Introduction
The Q-Submission program offers a structured process for dialogue between the U.S. Food and Drug Administration (FDA) and entities developing medical devices. It provides a path for obtaining the FDA's input on device development and clinical trial design, facilitating pre-regulatory discussions. The program includes various types of Q-Submissions tailored to different stages and questions in the development process, providing a roadmap for manufacturers.
The FDA's draft guidance on the Q-Submission Program emphasizes the commitment to refining this pathway and offers detailed advice on securing feedback efficiently. Stakeholders are encouraged to submit comments on the draft guidance to contribute to the final version. The importance of the Q-Submission process lies in its role in facilitating device development and market access, ensuring manufacturers navigate both regulatory and post-market challenges.
By engaging in this process, manufacturers can effectively address FDA requirements and improve patient outcomes.
What is a Q-Submission?
The Q-Submission program represents a structured process for dialogue between the U.S. Food and Drug Administration (FDA) and entities developing medical devices. It facilitates pre-regulatory discussions and provides a path for obtaining the FDA's input on device development and clinical trial design, which can include premarket applications and investigational device exemptions. There are distinct types of Q-Submissions tailored to various stages and questions in the development process, offering a clearer road map for manufacturers to follow.
The FDA's draft guidance on the Q-Submission Program, issued June 2, 2023, illustrates the commitment to refining this pathway. The guidance delineates the types of Q-Submissions and offers detailed advice on securing feedback efficiently, whether through informal communications or formal Pre-Submission meetings and other Q-Submission categories.
In response to this draft guidance, stakeholders are encouraged to submit comments electronically or in written form, taking care to avoid including confidential information in public postings. Comments should be submitted by May 14, 2024, to be considered in the final version of the guidance.
The FDA plays a pivotal role in ensuring the safety and efficacy of medical devices in the United States. Following FDA approval or clearance, the responsibility then shifts to payors and healthcare providers to make decisions on coverage, payment, and device use. The data required by the FDA for regulatory purposes may not fully align with the data needed by payors for coverage decisions, which can result in coverage, payment, or usage delays post FDA clearance. This highlights the importance of the Q-Submission process as a tool to facilitate device development and market access, ensuring that manufacturers effectively navigate both regulatory and post-market challenges.
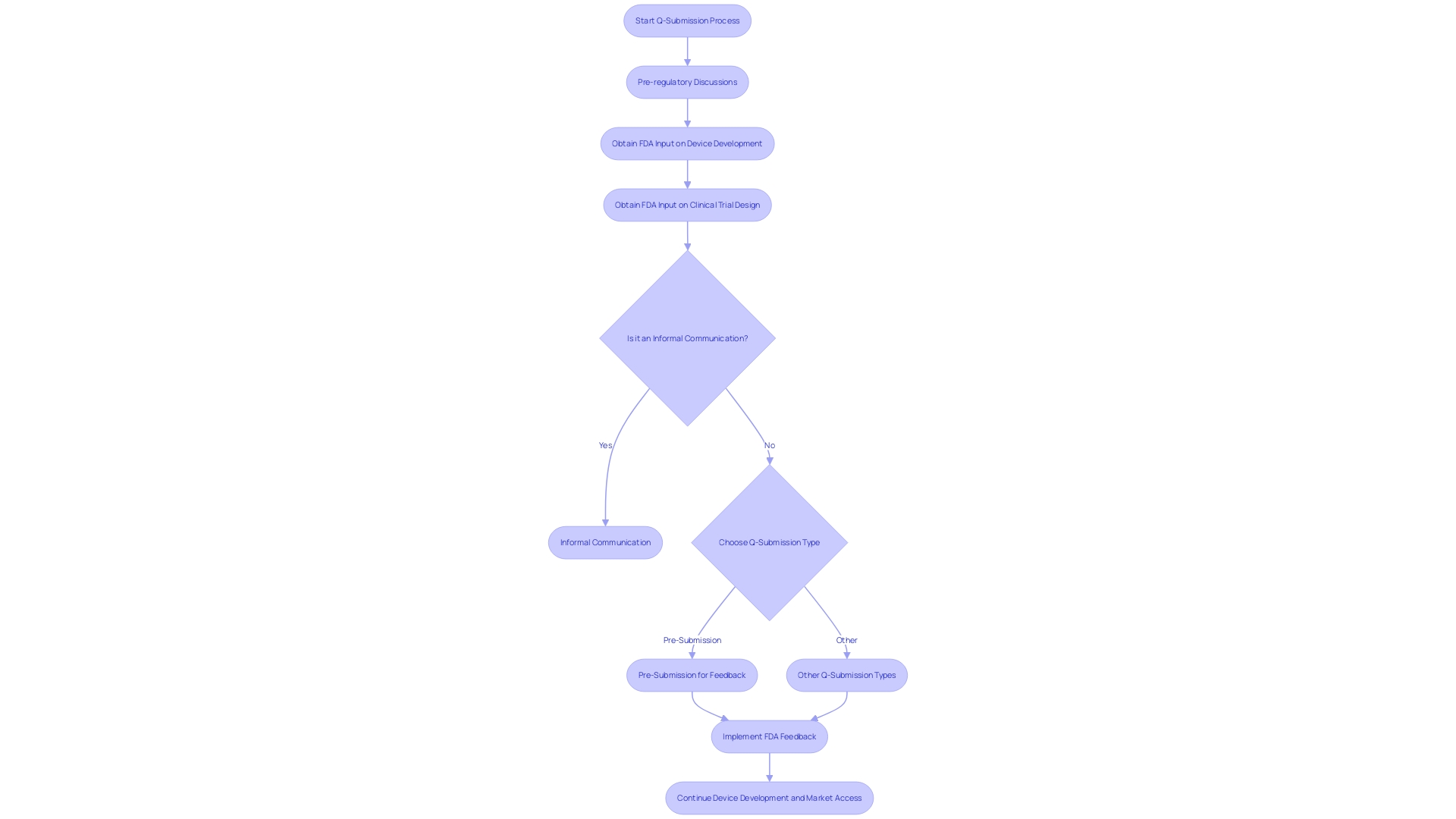
Types of Q-Submissions
The FDA's draft guidance on "Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program" delineates the various types of Q-Submissions manufacturers and researchers can utilize for FDA interactions. These include:
-
Pre-Submission Q-Submissions, which provide an opportunity to clarify specific questions or issues prior to formal regulatory application submission, aligning development plans with FDA expectations.
