Introduction
The FDA's design controls are an essential part of medical device regulation, ensuring the safety and effectiveness of devices. From concept to release, manufacturers must adhere to these controls to manage risks, rectify design defects, and substantiate device safety. However, navigating the regulatory landscape can be challenging, with fragmented information and the presence of "grandfathered" devices.
To enhance postmarket surveillance, the FDA is working towards establishing an active surveillance system. Manufacturers bear the responsibility for compliance with quality system requirements, and as regulatory demands increase, industry professionals emphasize the importance of efficiency and safety in the device development process. Understanding the scope and applicability of design controls is crucial for medical device manufacturers, as these controls apply to all classes of devices.
The integration of design controls and risk management is vital for proactive risk identification and mitigation. Implementing design controls with a user-centered approach and following best practices can optimize the device development process and ensure the delivery of safe and effective medical devices. However, challenges such as limited resources and changing regulations must be overcome.
By embracing service design, user-centered design, and digitalization, manufacturers can enhance user experiences, comply with regulations, and foster innovation. Successfully implementing design controls requires a comprehensive approach that addresses various challenges and balances innovation and control.
What are FDA Design Controls?
The FDA's regulations on medical instruments play a crucial role in protecting public health, ensuring their safety and effectiveness. The range of these checks is extensive, encompassing the creation and advancement lifecycle from initial idea to ultimate product launch. By following these controls, manufacturers are expected to proactively handle risks, fix design defects, and validate safety and effectiveness of the product.
Despite the robust framework provided by the FDA, challenges remain in navigating the regulatory landscape. The absence of a unified database to verify the approval status of healthcare products can complicate the process. Different databases, like Drugs@FDA for small-molecule drugs, The Purple Book for biological products, and other resources for medical devices, offer fragmented information. Some products that predate the modern regulatory system are legally marketed without formal approval, known as 'grandfathered' items.
Medical instruments are categorized by the FDA into three classes based on risk, with class three appliances—such as life-sustaining implantables—undergoing the most stringent regulatory scrutiny. These high-risk products represent a portion of the marketplace but require extensive approval processes. Acknowledging the complexity, the FDA has published guidance for direct-to-consumer drug advertisements to ensure clarity and neutrality, particularly in the major statement of side effects and contraindications in TV and radio ads.
The FDA's recent efforts to enhance postmarket surveillance of products underscore the importance of ongoing vigilance. Challenges such as securing funding and identifying users of the equipment have been identified, but the FDA is actively working to address these issues. This effort comes in view of data connecting over 1.7 million injuries and 83,000 deaths to healthcare instruments in the United States within a decade. The implementation of a proactive monitoring system is a measure towards reducing the hazards related to healthcare equipment and improving patient well-being.
Manufacturers bear the ultimate responsibility for compliance with quality system requirements, as outlined in the QS regulation. This regulation, while offering some flexibility, does not allow the delegation of accountability, although the execution of tasks can be. As the healthcare equipment industry gets ready to address growing regulatory demands, perspectives from industry experts underscore the surge in regulatory prerequisites and the influence of regulatory undertakings. Strategies for navigating these evolving demands focus on improving efficiency in regulatory and safety document preparation, a task critical for market entry and patient safety.
