Introduction
The Code of Federal Regulations (CFR) is a comprehensive collection of rules that govern various aspects of federal governance and public administration. It serves as a vital tool for the government to manage complex societal needs and has a significant impact on everyone, from working families to small businesses. Understanding the CFR is crucial not only for compliance but also for comprehending its broader implications on efficiency and effectiveness.
This article explores the organization, structure, and numbering system of the CFR, as well as the process of citing CFR sections accurately. It also highlights the importance of regular updates to ensure the CFR remains current and relevant. Accessing the CFR is made easy through both print and digital formats, with online resources offering advanced search functionalities and navigation features.
Supplementary materials such as indexes, finding aids, and supplements provide additional support for understanding and applying the regulations. Historical and bulk data access further enhance research capabilities, enabling scholars to track regulatory changes over time and conduct comprehensive data analysis. Finally, the article emphasizes the importance of understanding common terms and their unique definitions within the CFR to ensure accurate interpretation and compliance.
Overall, the CFR plays a crucial role in shaping a government that is efficient, effective, and trusted by the public it serves.
What is the Code of Federal Regulations?
The Code of Federal Regulations (CFR) is more than just a collection of rules; it represents the codification of the general and permanent rules published in the Federal Register by the departments and agencies of the Federal Government. It's an essential tool for the government to manage complex societal needs, touching the lives of everyone from working families to small businesses. For example, the CFR encompasses policies that can act as catalysts or barriers to technological advancement within government services, as highlighted by an adaptation of Kurt Lewin's force field analysis, which helps agencies navigate the potential impact of policy changes.
According to a senior research fellow at the Mercatus Center, the overwhelming volume of federal regulations—which would take an average adult reading full-time approximately three years to read—slows economic growth. This underscores the importance of understanding the CFR not only for compliance but also for its broader implications on efficiency and effectiveness.
Furthermore, the Paperwork Reduction Act of 1995, mentioned within the CFR, mandates OMB approval for information collection from the public, emphasizing the need for accuracy and utility in government data collection. This act is a testament to the CFR's role in ensuring that federal agencies serve the public responsibly.
Definitions within the CFR are vital for clarity and compliance. For instance, 'Customer' refers to a consumer engaged in a continuing relationship with a financial institution, and 'Customer information' means records containing personal details about the customer, which must be handled with utmost care.
As the digital landscape evolves, the CFR continues to be relevant. Recently, the Democratic Party of Korea mandated crypto asset disclosure for its candidates, reflecting the need for transparency and high moral standards—principles that are also central to the CFR. Additionally, with recent FASB accounting rule changes affecting crypto holdings, the CFR's guidance on financial regulations becomes even more pertinent.