-
Investigational Device Exemption (IDE) Q-Submissions, tailored for medical devices under clinical investigation. This submission type seeks FDA input on study design and protocol, among other study-related topics.
-
Pre-Market Approval (PMA) Supplement Q-Submissions, allowing for feedback on changes to an already PMA-approved medical device, ensuring ongoing compliance and safety.
-
510(k) Q-Submissions, applicable to medical devices requiring clearance via the 510(k) pathway. This involves showcasing substantial equivalence to an existing legally marketed device.
-
New Drug Application (NDA) Q-Submissions, specifically for pharmaceuticals, encompass data on drug safety, efficacy, and manufacturing, aiming for FDA marketing approval.
The FDA emphasizes the importance of clear, non-confidential communication when submitting comments on the draft guidance, which is not yet finalized. This open dialogue ensures that the public and stakeholders can contribute to the development of the final guidance document. Additionally, the FDA's recent publication of the "Direct-to-Consumer Prescription Drug Advertisements" final rule highlights their commitment to ensuring information is presented clearly and is easily understandable, reflecting their broader regulatory approach including Q-Submission guidance.
Manufacturers and researchers are advised to follow detailed instructions when providing feedback, maintaining confidentiality where necessary, and to engage with this process before the specified deadline, contributing to the refinement of the Q-Submission Program guidelines.
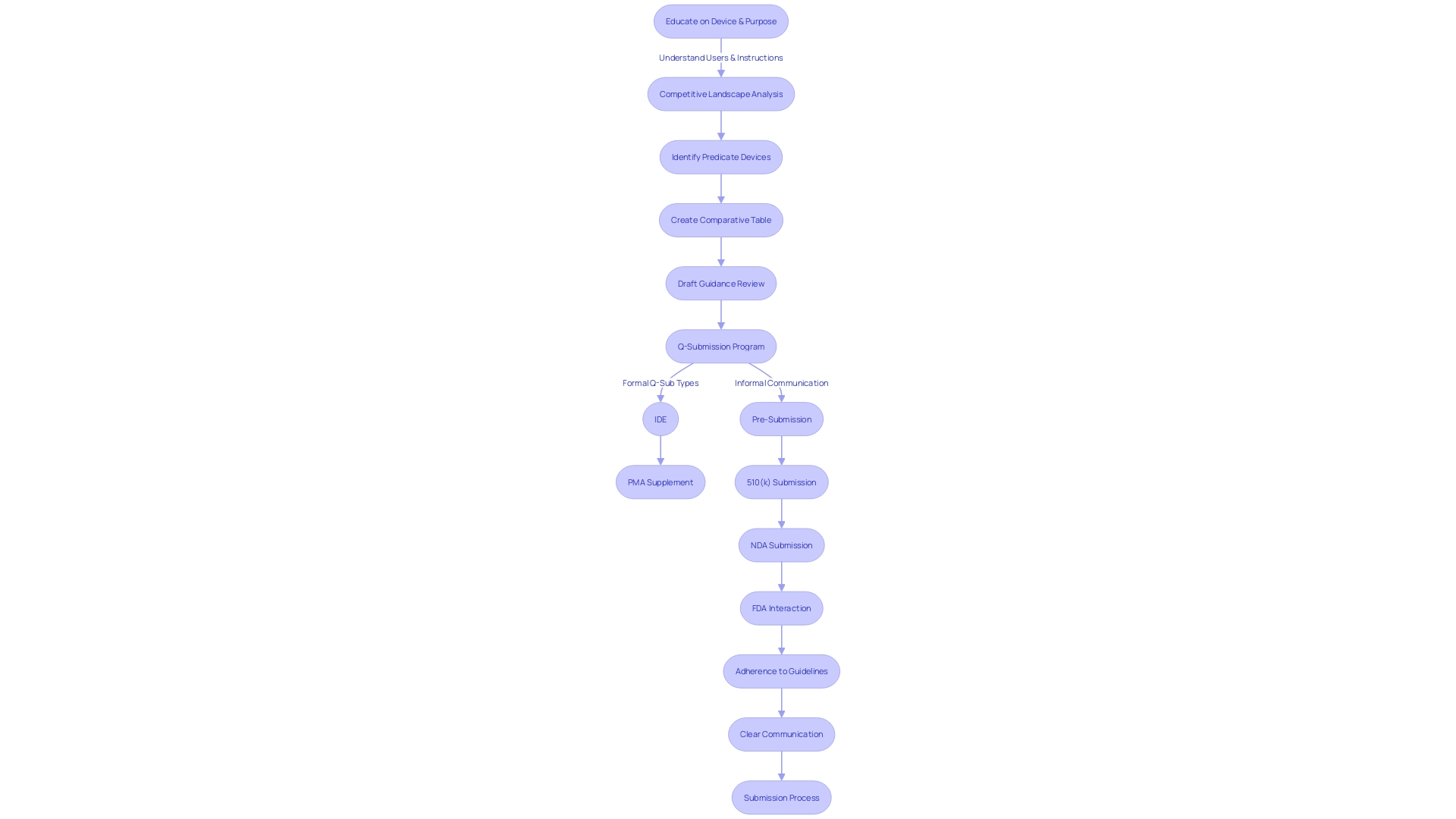
Pre-Submission Process
To streamline FDA compliance using the Q-Submission (Q-Sub) process, it's vital to execute a meticulous pre-submission strategy. This strategy must include identifying the purpose of the Q-Sub, which involves articulating the specific feedback required from the FDA on medical devices or products. A thorough collection of supporting data and documentation is crucial, ensuring that a comprehensive picture of the subject is presented. Familiarization with the FDA's most current guidance on Q-Submissions is imperative to align with regulatory expectations and to leverage the draft guidance provided for feedback mechanisms on medical device submissions. Compiling the submission package demands careful attention, inclusive of a cover letter and all pertinent documentation. Before the final step of submitting the Q-Sub to the FDA, an internal review should be conducted. This review should engage key stakeholders to validate the accuracy and completeness of the submission, ensuring it reflects the organization's objectives. It is also important to heed the FDA's instructions regarding the submission of comments to protect confidential information. This includes not only personal data but also proprietary business details. For comments containing confidential information not intended for public disclosure, a specific written/paper submission process must be followed, as detailed by the FDA's instructions. Submitters are advised to provide comments electronically or in writing by the specified deadline to allow adequate time for the FDA to consider the feedback before finalizing the guidance. Furthermore, an in-depth understanding of the device in question, including its users, instructions for use, and competitive landscape is essential. Comparing the device with predicate devices through research literature and clinical studies helps create a robust comparative table, which is an integral part of the Q-Sub process.
