Introduction
Securing 510(k) clearance from the U.S. Food and Drug Administration (FDA) is a pivotal step in bringing a medical device to the U.S. market. It ensures that the device is "substantially equivalent" to an existing legally marketed device in terms of safety and effectiveness. The FDA's thorough review process not only ensures patient safety but also fosters innovation by allowing similar devices to enter the market more swiftly.
In this article, we will explore the importance of 510(k) clearance, the eligibility criteria, the concept of predicate devices, the steps in the submission process, key components of a submission, tips for success, common challenges, and how to overcome them. Understanding the intricacies of the 510(k) clearance process is crucial for manufacturers looking to navigate this regulatory pathway successfully. So let's dive in and explore the world of 510(k) clearance.
What is 510(k) Clearance and Its Importance
Obtaining 510(k) clearance from the U.S. Food and Drug Administration (FDA) is a crucial stage in introducing a medical product to the U.S. market. The procedure requires that manufacturers demonstrate that their product is 'substantially equivalent' to a currently legally marketed item, referred to as a predicate item, in regards to safety and effectiveness. Obtaining this clearance is not only a regulatory formality; it's a thorough examination that guarantees new medical equipment is secure for patient use while promoting innovation by permitting equipment similar to those already accessible to enter the market more expeditiously.
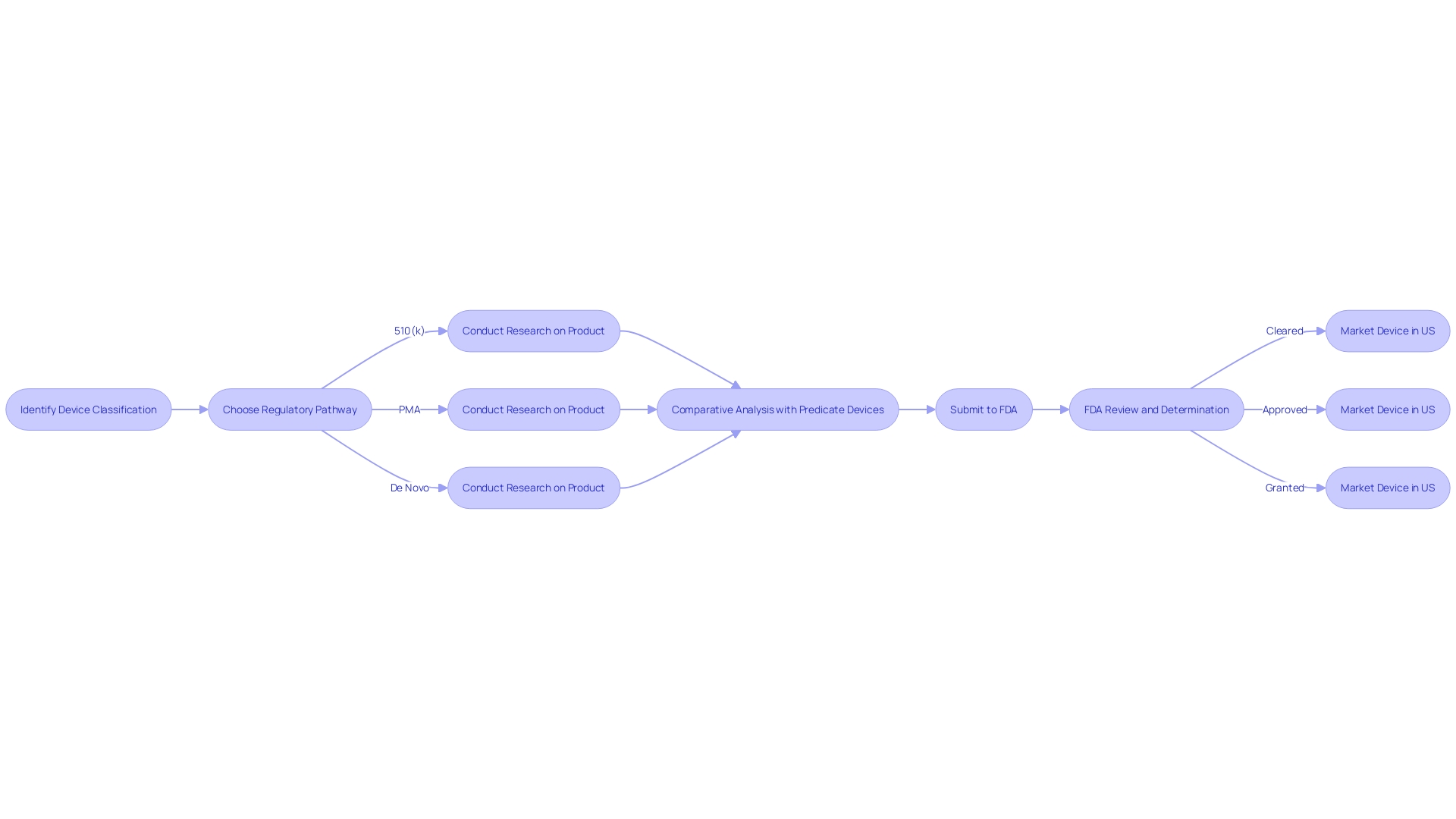
Starting the 510(k) clearance process necessitates a comprehensive comprehension of the classification of the apparatus. The FDA classifies medical equipment into three categories based on the level of control necessary to ensure the safety and effectiveness of the product. Once the classification is established, manufacturers must navigate the appropriate regulatory pathway – be it 510(k) for Premarket Notification, Pre-Market Approval (PMA), or the De Novo approach for innovative products without a comparable precedent.