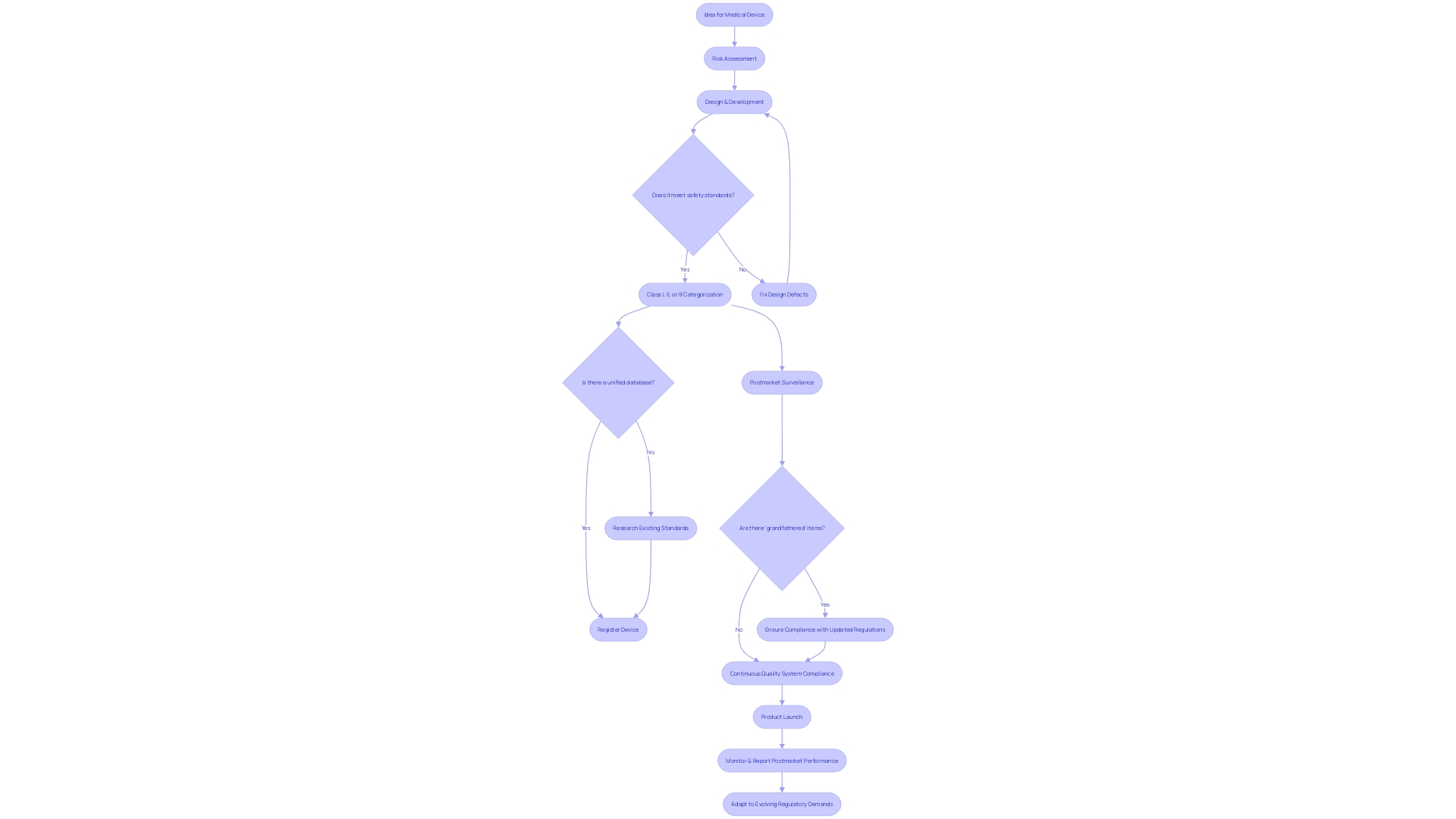
Scope and Applicability of Design Controls
For manufacturers of healthcare instruments, understanding the complete scope and demands of FDA regulations is essential. These rules are extensive and are applicable to all categories of healthcare tools, from basic instruments like tongue depressors to intricate, life-preserving machinery like heart pacemakers. The application of these management measures is not voluntary but a necessary stage to ensure the product's quality and safety. This is highlighted by a remarkable statistic: a 2018 study uncovered that during a span of 10 years, there were over 1.7 million injuries and 83,000 deaths in the U.S. potentially associated with healthcare equipment. The FDA has responded by improving post market surveillance to monitor the ongoing safety of healthcare instruments, a measure that emphasizes the significance of meticulous product management throughout the instrument's lifespan. Moreover, with professionals like Chris, a biomedical engineer with 13 years of experience in the field, contributing to the development and management of clinical studies, the industry is continually learning and advancing the standards for safety and effectiveness. The FDA's responsibility in protecting public health by ensuring the safety of healthcare equipment further highlights the necessity for manufacturers to strictly follow product planning measures as a vital component of the regulatory structure.
Key Components of FDA Design Controls
The intricacies of FDA control guidance for medical equipment are multifaceted, with each component playing a critical role in ensuring the safety and efficacy of the product. A meticulous approach to creation and development planning is paramount, with an established comprehensive plan that delineates timelines, resources, and responsibilities. Precise and clearly defined inputs, based on user requirements, intended use, and regulatory obligations, establish the groundwork for the specifications and requirements of the product. The resulting outputs must be detailed, encapsulating the physical and functional attributes of the apparatus through precise specifications, drawings, and documentation.
