is a critical first step. Manufacturers are encouraged to conduct thorough research, leveraging everything from clinical studies to competitor analysis, to identify a suitable predicate device. This can involve creating a comparative table to clearly illustrate the similarities and differences between the new device and existing ones with FDA clearance.
It's important to note that all communications with the FDA, including electronic submissions and any attachments, become part of the public record. Manufacturers must be diligent in excluding any confidential or sensitive information from these submissions unless they opt for a written/paper submission following the specified guidelines.
The FDA's responsibilities extend beyond medical devices to ensuring the safety and efficacy of a wide range of products, emphasizing the importance of compliance in all public communications. For a robust understanding of the FDA's expectations, manufacturers can review the Summaries of Safety and Effectiveness Data (SSED) from the FDA’s 510(k) database, comparing their device to those previously cleared.
By utilizing these FDA resources, from webinars to online databases, manufacturers can navigate the regulatory landscape more effectively, ensuring that their submission is comprehensive and compliant. This not only facilitates a smoother clearance process but also underscores the manufacturer's commitment to public health and safety.
Conclusion
In conclusion, navigating the FDA's regulatory framework is essential for medical device manufacturers entering the U.S. market. Understanding FDA clearance and approval processes ensures patient safety and product efficacy. Determining device classification determines the registration pathway, such as 510(k) clearance for substantially equivalent devices, PMA for novel devices, or De Novo for low to moderate risk devices.
Preparing a comprehensive 510(k) submission is crucial, including thorough documentation, comparative analysis with predicate devices, and providing performance testing, sterilization, and biocompatibility data. Finding and using predicate devices is vital, requiring evaluation of devices on the market and understanding the competitive landscape.
The FDA review process involves an initial acceptance review and substantive review, comparing the device to predicate devices to demonstrate substantial equivalence. Ongoing responsibilities post-clearance include post-market surveillance, adverse event reporting, and compliance with quality system regulations.
Different types of 510(k) submissions exist, such as traditional, special, and abbreviated, each with specific criteria. Protecting sensitive information when submitting to the FDA is vital.
The FDA provides resources and guidance documents to streamline the submission process. Thorough research, leveraging clinical studies and competitor analysis, helps manufacturers navigate the regulatory landscape effectively.
In conclusion, manufacturers must navigate the FDA's regulatory framework precisely to bring their medical devices to market successfully. Understanding requirements, following necessary steps, and ensuring compliance with FDA regulations are essential for patient safety, product efficacy, and market success.