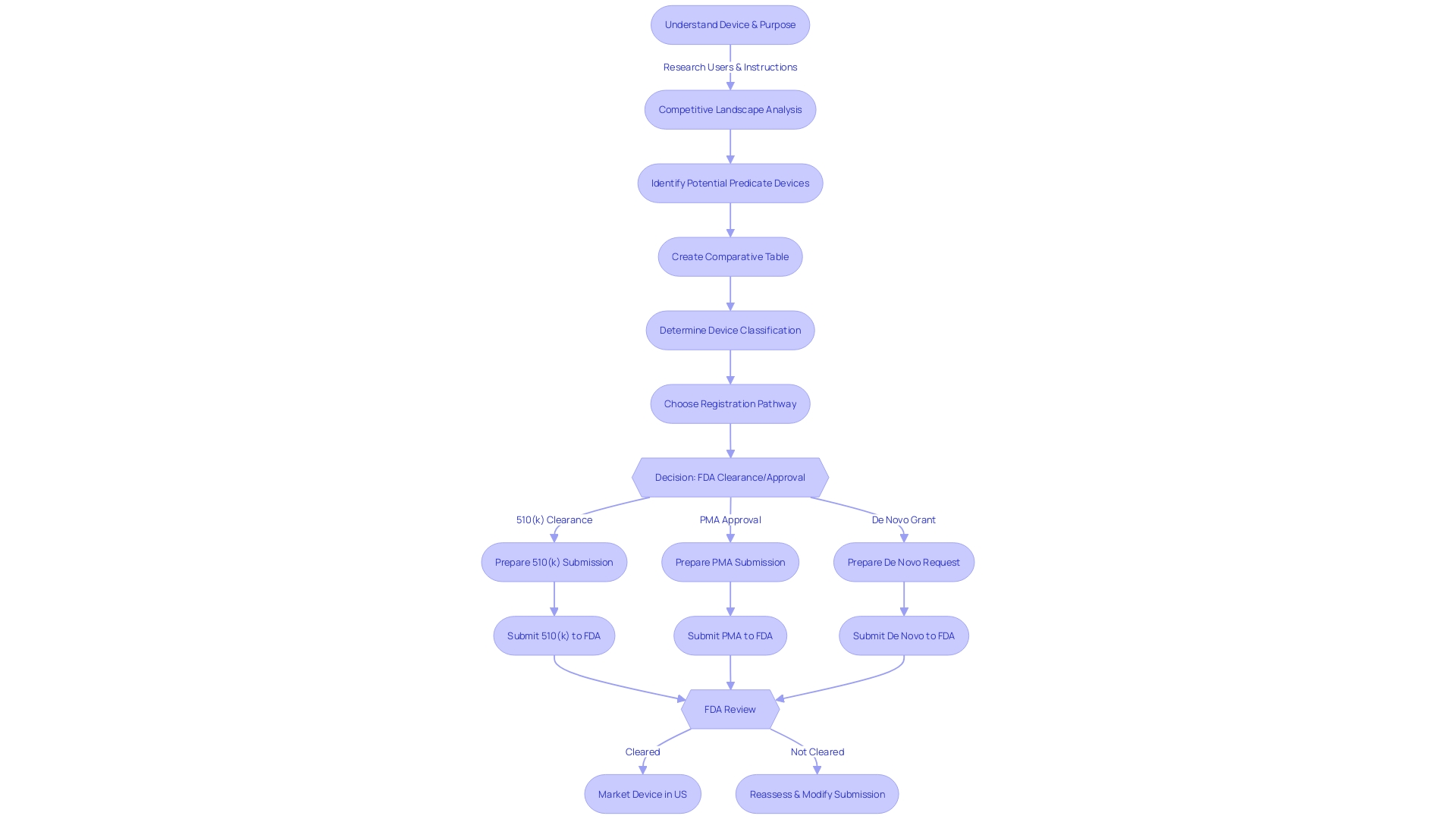
The distinction between being FDA 'Registered,' 'Cleared,' 'Approved,' or 'Granted' is crucial, each representing a different level of FDA evaluation and oversight. While 'Registered' implies a manufacturer has made the FDA aware of their product, 'Cleared' indicates that the FDA has reviewed a 510(k) submission and determined the product is safe and effective for use. 'Approved' is a designation typically reserved for high-risk objects that have undergone a more rigorous PMA process, and 'Granted' is used for items that successfully go through the De Novo pathway.
To guarantee a successful 510(k) submission, it's crucial to conduct thorough research on the product, including its intended use, user needs, and the market landscape. A comparative analysis with potential predicate mechanisms is essential, examining technological characteristics, clinical studies, and labeling, to solidify the claim of substantial equivalence.
The FDA's function goes beyond medical devices, covering a broad spectrum of public health responsibilities. For instance, the agency has recently taken steps to enhance the clarity and neutrality of direct-to-consumer prescription drug advertisements, ensuring that the major side effects and contraindications are communicated effectively to the public.
Recently, there has been increased focus on the scrutiny surrounding the 510(k) procedure, with discussions revolving around the sufficiency of the clearance method and the well-being of patients. This discussion has resulted in demands for change and a more thorough investigation of the clearance pathway to guarantee that equipment does not jeopardize patient well-being.
For those navigating the 510(k) clearance process, it's more than just a regulatory hurdle; it's about comprehensively understanding the equipment's purpose, its market context, and the commitment to patient safety that the FDA upholds.
Eligibility Criteria for 510(k) Clearance