Regular reviews are a foundation for monitoring progress, identifying potential concerns, and confirming alignment with inputs and regulatory stipulations. These reviews are complemented by rigorous verification of the product's structure, where manufacturers are tasked with substantiating that the device conforms to specified requirements via objective evidence and systematic testing. Moreover, validation of the blueprint is crucial for confirming the performance of the apparatus in its practical use, guaranteeing that it meets user requirements and its intended function.
The shift from creation to production, referred to as transfer, requires a concentrated effort to ensure that all processes and specifications are accurately relayed. In the ever-evolving realm of device creation, any adjustments to the blueprint are subject to meticulous evaluation, documentation, and management to uphold the product's integrity and safety. The culmination of these efforts is chronicled in the Design History File (DHF), a comprehensive repository of records that documents the object's design control activities from inception through to manufacturing.
The FDA's commitment to public health is reflected in its exhaustive regulatory databases for various products, yet it faces challenges such as the absence of a 'single source of truth' and complexities surrounding the regulatory status of some products. These issues emphasize the requirement for transparency and explainability in the development process of the apparatus, ensuring that information impacting risks and patient outcomes is effectively communicated. In this spirit, the FDA has established clear, conspicuous, and neutral standards for direct-to-consumer prescription drug advertisements to enhance consumer understanding.
The FDA's categorization of healthcare instruments into three tiers based on patient risk values determines the suitable registration pathway, whether it be Premarket Notification (510(k)), Pre-Market Approval (PMA), or the De Novo process. Understanding the nuances between terms like Registered, Cleared, Approved, and Granted is essential for regulatory professionals navigating this landscape. Compliance with Current Good Manufacturing Practice (CGMP) requirements is also critical, ensuring safety and effectiveness in accordance with the Federal Food, Drug, and Cosmetic Act.
The need for a proactive system to monitor products after they are sold has been emphasized by alarming data: during a recent decade, there were over 1.7 million injuries and 83,000 deaths in the U.S. that could potentially be associated with healthcare equipment. This highlights the need for continuous evidence review to identify safety concerns regarding healthcare equipment that may otherwise remain unreported. The FDA is actively taking measures to set up such a monitoring system, tackling the difficulties of funding and patient identification to strengthen the supervision of safety in healthcare instruments.
