Introduction
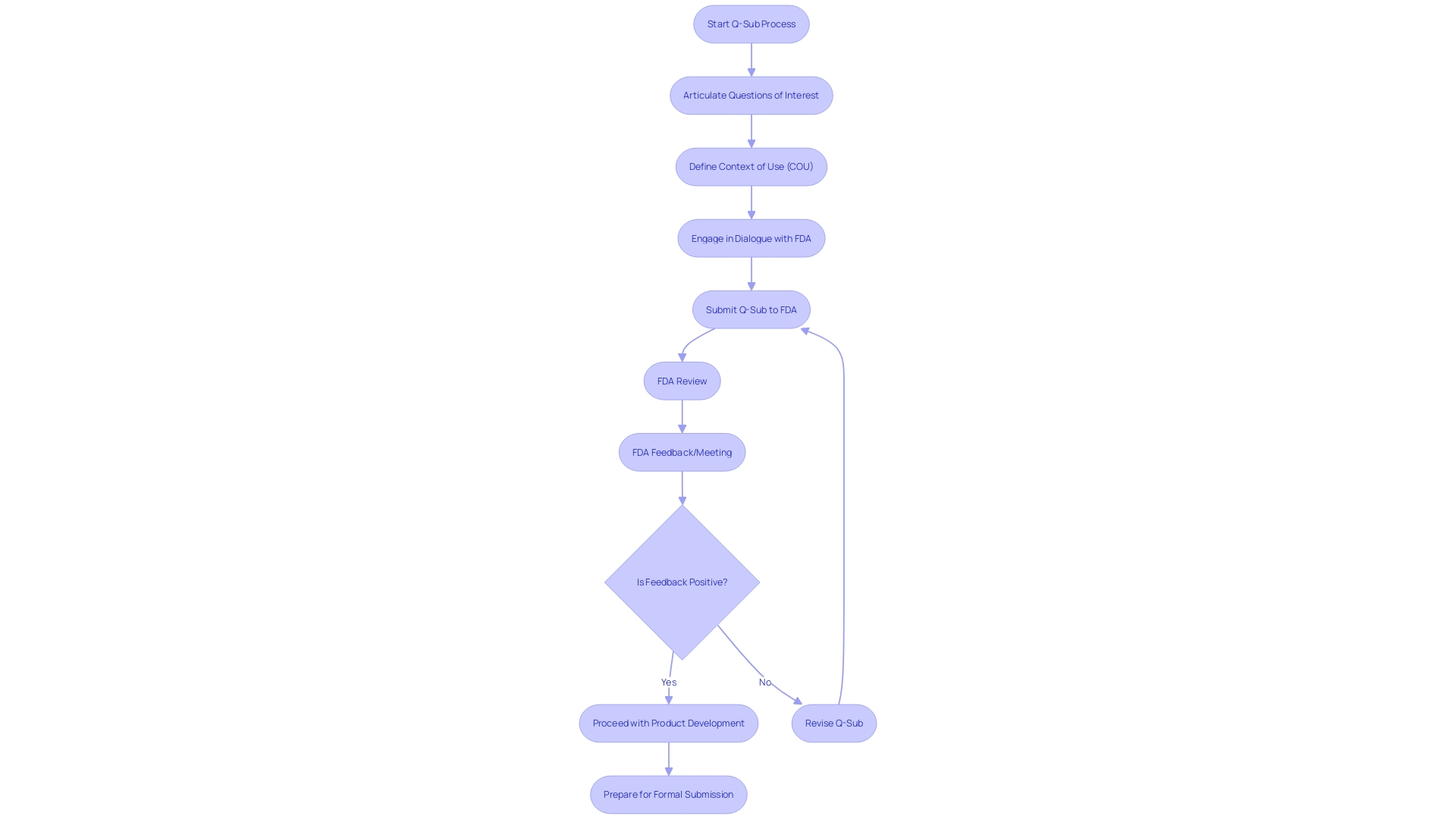
The Qualification Advice Submissions (Q-Sub) process is a crucial strategy for medical device manufacturers to engage with the FDA. This process allows for a dialogue between manufacturers and the FDA to ensure that regulatory requirements are clear and attainable. It is especially beneficial when dealing with complex computational models that are integral to a device's function.