For a medical instrument to obtain 510(k) clearance, it is necessary to meet specified criteria, ensuring it's safe and effective for human use. First and foremost, the apparatus must not be previously mandated to undergo a premarket approval (PMA) application, which is generally reserved for high-risk devices. Additionally, it must not be listed under any exclusions or exemptions in the regulatory framework.
A crucial stage in the 510(k) procedure is establishing 'substantial equivalence' to a legally marketed predicate product. This entails a thorough comparison that shows that the new apparatus is as secure and efficient as an already available one on the market with the same intended application and comparable technological features.
The categorization of the equipment plays a crucial part in determining the suitable regulatory route. There are three FDA classifications, each reflecting the level of risk they pose to the patient, with Class I posing the least risk and Class III the most. Understanding these classifications is crucial for determining whether to pursue 510(k) clearance, PMA, or the De Novo pathway for items without a clear precedent.
The FDA aims to safeguard public health by guaranteeing the safety and efficacy of medical instruments. The agency's comprehensive approach to regulation includes not only premarket evaluation but also active postmarket surveillance to monitor ongoing safety and effectiveness.
Despite the thorough evaluation conducted by the FDA for medical device approval, it is important to note that not all products require clinical trials before entering the market, as highlighted in the 2018 documentary 'The Bleeding Edge.' This revelation highlights the intricacy and subtleties of the clearance procedure, which are essential for industry professionals to fully comprehend. Furthermore, the significant count of injuries and deaths potentially associated with medical instruments reported in a 2018 research highlights the significance of upholding rigorous regulatory supervision throughout a device's lifespan.