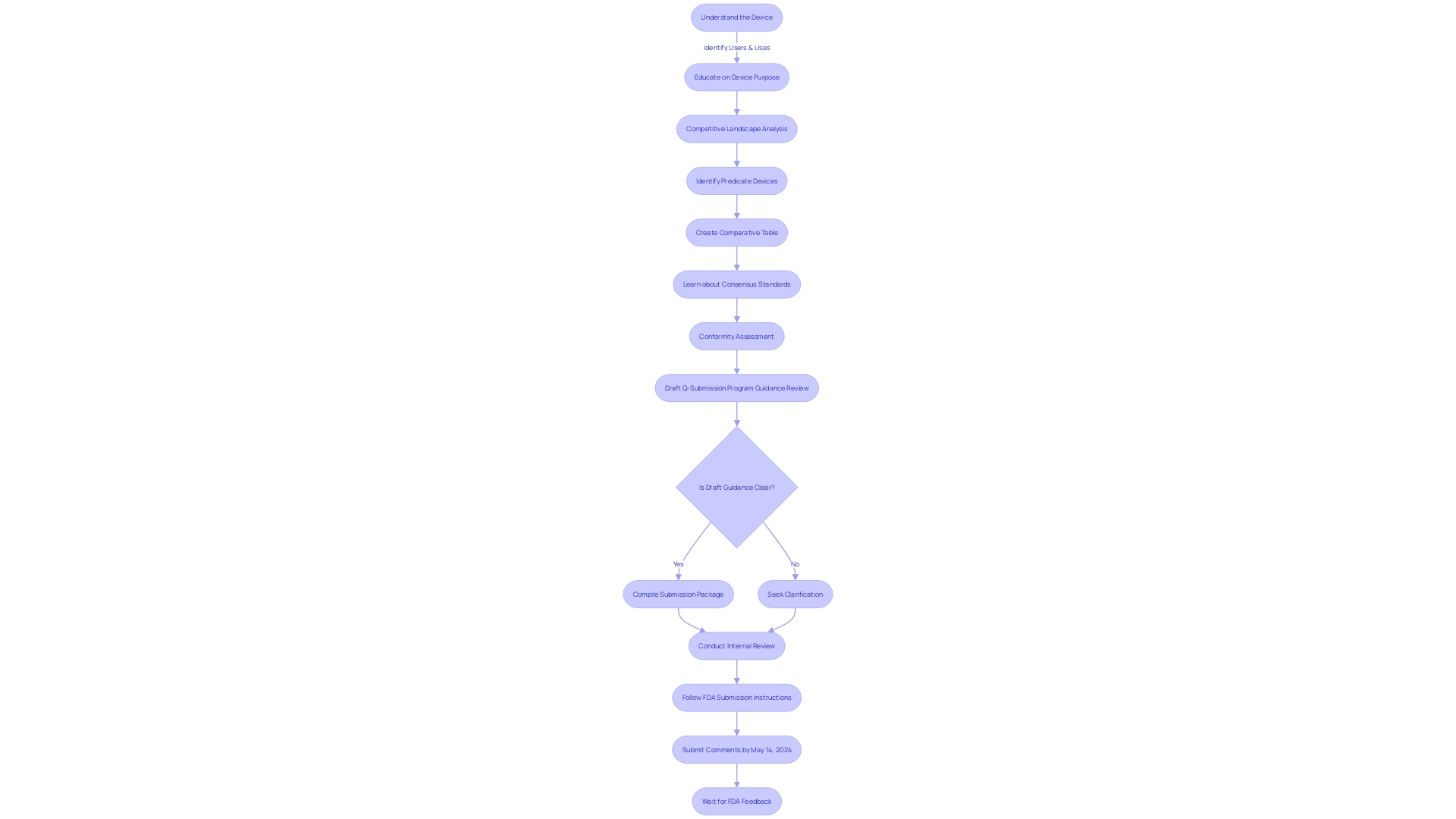
Components of a Pre-Submission
When preparing a pre-submission for Q-Submission to the FDA, it's crucial to assemble a comprehensive package that facilitates a clear and productive dialogue regarding your medical device or product. Start with a Cover Letter that succinctly outlines the intent of your submission, including specific queries and the type of feedback you're soliciting from the FDA.
Next, present Background Information that gives a detailed description of your product or device, highlighting its purpose, indications for use, and any pertinent historical data. This contextual groundwork is essential for the FDA to understand the scope and implications of your submission.
Including Supporting Data and Evidence is a pivotal component, as it substantiates your product's claims and addresses potential questions. This evidence can comprise scientific data, clinical trial results, and any ancillary information that reinforces your position.
Your Regulatory Strategy should be clearly documented, specifying the regulatory route you plan to take and any subsequent submissions you anticipate. This strategy will aid the FDA in assessing your long-term compliance and approval plan.
For instances where you're seeking the FDA's input on challenges or uncertainties, propose Proposed Solutions or Alternatives for the FDA to evaluate. This proactive approach demonstrates your commitment to adhering to regulatory expectations and finding mutually agreeable solutions.
Finally, an Appendix should be included to house any extra documentation or references, ensuring they are easily accessible and well-organized for review.
Be mindful that when submitting comments electronically, they will be made public; hence, it is your responsibility to exclude any confidential information, such as proprietary data or personal identifiers. If your comments contain such sensitive information, consider submitting them as a written/paper submission, following the detailed instructions provided by the FDA.
As the FDA continuously works to assure public health and safety, staying informed on the latest draft guidance, such as the 'Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program,' and submitting comments by the specified deadlines, will keep your regulatory approach aligned with current standards and expectations.