Manufacturers must articulate their core concerns and define how the model's outputs will be utilized in the regulatory submission. The FDA emphasizes the importance of transparency and stakeholder engagement in the development of medical devices, while safeguarding sensitive information. Compliance with FDA regulations and voluntary consensus standards is essential to demonstrate conformity and ensure the highest standards of quality and safety.
The FDA's approach to regulation extends to the digital space, emphasizing clarity and presentation of information. Manufacturers must navigate FDA regulations by understanding which data is critical and how to organize it effectively. This strategic digitalization is crucial for regulatory compliance and market success.
What is a Q-Sub?
The Qualification Advice Submissions (Q-Sub) approach is a crucial pre-submission strategy for medical product manufacturers to interact with the FDA. It allows for a dialogue between manufacturers and the FDA to ensure that the regulatory requirements are clear and attainable. This procedure is especially advantageous when handling intricate computational models that are essential to the function of the apparatus. Manufacturers must articulate their Questions of Interest clearly, which are the core concerns the computational model aims to address in the regulatory submission. Moreover, they must define the Context of Use (COU), detailing how the model's outputs will be utilized to answer these questions, thus aligning the development process with the FDA's expectations.
As a component of its objective to guarantee general well-being, the FDA requires that health-related apparatuses fulfill stringent safety and efficiency criteria before they are made available to the public. The FDA has recently highlighted the significance of openness and involvement of interested parties in the advancement of healthcare equipment. Therefore, manufacturers of healthcare equipment are encouraged to provide feedback and participate in the administrative procedure, although it is advisable to refrain from publicly revealing any confidential details. This open dialogue underscores the FDA's commitment to collaboration with industry stakeholders while safeguarding sensitive information.
Compliance with premarket requirements is not only about adherence to FDA regulations but also involves the integration of voluntary consensus standards recognized by the FDA. These standards are developed through a process that embodies transparency and openness, allowing for balanced representation and due process. The FDA's recognition of such standards is meticulously documented and available to the public, providing a clear pathway for manufacturers to demonstrate conformity. This rigorous conformity assessment is a critical component of the FDA's regulatory framework, ensuring that healthcare instruments meet the highest standards of quality and safety before reaching consumers.
The FDA's approach to regulation also extends to the digital space, where the clarity and presentation of information are paramount. For instance, the FDA's final rule on direct-to-consumer prescription drug advertisements requires that major side effects and contraindications be presented in a manner that is easily understandable to consumers, utilizing both audio and text in television ads to ensure the message is conveyed effectively. This attention to detail in communication reflects the FDA's overarching goal of ensuring that all stakeholders, including consumers, have access to clear and accurate information about healthcare products.
As the industry navigates the complexities of FDA regulations, experts emphasize the importance of smart digitalization—understanding which data is critical and how to organize it effectively. This strategic digitalization is not only about converting documents into a digital format but about enhancing the business and compliance status by making informed decisions based on valuable data. It's about setting a course not just for where companies want to go, but where they need to go in terms of regulatory compliance and market success.