Understanding the Predicate Device Concept
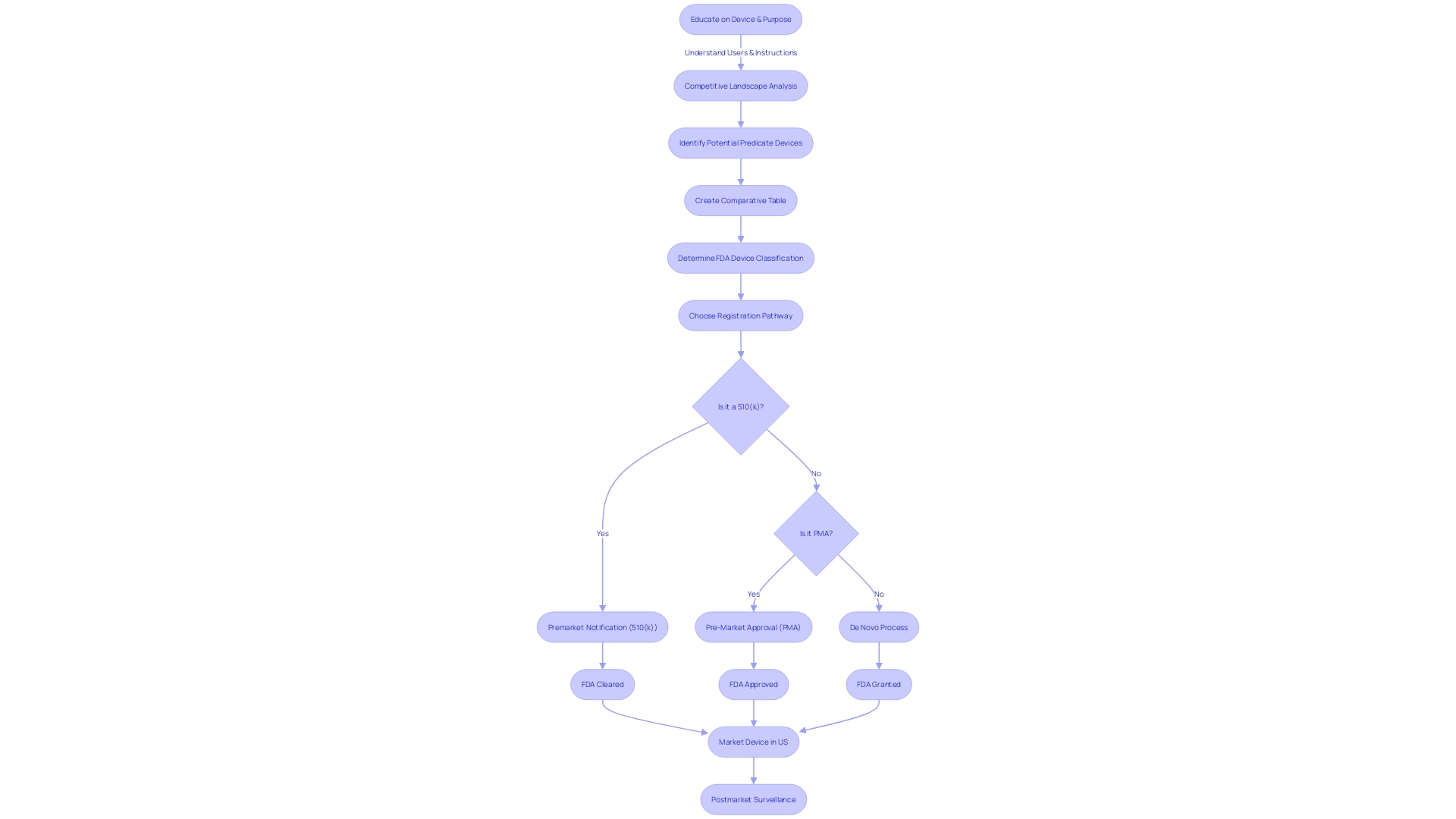
Essential to the 510(k) clearance process is the idea of a precedent instrument, which acts as a benchmark for new medical apparatus seeking FDA approval. To obtain approval, a new apparatus must show comparable safety and efficacy to a current, lawfully sold prototype. This comparison is critical, as any differences between the new instrument and its predicate must not compromise safety or effectiveness. A thorough comprehension of the subject matter, encompassing its target audience, usage guidelines, alerts, and precautions, is crucial. 'Research into the competitive landscape aids in identifying potential predicate items with similar intended uses and technological characteristics.'. Building a comparative table using this research, incorporating information from the FDA's 510(k) database, is an essential step in validating the safety and effectiveness of the new apparatus. The FDA's modernization efforts include developing best practices for selecting predicates, as outlined in a draft guidance document released in September 2023. This initiative highlights the significance of selecting lawfully sold predecessors and assessing their technological attributes to guarantee they match with the new equipment without raising safety concerns. The utilization of older predicates can offer advantages, such as the availability of long-term safety data. The FDA's dedication to public health is emphasized by its thorough clearance procedure, which guarantees the safety and efficacy of medical equipment available for public use.