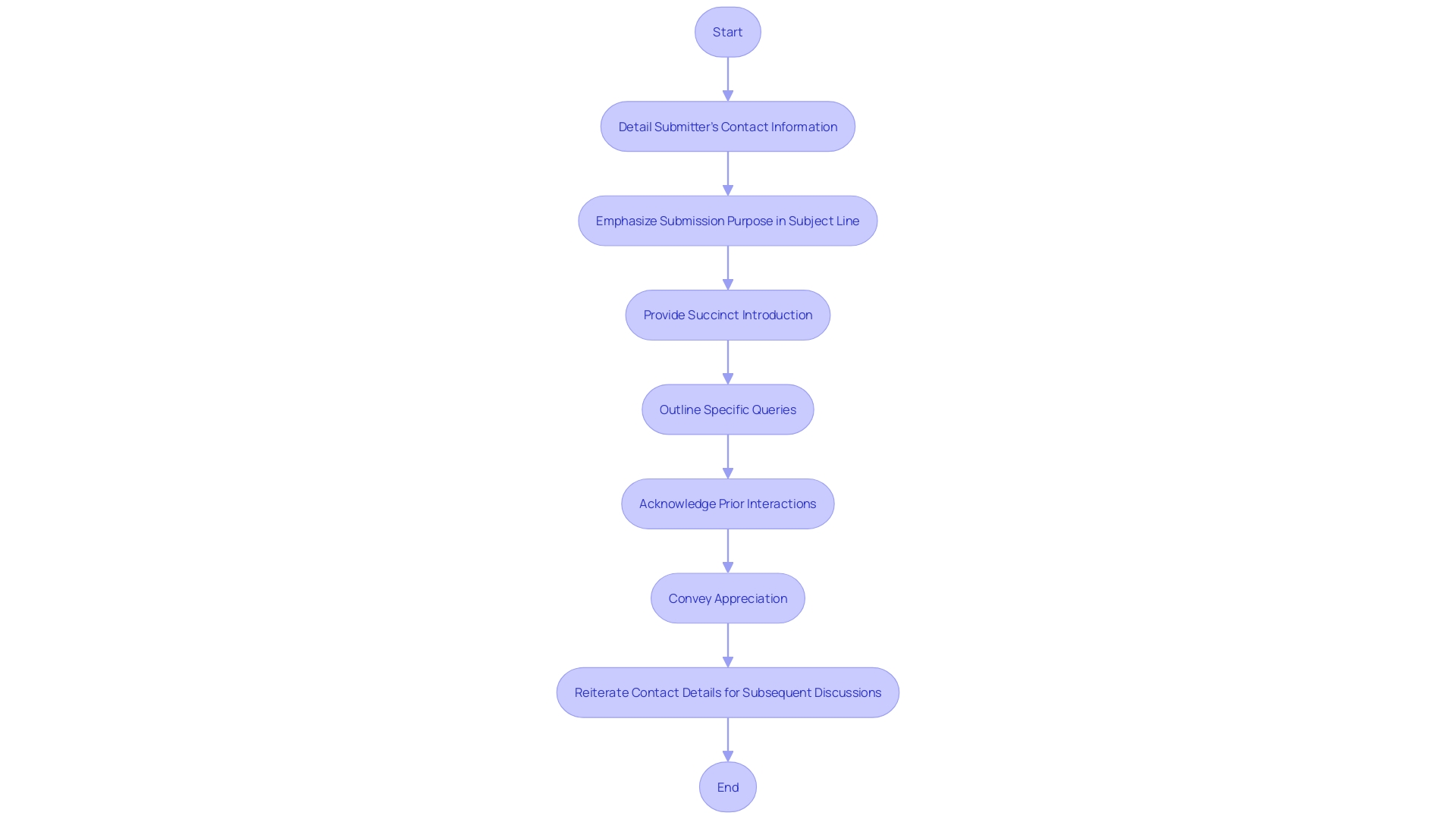
Cover Letter Requirements
Crafting a compelling cover letter for your Q-Submission is essential for successful FDA interactions. Begin by detailing the submitter's contact information to facilitate clear communication channels. Emphasize the submission's purpose in the subject line to immediately bring the FDA's attention to the intent of your correspondence. Provide a succinct introduction to your submission, describing the medical device or product and setting the stage for the detailed content to follow.
Outline specific queries where FDA guidance is requested, ensuring they are well-defined and directly related to your submission's context. It's crucial to express the nature of feedback you seek, whether it's regarding study design elements, manufacturing procedures, or other pivotal aspects of your product's development. If your submission is part of an ongoing dialogue with the FDA, acknowledge prior interactions to maintain continuity in your regulatory narrative.
In closing, convey appreciation for the FDA's consideration and reiterate contact details for subsequent discussions. Remember, transparency is key; avoid including confidential information within your public comments to safeguard sensitive data. By adhering to these guidelines, your cover letter will serve as a clear and professional invitation for constructive feedback from the FDA.
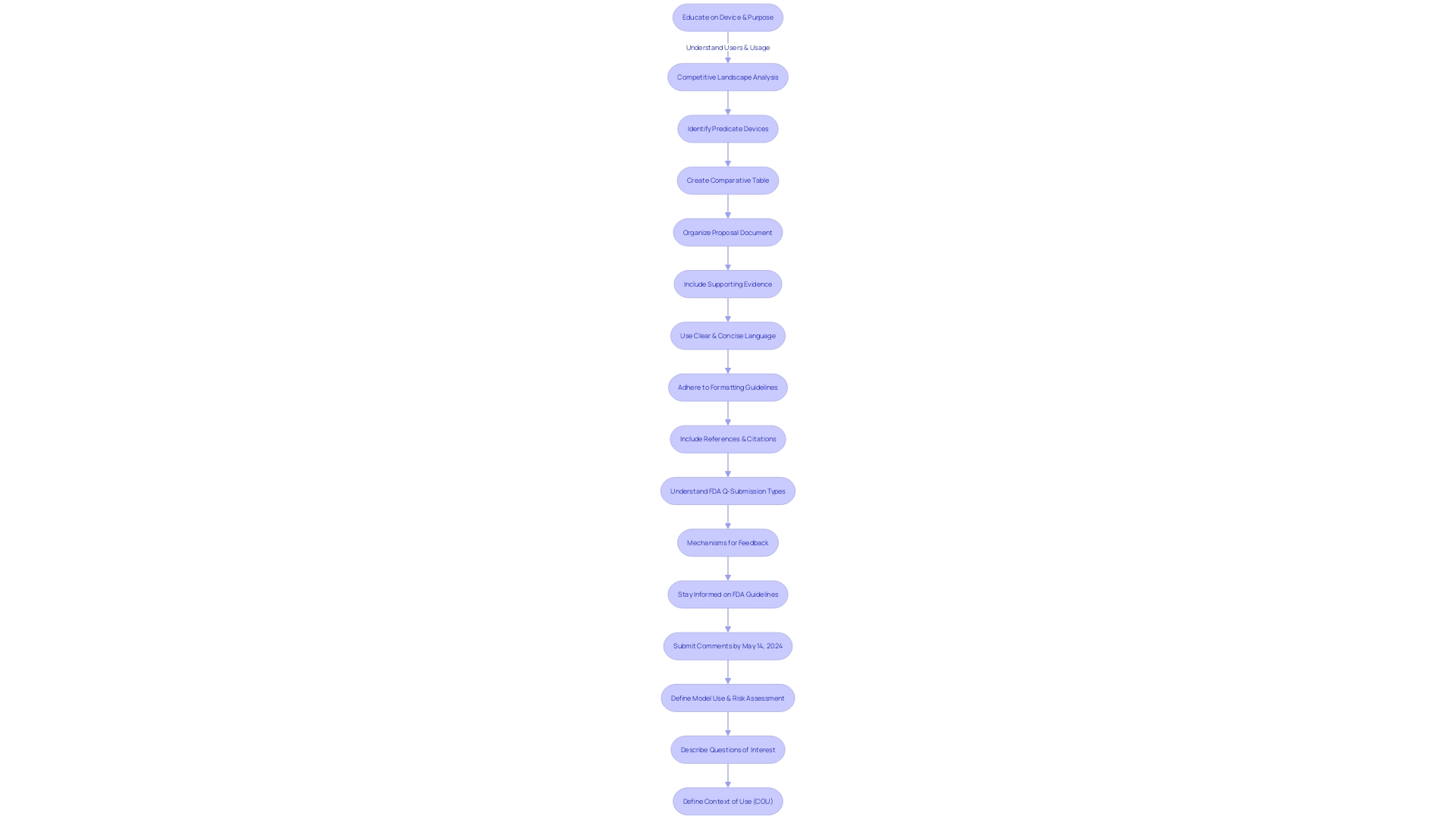
Body of the Submission
To ensure successful FDA feedback for medical device submissions through the Q-Submission process, it is critical to submit a well-structured proposal. Begin by organizing the document with clear headings and subheadings for navigational ease. Each section should present supporting evidence, including scientific data, clinical studies, or other relevant materials, to substantiate your statements. Employ clear and concise language to facilitate FDA reviewers' understanding. Adherence to formatting guidelines, such as font size, margins, and spacing, is essential for compliance. Lastly, include thorough references and citations for all sources utilized within your submission.
As highlighted in the FDA's draft guidance on the Q-Submission Program, submitters should be cognizant of the different types of Q-Submissions and the mechanisms for obtaining feedback. It is also crucial to understand the confidentiality of the information submitted, as electronic comments become part of the public record, whereas written comments can preserve confidentiality.