Types of Q-Subs
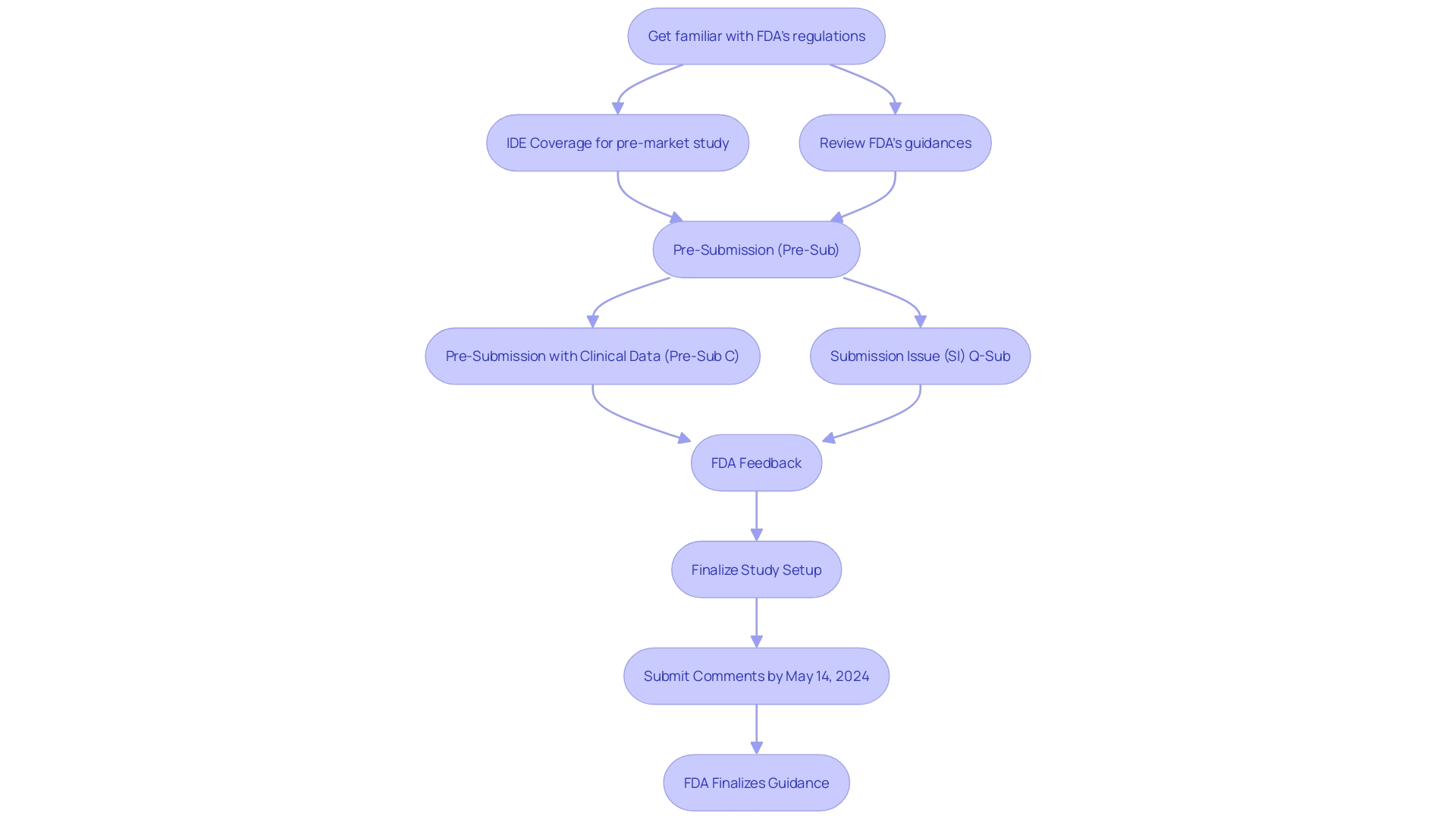
The FDA offers various Q-Submission (Q-Sub) pathways for medical instrument manufacturers, each tailored to support distinct regulatory objectives. A Pre-Submission (Pre-Sub) is suitable for those seeking early feedback before submitting their formal application. If clinical data is integral to the device's review, a Pre-Sub with Clinical Data (Pre-Sub C) can be utilized. For issues arising post-submission, the Submission Issue (SI) Q-Sub offers a structured approach for resolution. These submission types are not only gateways to FDA's advisory input but are part of the agency's broader commitment to fostering innovation and ensuring safety and efficacy in healthcare products. The FDA's explicit guidance on these procedures assists manufacturers in navigating the regulatory landscape, ensuring that innovative healthcare tools, which could greatly improve patient care, are brought to market in an efficient and effective manner.