Steps in the 510(k) Submission Process
In order to successfully navigate the process of submitting 510(k), manufacturers must meticulously adhere to a series of steps to obtain FDA clearance for their medical equipment. The journey begins with the identification of a suitable benchmark predicate, which serves for comparison. This involves a detailed examination of the users, such as clinicians and patients, as well as a comprehensive review of the usage instructions, including any warnings. Additionally, manufacturers are encouraged to collaborate with their marketing teams to analyze the competitive landscape, drawing insights from a variety of sources like research literature, clinical studies, and competitor marketing materials. This research helps in constructing a comparative table to demonstrate the new apparatus's congruence with existing ones in terms of intended use and technological features.
Subsequently, establishing a robust Quality Management System is crucial to align with FDA's stringent regulatory standards. An in-depth understanding of the FDA's guidance documents is essential, as they provide a clear outline of what is expected in a submission. Manufacturers must ensure all required data is meticulously compiled, reflecting a commitment to safety and efficacy. This includes conducting rigorous testing to assess its performance and to substantiate claims of substantial equivalence.
The final stage of this procedure is the completion and submission of the 510(k) application, which must be accompanied by comprehensive documentation, including a representative sampling of advertisements and any other labeling that makes promotional claims about the product. It's imperative to follow the FDA's instructions for submitting comments and to safeguard any confidential information.
Given the initiative of Everly Health Solutions to improve early detection and prevention of chronic kidney disease through advanced diagnostics, it is crucial to adhere to the 510(k) submission protocol. It is a critical step to bring innovative medical equipment to market, facilitating advancements in healthcare and providing new treatment options to patients.