To be well-informed on the device in question, delve into research literature and competitive analysis, creating comparative tables where applicable. By staying abreast of the latest FDA guidelines and news, such as the recent announcement regarding draft guidance for medical device submissions, submitters can better navigate the Q-Submission program and contribute to the FDA's mission of ensuring public health and safety.
Regulatory History and Previous Interactions
When documenting interactions with the FDA regarding a medical product or device, it's crucial to include a comprehensive summary of past regulatory history. This summary should encapsulate all prior communications, such as pre-submissions and meetings, providing clear insight into the development journey and any guidance from the FDA. For instance, the Impella Connect System's premarket authorization process highlighted its software functions that are critical for patient monitoring, as outlined in the website instructions. Such detailed reporting is vital, as the FDA's role extends beyond the initial approval or clearance of medical devices. It involves continuous oversight to ensure the safety and effectiveness of these devices, which directly impacts patient care and treatment outcomes.
Understanding the FDA's classification system for medical devices, which ranges from Class I to Class III based on patient risk, is a foundational step in navigating regulatory pathways. Whether a device follows the 510(k) clearance, Pre-Market Approval (PMA), or De Novo process, the goal is to achieve FDA clearance, approval, or grant status before marketing in the U.S. The importance of clarity and transparency in these submissions cannot be overstated, as they convey critical information that could influence risks and patient outcomes.
Moreover, the FDA's evaluation of medical devices for U.S. use is just the beginning. Coverage decisions by payors and healthcare providers can delay or deny patient access to devices even after FDA clearance. This underscores the need for manufacturers to provide data that meets both FDA requirements and payor criteria for coverage determinations. The case of devices scrutinized in the 2018 documentary 'The Bleeding Edge' serves as a stark reminder of the potential consequences of inadequate clinical trials and regulatory scrutiny, with instances of patient harm underscoring the gravity of thorough FDA review processes.
Requesting Feedback and Meetings
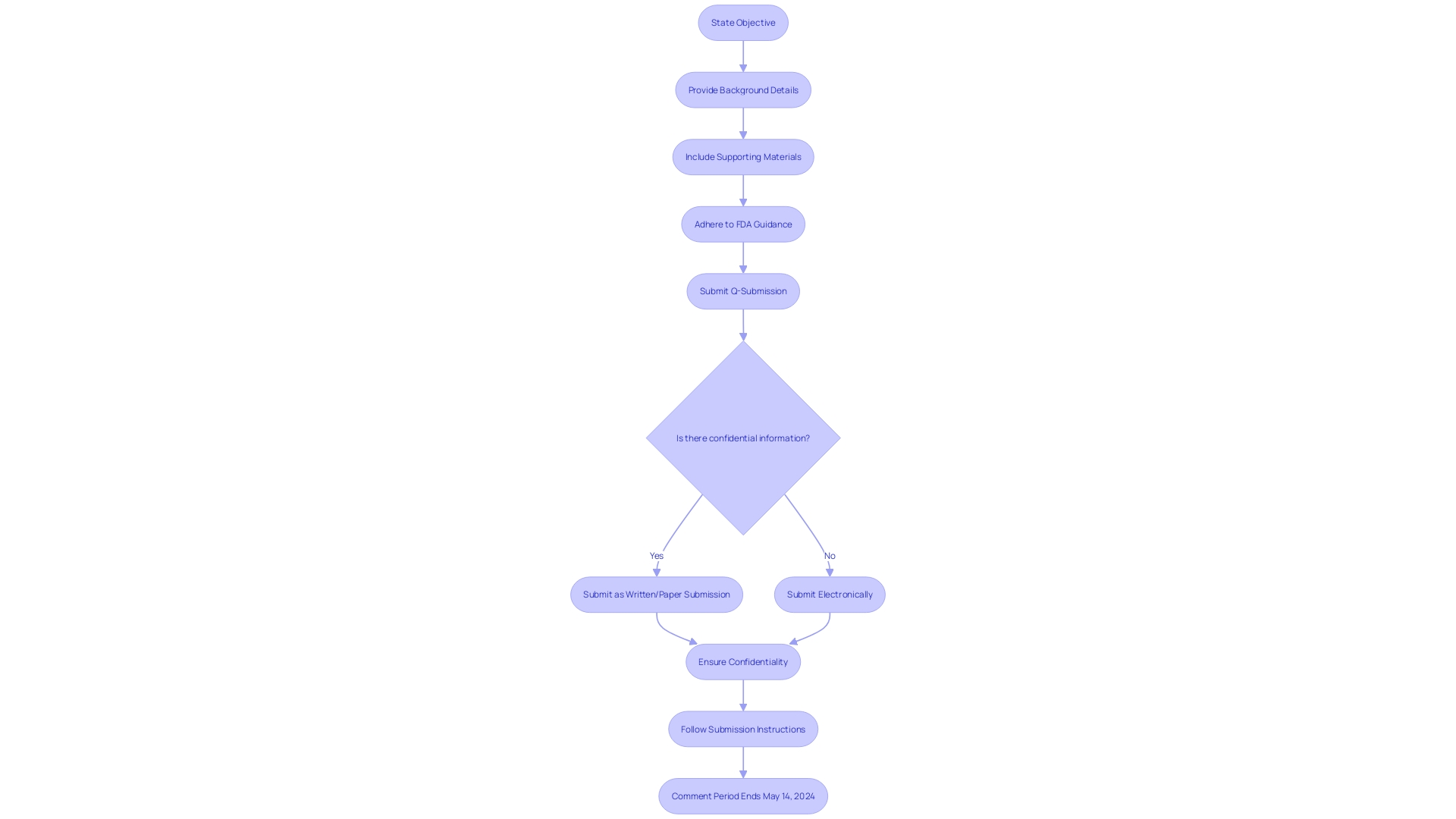