Pre-Submission (Pre-Sub) Overview
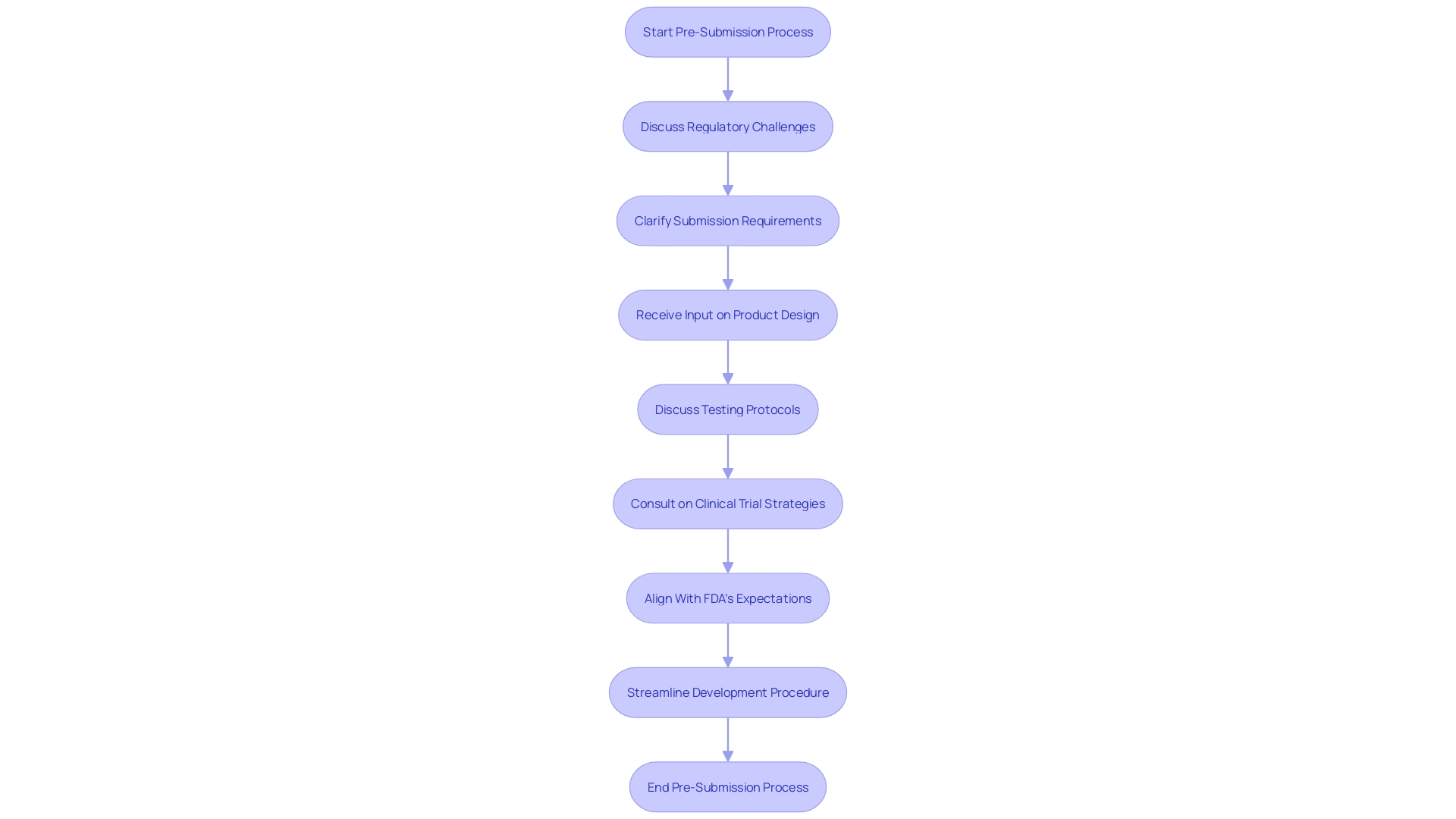
The Pre-Submission (Pre-Sub) component of the Quality Submission (Q-Sub) Program represents a critical juncture in medical apparatus development, as it enables manufacturers to engage with the FDA at an early stage. During this phase, companies can discuss potential regulatory challenges, clarify submission requirements, and receive valuable input on product design, testing protocols, and clinical trial strategies. This proactive approach facilitates a mutual understanding between the FDA and manufacturers, aiming to streamline the development procedure and enhance alignment with the Agency's expectations, potentially conserving time and resources.