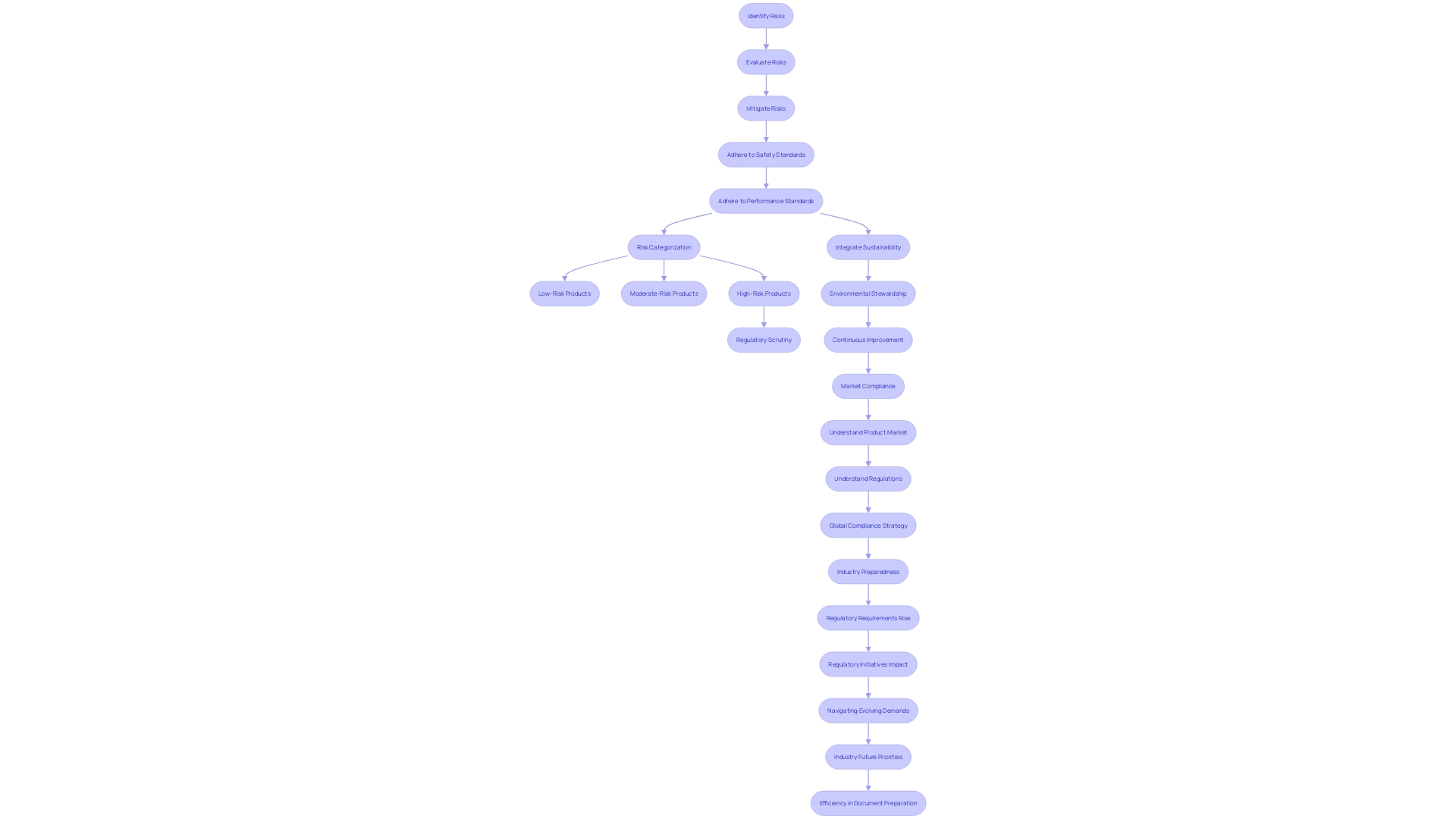
Linking Design Controls with Risk Management
In the field of healthcare equipment development, it is crucial to integrate design controls with strong risk management practices. This integration is crucial for manufacturers to proactively identify, evaluate, and mitigate potential risks throughout the lifecycle of an item. Not only does this approach ensure adherence to safety and performance standards, but it also plays a crucial role in safeguarding patient health.
Experts such as Bijan Elahi, an experienced practitioner with more than 29 years in safety risk management for healthcare tools, stress the significance of adopting a holistic approach to risk management. Elahi, who has equipped more than 10,000 individuals with best practices in the field, underscores that robust risk management extends beyond simply following ISO 14971 standards. It is about comprehending the intricacies, implementing practical strategies for success, and engaging in hands-on exercises for intricate assessments.
Since medical apparatus vary from basic structure to intricate machinery, they are categorized by the FDA into three levels of risk: class one represents the least risk, while class three denotes the highest level, encompassing life-sustaining apparatus such as pacemakers. Approximately 10% of equipment falls into class three and is subject to rigorous regulatory scrutiny due to its crucial role in healthcare. It's important to highlight that the timeframes for approval can be extensive for such high-risk products, requiring a well-coordinated development and risk management approach.
The incorporation of controls and risk management not only satisfies regulatory requirements but also aligns with industry moves towards more sustainable practices. The WEEE directive, for example, requires manufacturers of healthcare equipment to participate in the appropriate disposal and recycling of electronic waste, showcasing the industry's dedication to environmental stewardship and public safety.
To summarize, manufacturers must navigate the intricacies of regulations for devices in the healthcare field with a comprehensive approach to development and risk control. This includes keeping abreast of evolving industry standards, engaging in rigorous testing, and committing to ongoing education in risk management methodologies. By doing so, they can ensure the delivery of secure, dependable, and efficient healthcare products to the market.