When initiating Q-Submissions, it's vital to streamline the process for both the submitter and the FDA. Start by succinctly stating the feedback or meeting's objective, ensuring all necessary background details are presented. Equally important is the inclusion of all relevant supporting materials that could aid the FDA's comprehension and decision-making process.
Adherence to the FDA's guidance is crucial. The Agency has outlined detailed protocols for Q-Submissions, as seen in the draft guidance titled 'Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program.' This document sheds light on the appropriate methods for interaction with the FDA, differentiates between types of queries such as informal communications versus Pre-Submissions, and provides improved examples. Although this guidance is in draft form and not for current implementation, stakeholders should submit comments by May 14, 2024, to influence the final document.
Maintaining professionalism is imperative throughout your interactions with the FDA. Recognize the Agency's authority and substantial workload by conveying your requests respectfully. Remember, any electronic comments submitted, including attachments, will be publicly available and unaltered in the docket. It is the submitter's responsibility to omit any confidential information, such as proprietary business details or personal identifiers, from these comments. For confidential submissions, follow the instructions for written/paper submissions.
With the FDA's role in ensuring the safety and efficacy of medical devices, as well as its oversight of various other health-related areas, it's important to respect the formal processes established for feedback requests. The upcoming virtual public meeting titled 'Listening Session: Optimizing FDA's Use of and Processes for Advisory Committees,' scheduled for June 13, 2024, emphasizes the FDA's commitment to transparency and stakeholder engagement. Keep abreast of such opportunities to participate and provide input that could shape future regulatory processes.
FDA Response and Meeting Logistics
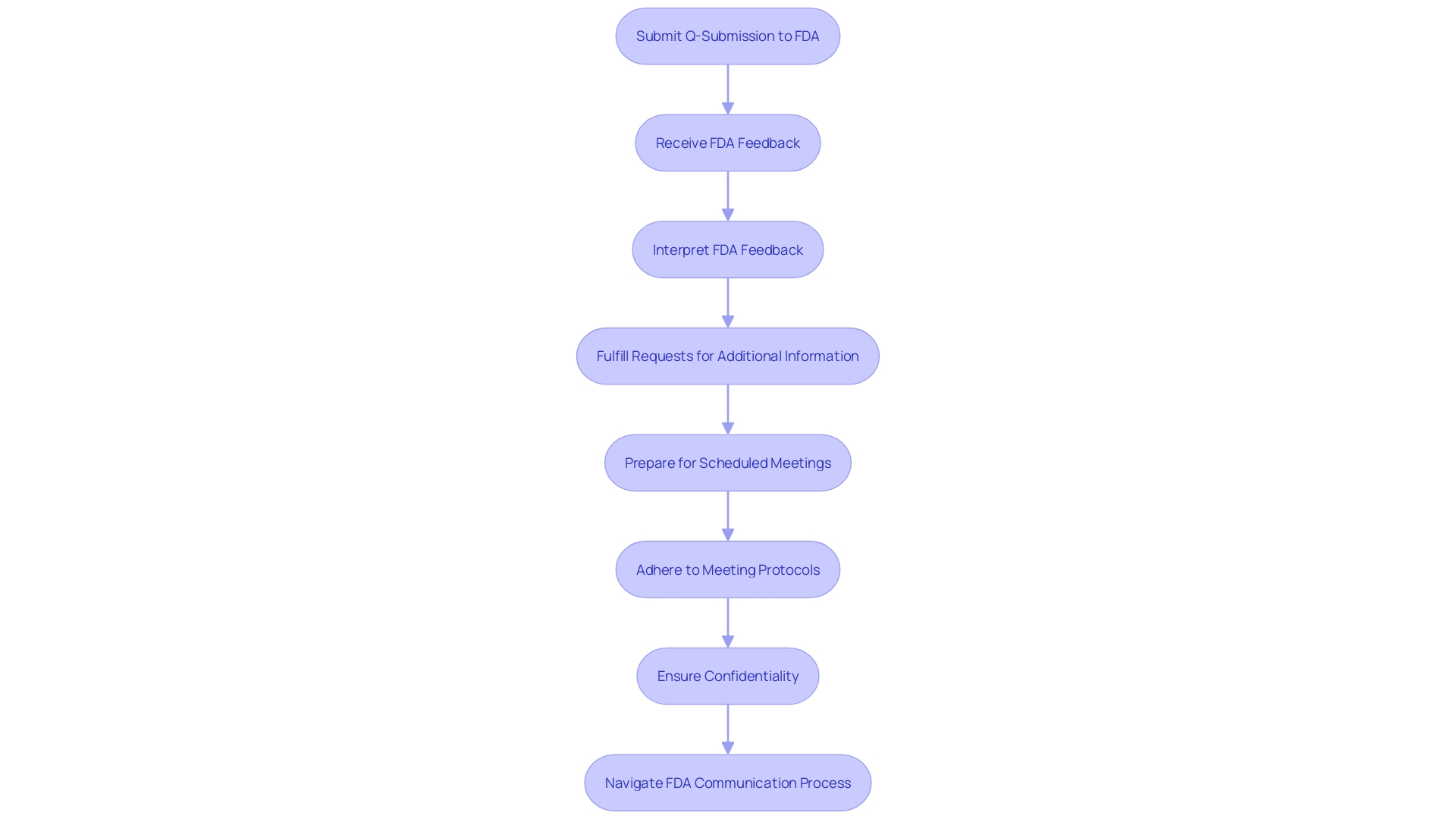
Upon submitting a Q-Submission to the FDA, it is vital to attentively navigate the ensuing communication process. The FDA's prompt and structured response will typically encompass written feedback, additional information requests, or the coordination of a meeting. To effectively engage with the FDA's response, several critical steps should be taken:
-
Interpret the FDA's Feedback: It is imperative to meticulously analyze the feedback or clarification provided by the FDA. This can involve parsing through complex regulatory language or technical details to ensure a comprehensive understanding of the feedback.