Ongoing communication with the FDA is emphasized by the Agency's latest draft guidance on the Q-Sub Program, which delineates the available interaction mechanisms for equipment submissions. The document, titled "Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program," is poised to replace the previous guidance from June 2, 2023, upon finalization. It offers explicit clarifications on Q-Sub types, outlines methods for acquiring feedback on various questions, and provides enriched examples to aid submitters. Stakeholders are invited to submit comments on the draft guidance by May 14, 2024, ensuring that all feedback is considered prior to the commencement of the final guidance document.
The FDA encourages public participation in the shaping of guidance documents, reminding that all comments will be publicly accessible. Individuals providing feedback are responsible for omitting confidential information and are advised that any personal data included in comments will be made publicly available. For those wishing to submit confidential comments, the FDA specifies a written/paper submission process, underscoring the importance of secure and responsible sharing of sensitive information.
Content and Purpose of Pre-Submissions
Producers aiming to navigate the regulatory pathways for medical products must understand the critical role of a Pre-Submission to the FDA. This submission should encompass a comprehensive dossier detailing the apparatus's intended use, technological attributes, performance benchmarks, and any pertinent clinical information. The Pre-Submission's goal is to equip the FDA with enough detail to facilitate a constructive dialogue, providing manufacturers with essential feedback on their product's regulatory journey, testing protocols, and clinical trial design.
Transparency during this procedure is crucial, as it directly affects risk management and patient outcomes. Manufacturers are advised to ensure that their documentation effectively communicates the product's purpose and development process to the relevant stakeholders. It's also essential to present this information considering the user's context, employing optimal communication methods and timing.
To further clarify the application of the equipment, manufacturers should provide a clear and thorough explanation of the equipment's function, the intended user base, usage contexts, and the target population. It's equally beneficial to explain how the apparatus integrates into the current healthcare workflow, outlining its inputs and outputs.
Since the FDA is responsible for protecting public health by ensuring the safety and effectiveness of healthcare tools, compliance with its guidelines is not only a regulatory necessity but also a dedication to public welfare. The FDA's standards, such as those established for direct-to-consumer prescription drug advertisements, exemplify the agency's insistence on clear, conspicuous, and neutral presentation of information, principles that are equally applicable to Pre-Submission materials.
It is crucial for manufacturers to be aware of the public nature of the comments and information submitted, taking care to exclude confidential or sensitive data unless it is through a secure and specified written/paper submission process. As the landscape of healthcare equipment regulation continues to evolve, staying informed and compliant with FDA guidelines remains a top priority for manufacturers seeking to bring innovative and safe products to market.
Preparing a Pre-Sub: Key Elements
When creating a Pre-Submission package for the FDA, it is crucial to incorporate comprehensive elements that enable the agency to thoroughly evaluate the medical product. This should encompass a succinct cover letter, an in-depth description of the equipment, proposed labeling, and a thorough risk analysis. Additionally, detailed testing and validation strategies should be outlined, alongside clinical trial protocols when applicable. To strengthen the submission, manufacturers must also provide supporting data and relevant literature that substantiate the safety and efficacy of the equipment.
To achieve success in the intricate regulatory environment, educational efforts on the apparatus and its intended use are paramount. A deep understanding of the user's needs—be it clinicians, physicians, dentists, or patients—is necessary, and this understanding should be reflected in the clear instructions for use, including any warnings and cautions. Moreover, knowledge of the competitive landscape is crucial, requiring an examination of research literature, clinical studies, and other competitive devices to ensure the uniqueness and potential market impact of the new device.