Best Practices for Implementing Design Controls
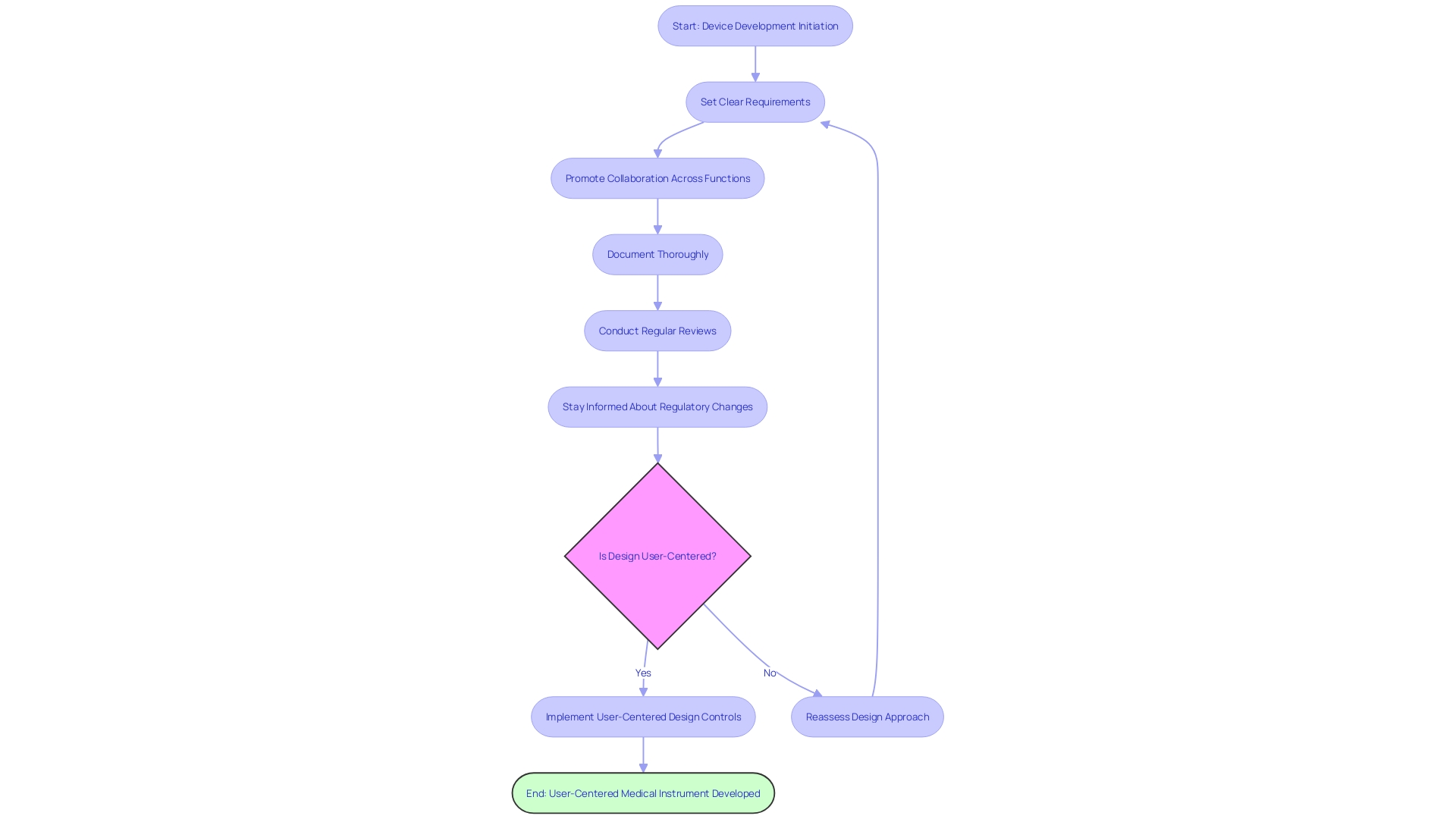
Successfully implementing management measures is not only a regulatory obligation but also a strategic method for the advancement of medical instruments that can greatly influence user satisfaction and product achievement. To enhance the efficiency of managing procedures, it's crucial to incorporate user-centered principles from the beginning. This means understanding the behaviors, needs, and motivations of all users, which may include clinicians, patients, and even support staff like maintenance and sterilization teams, to develop products that deliver impactful and relevant experiences.
Key best practices for implementing design controls with a user-centered approach include:
-
Starting Early: Integrating design controls from the initial stages of device development is crucial to minimize redesign efforts and associated costs. Early implementation allows for a more nuanced understanding of the device's functions and intended use, which is critical for identifying potential risks to users and ensuring the security of the device.
-
Setting Clear Requirements for Design: Well-defined requirements are the foundation of a development process that satisfies both user needs and regulatory standards. This clarity aids developers in making informed decisions and fosters transparency, which is vital for conveying information that could impact risks and patient outcomes.
-
Promoting Collaboration Across Functions: Achieving effective implementation of management demands collaboration across different teams such as engineering, quality assurance, and regulatory affairs. This collaborative approach ensures a holistic understanding of user environments and workflows.
-
Documenting Thoroughly: Comprehensive documentation is not only a regulatory necessity but also enables a smart digitalization process that enhances the work product by focusing on critical data. It facilitates future audits and ensures a secure, informed pathway to innovation.