Key Components of a 510(k) Submission
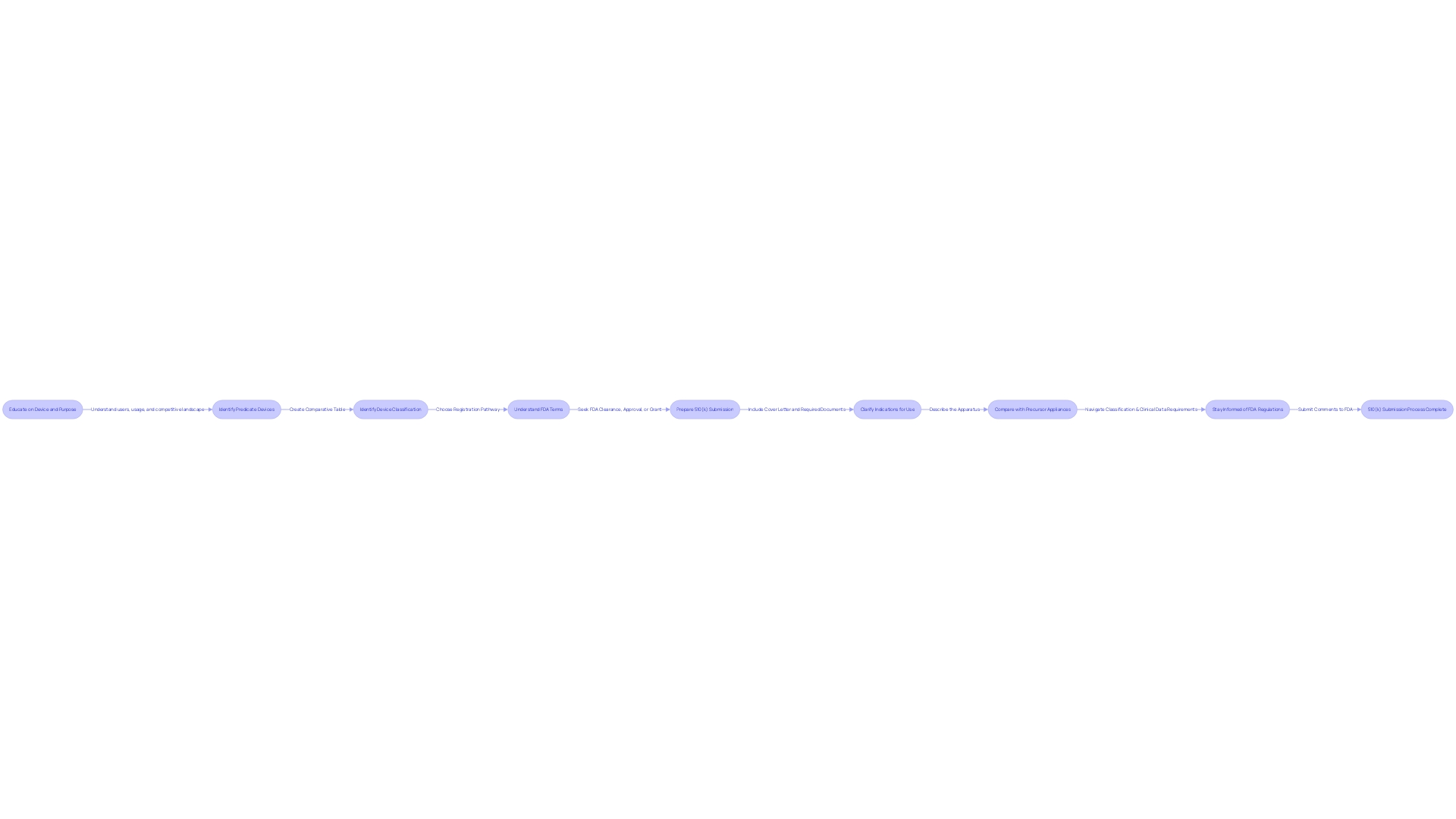
The 510(k) submission process is a comprehensive and meticulous journey, beginning with a cover letter that sets the stage for the FDA's evaluation. This introduction summarizes the entire submission, highlighting the importance of the medical equipment. Next, the submission must clarify the indications for use and provide a comprehensive description, outlining the intended application, design, and specifications of the apparatus. It's crucial to have a profound understanding of the instrument, involving with its users, and grasping the subtleties of its operation, including any warnings and precautions.
To guarantee the apparatus aligns with FDA expectations, manufacturers must scrutinize research literature, clinical studies, and competitor devices. This entails constructing a comparative table to establish connections between the new apparatus and current, comparable appliances with FDA approval, referred to as precursor appliances. Such comparisons can be facilitated by examining Summaries of Safety and Effectiveness Data (SSEs) from the FDA's 510(k) database, which provides insights into the similarities and differences between products.
The submission's complexity is determined by the classification of the equipment, which determines the clinical data required. Manufacturers must navigate this landscape carefully, choosing the right 510(k) pathway based on the innovation level and risk profile of the product. To this end, they must stay informed of the latest FDA regulations and guidance, such as the recent final rule on direct-to-consumer prescription drug advertisements, which stipulates the clear, conspicuous, and neutral presentation of a drug's major side effects and contraindications in advertisements.
As regulatory landscapes evolve, professionals submitting comments to the FDA must do so with caution, ensuring that no confidential information is made public inadvertently. This thorough procedure emphasizes the FDA's dedication to protecting public health by guaranteeing that medical equipment is both secure and efficient before it can be sold.