-
Fulfill the FDA's Requests: Should the FDA request further information, it is crucial to respond swiftly and thoroughly, supplying the requested data or documentation to advance the submission process.
-
Prepare for Scheduled Meetings: In the case of a meeting, preparation is key. This includes organizing pertinent materials, formulating a clear agenda, and pinpointing essential topics for discussion to optimize the value of the interaction.
-
Adhere to Meeting Protocols: During any meetings with the FDA, it is essential to maintain a professional demeanor, engage actively and listen attentively. Documenting the proceedings and noting any agreed-upon actions or necessary follow-ups is equally important.
When participating in this process, it is also critical to ensure that no confidential information is inadvertently disclosed. Comments and attachments submitted electronically become part of the public record. Therefore, safeguarding sensitive personal, medical, or business information is of paramount importance. In circumstances requiring confidentiality, comments should be submitted following the FDA's detailed written/paper submission guidelines.
Engagement with the FDA is not only a regulatory hurdle but also a collaborative effort to ensure the safety, efficacy, and security of medical products. As such, staying abreast of the FDA's systematic processes, including post-market assessment of chemicals in food and the approval of new drugs and biological products, is essential for a successful partnership and advancement of public health initiatives. Understanding these intricacies, while navigating the regulatory environment, can significantly impact the timely advancement of medical products and therapies.
Best Practices for Submitting Q-Subs
To enhance the effectiveness of the Q-Submission process and secure valuable feedback from the FDA, consider incorporating these best practices:
-
Initiate Early: Engage with the FDA at the onset of your product or device development. Early interaction paves the way for timely guidance and potential adjustments.
-
Adhere to Guidance: Immerse in the FDA guidance on Q-Submissions, ensuring your submissions are in line with stipulated procedures and requisites. The draft guidance entitled 'Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program' is a vital resource for understanding the Q-Sub mechanisms and how to solicit different types of feedback.
-
Clarity and Specificity: Your submission should transparently state its purpose and pinpoint the exact feedback or queries you need addressed. As one expert puts it, "Educating yourself on the subject device and its purpose is extremely important."
-
Comprehensive Information: A robust submission is replete with all pertinent documentation, data, and evidence, offering a thorough perspective of the product or device.
-
Internal Review: Prior to submission, conduct a meticulous internal review to verify the accuracy, comprehensiveness, and alignment with your organization's objectives.
-
Professionalism: Maintain a professional demeanor throughout your interactions, acknowledging the FDA's authority and expertise in safeguarding patient safety and product efficacy.
Remember, the FDA's role extends to ensuring the safety and effectiveness of medical devices, and your compliance with their guidance and submission protocols is crucial. Your comments on the draft guidance are also valuable and should be submitted by the specified deadline, May 14, 2024, taking care to exclude any confidential information unless it is through a secure written/paper submission.
Common Mistakes to Avoid
When submitting Q-Submissions, attention to detail and adherence to FDA guidelines are paramount for a successful review process. To ensure that your submission is not delayed due to avoidable errors, consider the following best practices:
- Accuracy and Completeness: Double-check your submission for precision and ensure that no relevant information is omitted. All data should be current, supported by appropriate documentation, and concisely presented.
- Clear Objectives: Articulate the intent of your submission and pinpoint the exact feedback you are seeking from the FDA. This clarity will streamline the review process and prevent unnecessary back-and-forth communication.
- Regulatory Adherence: Familiarize yourself with the latest FDA requirements, including the draft guidance on Q-Submission Program interactions. Ensure your submission meets the current standards for format, procedures, and deadlines.
- Evidence-Based Support: Your submission should be fortified with comprehensive evidence, including clinical study data and other pertinent documentation, to substantiate the safety and efficacy of your medical device.
- Professional Communication: Engage with the FDA with professionalism, promptly addressing any additional information requests and keeping communication channels open for any clarifications.
- Proactive Follow-Up: Be diligent in addressing any action items or queries from the FDA. Timely responses and proactive engagement are crucial for the expeditious processing of your submission.
By avoiding these common pitfalls and incorporating a strategic approach, you can facilitate a smoother FDA review process for your medical device submissions.