-
Conducting Regular Reviews: These reviews are crucial for early identification of issues and ensure that the development progresses according to plan. Regular assessments maintain the pace of innovation while addressing potential risks, including quality, safety, and cybersecurity concerns.
Staying informed about changes in regulations: With regulations for devices used in healthcare constantly changing, manufacturers need to adjust their control processes to ensure compliance. This includes using established cryptographic communication protocols like Transport Layer Security (TLS) to ensure secure communications with healthcare equipment.
By integrating these practices with user-centered approach, manufacturers can achieve a balance between creating user-focused products and complying with strict regulatory requirements. The recent establishment of UL Solutions' testing laboratory in Michigan, for example, shows how the industry is addressing the need for facilities that can rapidly adjust testing approaches to manufacturer requirements, thereby promoting the development of safe and efficient healthcare products.
Common Challenges and Solutions
As the healthcare equipment industry progresses, the application of regulatory measures offers both prospects and challenges. Key challenges include limited resources such as time, budget, and expertise, which can be surmounted through strategic resource allocation, comprehensive training, and judicious outsourcing. A collaborative approach with regular communication and clear documentation is imperative to bridge communication gaps among diverse teams and stakeholders, preventing misunderstandings and project delays.
Staying abreast of the frequently changing medical device regulations is essential for compliance. Being proactive in monitoring and adjusting processes is essential in this ever-changing regulatory environment. Furthermore, achieving the balance between flexibility and control in processes of creation is essential; an excess of rigidity can hinder innovation, while an extreme amount of flexibility may compromise safety and quality. This balance is achieved through meticulous evaluation and robust risk management.
Incorporating service planning and user-centered development principles is increasingly recognized as beneficial in this sector. Service planning recognizes the wider scope of participants beyond the patient, including clinicians and hospital personnel, ensuring that healthcare equipment fulfills the requirements of all users in a healthcare environment. Meanwhile, user-centered approach explores the users' behaviors, needs, and motivations, encompassing not only the clinicians and patients but also caregivers, support staff, and technicians. It aims to enhance individual user experiences with healthcare equipment through user research, usability testing, and iterative development procedures.
The emotional interaction with healthcare tools also plays a crucial role. A product's aesthetics can significantly impact adoption, compliance, usability, and clinical outcomes. Taking into account and addressing users' emotional reactions through the creation can result in favorable experiences with healthcare equipment.
Embracing digitalization is another aspect that cannot be overlooked. It involves discerning the critical data and structuring work products accordingly, using digitalization to enhance not only where businesses want to go but also where they need to be. For example, UL Solutions has recently introduced testing services for healthcare equipment in Michigan, emphasizing the significance of adjusting to manufacturers' requirements and mitigating risks such as quality, safety, and cybersecurity while promoting innovation.
To summarize, effectively implementing controls in medical products necessitates a holistic approach that tackles limitations in resources, difficulties in communication, adherence to regulations, and the requirement for a balance between creativity and regulation. It also involves a user-centered design ethos that accounts for the emotional impact of devices and leverages digitalization to optimize both business and compliance objectives.
Conclusion
In conclusion, FDA design controls are crucial for ensuring the safety and effectiveness of medical devices. Manufacturers must adhere to these controls throughout the design and development process to manage risks and substantiate device safety. Challenges such as limited resources and changing regulations must be overcome.
Understanding the scope and applicability of design controls is crucial for medical device manufacturers. By integrating design controls with risk management and following best practices, manufacturers can optimize the device development process and deliver safe and effective medical devices.
To address challenges, manufacturers can embrace service design, user-centered design, and digitalization. These approaches enhance user experiences, ensure compliance with regulations, and foster innovation.
Effectively implementing design controls requires starting early, establishing clear design requirements, fostering cross-functional collaboration, documenting thoroughly, conducting regular design reviews, and staying updated on regulatory changes.
Common challenges in implementing design controls include limited resources, changing regulations, and finding the right balance between flexibility and control. These challenges can be overcome through strategic resource allocation, comprehensive training, proactive monitoring of regulatory changes, and a user-centered design approach.
In summary, successfully implementing design controls requires a comprehensive approach that addresses challenges and balances innovation and control. By incorporating user-centered design, embracing digitalization, and staying informed about regulatory changes, manufacturers can ensure the delivery of safe, reliable, and effective medical devices to the market.