Tips for a Successful 510(k) Submission
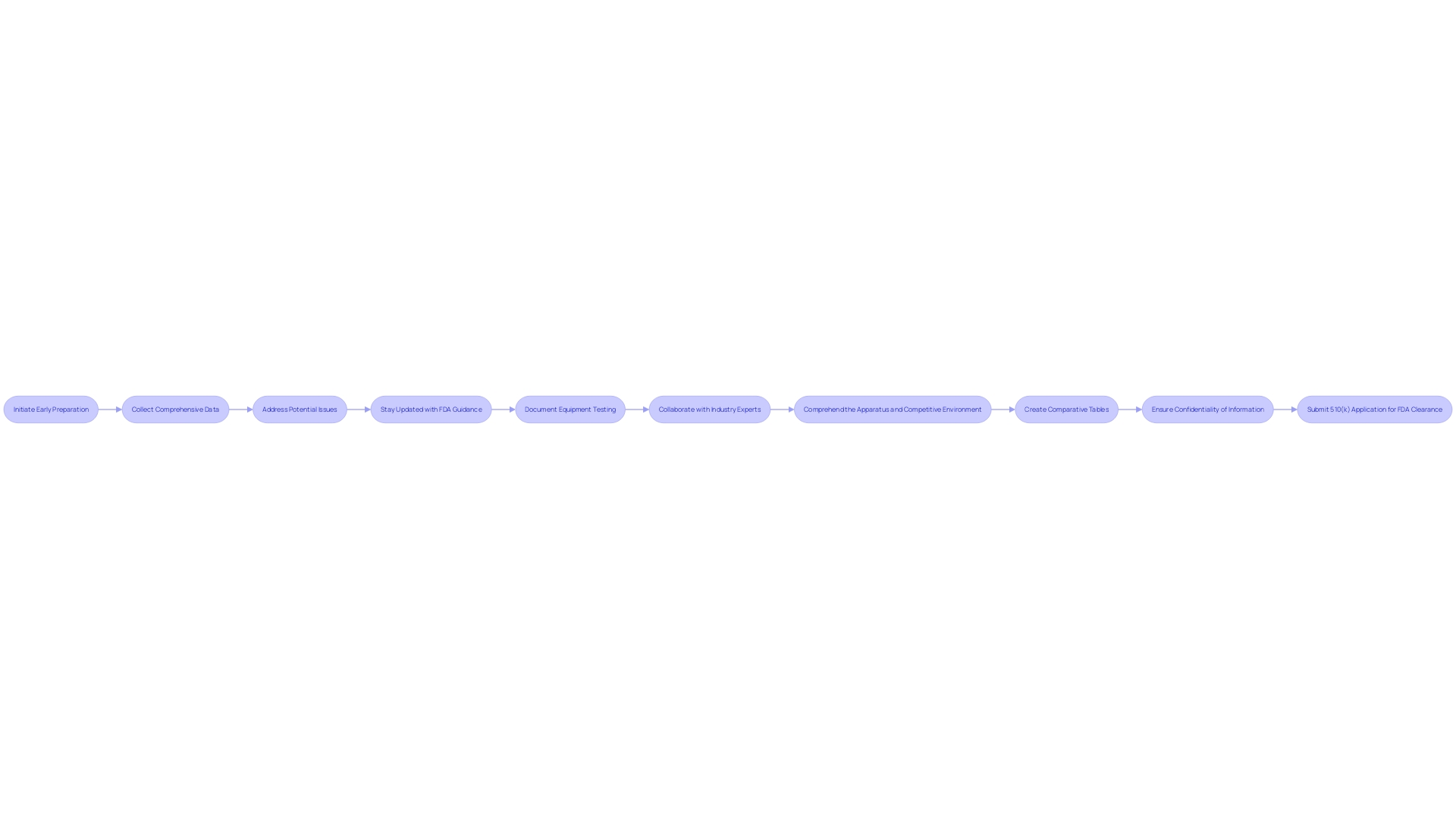
A robust and well-constructed 510(k) application is vital for obtaining FDA clearance, and there are strategic measures that can be taken to improve the likelihood of success. Initiating the preparation early allows ample time for comprehensive data collection and addressing potential issues. It's crucial to stay abreast of the FDA's current guidance documents, ensuring their recommendations are integrated into your application.