Benefits of Early Interaction with the FDA
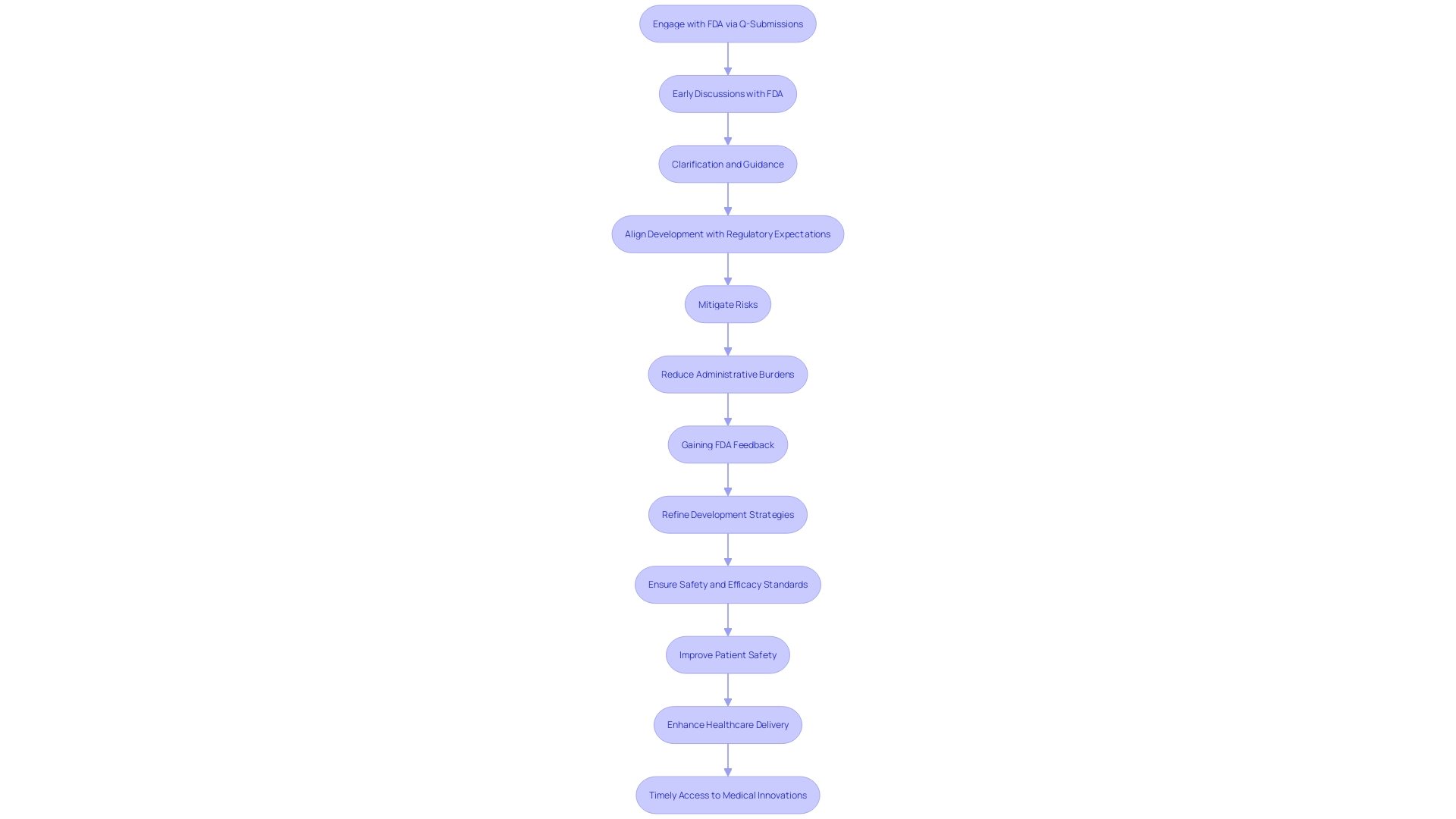
Proactive engagement with the FDA via Q-Submissions can significantly streamline the development process for medical devices and products. Early discussions provide critical clarification and guidance, aligning development with regulatory expectations. This proactive approach is exemplified by the FDA's draft guidance on the Q-Submission Program, which, when finalized, will offer improved pathways for feedback on medical device submissions.
Mitigating risks early on is vital. By addressing potential challenges at the outset, manufacturers can avert costly delays. The experience of healthcare providers, who spend a substantial portion of their time on administrative tasks, underscores the importance of efficiency. By reducing administrative burdens, professionals can focus more on patient care, thus enhancing overall healthcare delivery.
Efficiency and cost savings are intertwined. Gaining FDA feedback early allows manufacturers to refine development strategies, which may reduce unnecessary expenses and expedite market entry. Companies like Teal, dedicated to improving women's health through convenient cervical cancer screening, demonstrate the impact of aligning medical services with patient needs and preferences.
Understanding the product deeply is paramount. A thorough comprehension of the device, its users, and the competitive landscape, as well as the regulatory requirements, contributes to a more efficient development and approval process. This understanding also ensures that the product adheres to safety and efficacy standards, thus safeguarding public health.
Ultimately, these interactions with the FDA are geared towards improving patient safety and product efficacy. As the Agency evaluates the safety and effectiveness of medical devices, manufacturers must recognize that the data submitted to the FDA may differ from that required by payors for coverage decisions. This could lead to potential delays or denials in coverage, affecting patient access to new medical devices. Therefore, early interaction with the FDA is not just about regulatory compliance, but also about enhancing patient outcomes and ensuring timely access to medical innovations.
Conclusion
In conclusion, the Q-Submission program serves as a valuable pathway for dialogue between the FDA and medical device developers. It enables pre-regulatory discussions and provides a roadmap for obtaining FDA input on device development and clinical trial design. The FDA's draft guidance on the Q-Submission Program demonstrates their commitment to refining this process and offers detailed advice on securing feedback efficiently.
Engaging in the Q-Submission process allows manufacturers to effectively address FDA requirements and improve patient outcomes. It is crucial to understand the different types of Q-Submissions and the mechanisms for obtaining feedback as outlined in the FDA's draft guidance. By following a meticulous pre-submission strategy and assembling a comprehensive package, manufacturers can facilitate a clear and productive dialogue with the FDA.
Documenting interactions with the FDA requires a comprehensive summary of past regulatory history and an understanding of the FDA's classification system for medical devices. Requesting feedback and meetings should be done respectfully and in accordance with FDA guidance.
Upon submitting a Q-Submission, it is important to navigate the ensuing communication process attentively. This includes interpreting the FDA's feedback, fulfilling requests for additional information, and adhering to protocols. Safeguarding confidential information is crucial throughout the process.
To enhance the effectiveness of the Q-Submission process, manufacturers should incorporate best practices such as initiating early engagement with the FDA, adhering to guidance, ensuring clarity and specificity in submissions, providing comprehensive information, conducting internal reviews, and maintaining professionalism. Avoiding common mistakes, such as inaccuracies and omissions, will contribute to a successful review process.
Proactive engagement with the FDA through Q-Submissions streamlines the development process, mitigates risks, and enhances efficiency. Ultimately, these interactions aim to improve patient safety, product efficacy, and timely access to medical innovations. By understanding and following the FDA's guidance, manufacturers can navigate the regulatory landscape effectively and contribute to the advancement of public health initiatives.