The FDA's commitment to public health and safety underscores the importance of a well-crafted Pre-Submission. The agency's recent standards for direct-to-consumer prescription drug advertisements highlight the need for clarity and consumer-friendly communication. Similarly, manufacturers must ensure their submissions present information in a manner that is easily digestible and straightforward for FDA reviewers.
The successful navigation of the pre-market submission is indicative of a company's expertise in bringing new products to market. In a digital health-driven landscape, the integration of software and hardware components is increasingly valuable. As submissions can be voluminous, drawing from diverse company resources, the assembly and formatting of information are critical steps that can extend timelines significantly. Thus, an efficient and effective Pre-Submission not only facilitates the FDA's review but also reflects a company's comprehensive preparation for market entry.
Submission Process for Q-Subs
When preparing a Q-Submission (Q-Sub) for the FDA, manufacturers must meticulously compile all necessary documentation, adhering closely to the FDA's comprehensive guidance. This includes the 'Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program' draft guidance, which delineates the available interaction mechanisms with the FDA. The draft, set to supplant the previous document issued on June 2, 2023, emphasizes a clearer demarcation of Q-Sub types, feedback procurement methods, and detailed examples, although it is not yet in effect.
Public comments are crucial for the FDA's review, and therefore, the FDA welcomes electronic or written feedback on the draft guidance by May 14, 2024, which can be submitted following detailed instructions to ensure consideration before finalizing the guidance. When submitting comments, the FDA stresses the importance of not including any confidential information in the electronic comments, as they will become public record. For submitting confidential information, a separate written/paper submission procedure is outlined.
The FDA's primary goal is to uphold the safety, effectiveness, and security of healthcare equipment, among other duties related to public health. In fulfilling this duty, the FDA evaluates Q-Subs thoroughly, providing feedback to manufacturers. It is important for manufacturers to promptly address any FDA inquiries to facilitate a seamless submission procedure and progress towards achieving compliance and approval for their healthcare equipment.
Timing and Feedback Mechanisms
Improving the timing of a Q-Submission (Q-Sub) to the FDA is a strategic measure that can greatly influence the approval processes for healthcare instruments. While progressing through the stages of creating healthcare instruments, it is crucial to match regulatory inquiries with the appropriate Q-Sub category. The FDA, an authoritative body ensuring the safety and effectiveness of medical instruments, provides comprehensive draft guidance on this topic. This includes comprehensive details regarding the range of Q-Sub types and the procedures for acquiring feedback for different inquiries, whether through informal communication or a formal Pre-Submission method.
Manufacturers are encouraged to thoroughly understand the subject product, including its intended users and usage instructions, and to assess the competitive landscape, possibly identifying predicate items with analogous intended uses and technological characteristics. By submitting comments on draft guidances, stakeholders can participate in shaping the regulatory framework. The FDA offers multiple feedback mechanisms, such as in-person meetings, teleconferences, or written communications, tailored to the complexity of the submission. Maintaining open communication with the FDA is crucial, as it guarantees valuable input that can expedite the development and evaluation procedure.
It is noteworthy that comments on draft guidances, submitted electronically or in writing, are made public post-review. Submitters must be diligent in excluding confidential information from their comments, adhering to the FDA's submission instructions. By May 14, 2024, stakeholders have the opportunity to comment on the draft guidance for 'Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program', ensuring their perspectives are considered before finalization.
Best Practices for Pre-Sub Questions and Meetings
For manufacturers of healthcare equipment, the path to FDA approval is a careful procedure that requires a strategic approach, especially during Pre-Submission (Pre-Sub) meetings and submissions. To ensure a successful interaction with the FDA, it is imperative to adopt certain best practices.
Firstly, a thorough understanding of the medical equipment, including its intended use, user instructions, warnings, and cautions, is crucial. Manufacturers should also analyze the competitive landscape, comparing their product to existing products with similar uses and technological characteristics, often using a comparative table for clarity.