A crucial aspect of the application is the careful documentation of equipment testing. Every protocol, result, and validation must be recorded in detail. Collaborating with industry experts can provide valuable insights into the process, potentially improving the quality and compliance of your submission.
Comprehending the apparatus, its users, and its competitive environment is fundamental. Marketing teams can help in identifying predicate products with similar intended purposes and technological features. Creating comparative tables based on research literature, clinical studies, and existing product information can significantly support your application.
An example of the importance of thorough preparation can be seen in the story of Artos, a company developed from the firsthand experiences of its founders with regulatory submissions. Their journey underscores the complexity and effort required to compile and format information for regulatory review.
As highlighted in recent discussions, it is paramount to ensure that any public comments or submissions to the FDA do not contain confidential information. This vigilance protects both personal and business-sensitive data. The procedure of regulatory submission is not only about meeting technical requirements but also involves strategic planning and an acute awareness of the information being made publicly available.
Common Challenges and How to Overcome Them
Navigating the intricacies of the 510(k) clearance process can be formidable for medical instrument manufacturers. To address common obstacles, a comprehensive approach is required. When a suitable predicate instrument is unavailable, it's critical to delve deep into research or evaluate alternative regulatory pathways. This situation emphasizes the significance of fully comprehending the subject equipment, including its intended users and usage instructions, as well as conducting a thorough examination of the competitive landscape to identify potential precedent objects with comparable intended uses and technological characteristics.
In the realm of testing, inadequate or insufficient testing methodologies can trigger delays or outright rejection of the 510(k) submission. A proactive strategy involves ensuring adherence to the pertinent standards and guidelines for all necessary testing, thereby mitigating the risk of submission setbacks. Additionally, the arrangement of documentation is crucial; a systematic compilation of all necessary documentation can greatly simplify the FDA's evaluation.
Keeping abreast of regulatory changes is also vital. The approval of medical equipment that fulfill unmet medical needs has been noticeably expedited, as evidenced by the collaborative efforts between regulatory agencies and industry stakeholders, particularly during the COVID-19 pandemic. This trend towards streamlined regulatory pathways emphasizes the need for manufacturers to stay knowledgeable and flexible to changes that could impact the 510(k) clearance procedure.
Lastly, it is crucial to acknowledge the diverse intricacy and linked endorsement durations of medical equipment throughout distinct regulatory entities. For example, the FDA classifies medical equipment into three categories, with class three tools, such as life-sustaining implantables, undergoing more rigorous regulatory processes. Understanding the categorization and regulatory environment not only in the US but also in Europe, where the EMA plays a role, can inform manufacturers' strategic planning and timeline estimations for device clearance.
Conclusion
In conclusion, securing 510(k) clearance is crucial for medical device manufacturers to bring their products to the U.S. market. It ensures safety, fosters innovation, and allows similar devices to enter the market swiftly. The process involves demonstrating substantial equivalence to a predicate device and navigating the submission process.
Thorough research and comparative analysis are essential for selecting the appropriate predicate device and ensuring the new device's safety and effectiveness. The FDA's commitment to public health is evident in its rigorous clearance process.
The submission process requires thorough investigation, collaboration, and the establishment of a robust Quality Management System. Meticulous data compilation, device testing, and detailed documentation are crucial. Compliance with FDA instructions and safeguarding confidential information are imperative.
Key components of a 510(k) submission include a cover letter, indications for use, device description, and comparative analysis. Manufacturers must stay informed of FDA regulations and guidance and ensure compliance with device classification.
To increase the likelihood of success, early preparation, integration of FDA guidance, and collaboration with industry experts are recommended. Thorough understanding of the device, its users, and the competitive landscape is crucial. Meticulous documentation of device testing and careful consideration of public comments are important.
Navigating the challenges of the 510(k) clearance process requires a comprehensive approach. Thorough research, adherence to testing standards, meticulous documentation organization, and staying informed about regulatory changes are key strategies.
In summary, the 510(k) clearance process is a comprehensive journey that ensures the safety and effectiveness of medical devices. By understanding the eligibility criteria, predicate device concept, submission process, and key components, manufacturers can navigate this process successfully and bring their innovative devices to market, advancing healthcare and patient treatment options.