In preparing for a regulatory submission, it's essential to articulate the specific regulatory questions or concerns that need addressing. This involves describing the Questions of Interest and defining the Context of Use, detailing how the model outputs will inform regulatory decisions.
To convey these questions effectively to the FDA, manufacturers should:
- Provide a clear statement of the regulatory questions or issues requiring clarification or guidance.
- Offer adequate background information, ensuring the FDA comprehends the context of the questions.
- Pose questions that are specific and succinct.
- Support inquiries with relevant data, analyses, or literature for a well-rounded understanding. Prepare for the meeting by becoming acquainted with relevant guidance documents and previous FDA feedback on similar products.
During the Pre-Sub meeting, active participation is key. Manufacturers should engage with the FDA, listen attentively to feedback, and seek clarifications when necessary. Such diligence can greatly improve the value obtained from Pre-Sub meetings, guiding product development with informed precision.
The FDA's recent draft guidance on the 'Q-Submission Program' provides further insights into the types of interactions available to submitters and how to procure feedback for different types of questions. Producers should assess and provide feedback on this preliminary guidance, as it outlines the official procedures and anticipations for healthcare instrument submissions when completed.
Additional Considerations: MDDTs and EUAs
Manufacturers in the medical field who are going through the Q-Sub process must take into account the strategic utilization of Medical Device Development Tools (MDDTs) and the consequences of Emergency Use Authorizations (EUAs). MDDTs encompass a variety of instruments, techniques, or materials that facilitate the evaluation of medical devices' safety, effectiveness, or performance. The FDA supports the use of Models to streamline development and inform regulatory decisions, thus it is beneficial for manufacturers to integrate information about MDDT usage in their Q-Sub submissions to emphasize the scientific grounding of the product.
For gadgets designed for emergency scenarios, EUAs provide a regulatory pathway to expedite their availability during public health crises. Manufacturers seeking EUAs must carefully align their Q-Sub with the specific EUA requirements, providing detailed data to support the emergency utilization.
Submissions should include a comprehensive breakdown of the design, supported by literature, research, and evaluations, as well as manufacturing processes and quality controls to ensure the integrity of the product. A comprehensive understanding of the uniqueness and clinical utility of the product can be obtained by providing a detailed description with diagrams, photos, and technical specifications, as well as summaries of previous models and similar devices available on the market. Clear identification, intended purpose, risk classification, and envisioned clinical performance are also critical elements that should be consistently documented across all materials, from the clinical evaluation plan to risk management files.
To illustrate the industry's drive for innovation and quality, companies like Medtronic exemplify the commitment to delivering healthcare technologies that address complex health challenges. With a worldwide presence and a wide range of products, Medtronic's mission to relieve pain, improve health, and prolong life reflects the sector's focus on patient-centered solutions.
Overall, a successful Q-Sub submission not only fulfills regulatory requirements but also demonstrates a profound comprehension of the role of the equipment in patient care. It links the technical aspects of the apparatus with clinical benefits, backed by a thorough examination of both primary and post-market surveillance data. As the medical device landscape evolves, particularly with the integration of AI and the growing importance of Sand, manufacturers must remain proactive in adapting to legislative and regulatory changes to ensure the safety and efficacy of medical technologies.
Conclusion
In conclusion, the Q-Sub process is vital for medical device manufacturers to engage with the FDA and ensure clear regulatory requirements. Compliance with FDA regulations and strategic digitalization are essential for market success. The FDA offers various Q-Sub pathways tailored to support different regulatory objectives, facilitating collaboration and innovation.
The Pre-Submission component enables manufacturers to discuss challenges, requirements, and receive valuable input. Transparency and adherence to FDA guidelines, including clear presentation of information, are crucial. Preparation of comprehensive documentation and active participation in the submission process are key to success.
Feedback mechanisms should be utilized to optimize timing and facilitate valuable interactions. Overall, the Q-Sub process plays a critical role in navigating regulatory requirements and ensuring the safety and effectiveness of medical devices.