In the words of the Chair of the Securities and Exchange Commission, the federal securities laws and the Sec's oversight have been instrumental in the United States' economic success. The CFR is a cornerstone in this regulatory framework, ensuring investor protection and fair markets. As Federal agencies increasingly turn to digital solutions to interact with the public, the CFR is instrumental in shaping a government that is efficient, effective, and trusted by those it serves.
Organization of the CFR
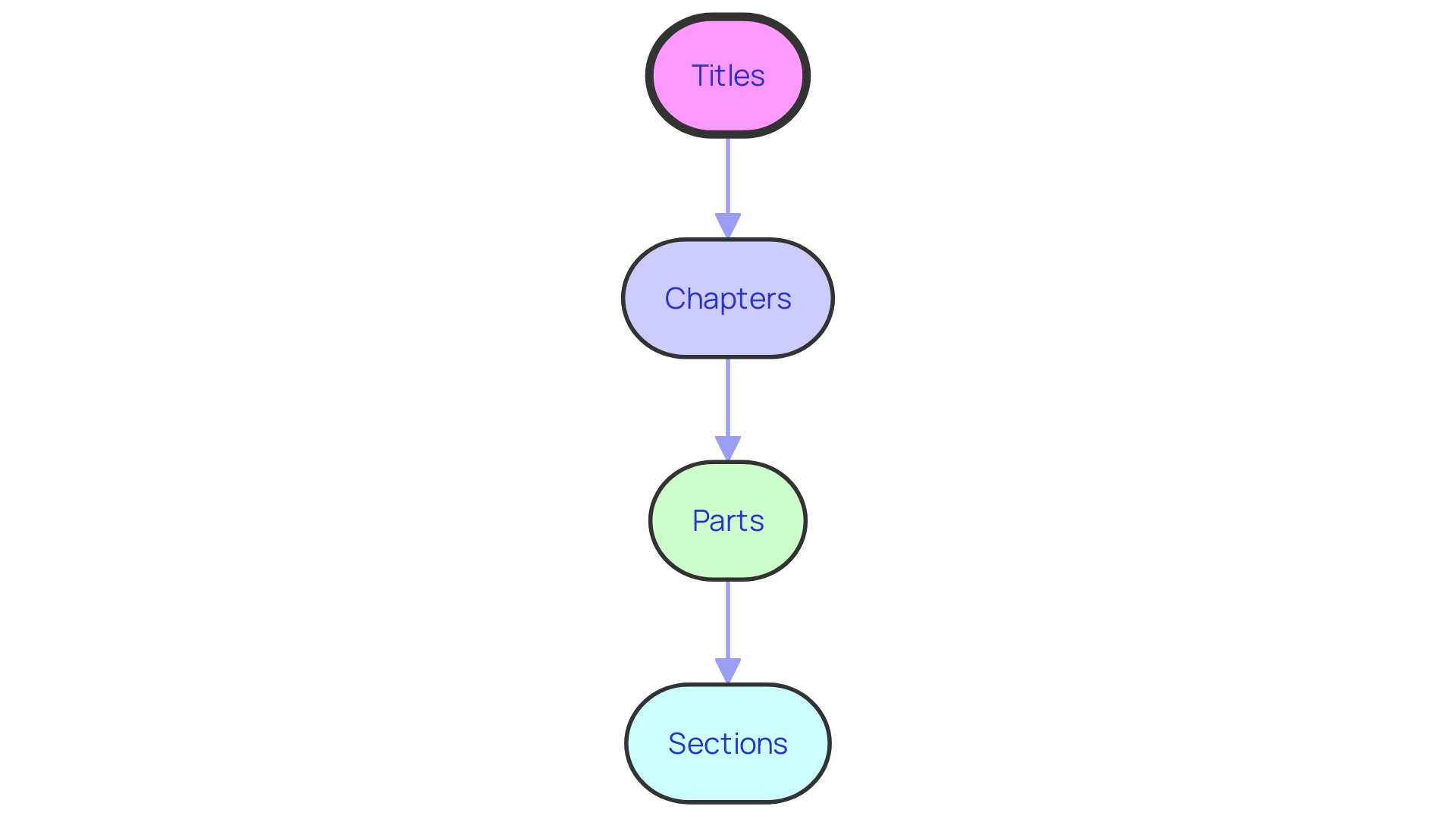
The Code of Federal Regulations (CFR) serves as the backbone of federal administrative law, segmented into 50 distinct titles that represent a broad range of subject matter. This extensive compilation is systematically structured for ease of access: titles branch into chapters, with further divisions into subchapters, parts, and nuanced sections. This hierarchical architecture is not just an organizational tool; it mirrors the complex interconnectedness observed in various systems, from technology to ecology.
Each component within the CFR, akin to a sub-assembly in a technology system, supports and refines the primary function of regulatory compliance. Just as the Nature of Technology suggests, the CFR's layered format ensures that the main assembly of legal stipulations is buttressed by detailed provisions that regulate function, clarify requirements, and delineate procedures. In essence, the CFR is a vertebrate of legal frameworks, its structured segments working in concert to guide compliance and governance across diverse federal agencies and industries.
Structure of the CFR
The Code of Federal Regulations (CFR) is meticulously organized to ensure that specific regulations are easily navigable and understandable. Starting at the top, the CFR is divided into titles that represent broad areas subject to Federal regulation. Each title is further broken down into chapters, which are typically aligned with the federal agencies responsible for the regulatory material found within.
These chapters are then segmented into subchapters that group related regulations together for clarity and ease of access.
Within these subchapters, we find individual parts that contain the granular details of the regulations. These parts are composed of sections - the most specific level of the CFR - where one will find the precise rules and guidelines that must be followed. For instance, in the healthcare and biosafety context, the Human Pathogens and Toxins Act (HPTA) and its accompanying Human Pathogens and Toxins Regulations (HPTR) in Canada illustrate a similar hierarchical structure, where stringent compliance and reporting protocols are legally mandated for work with pathogens.
Regulations such as these are not static; they evolve with industry practices and societal needs. For example, recent proposals seek to update Community Reinvestment Act (CRA) regulations to strengthen the core purposes of the statute while adapting to modern banking transformations like the rise of mobile and online banking. This indicates a dynamic regulatory environment where updates aim to provide greater clarity and consistency, catering to different bank sizes and business models, and considering local conditions.
The CFR also defines specific terms, such as 'restricted device' and 'initial importer,' to ensure unambiguous communication across all stakeholders. These clearly outlined definitions within the regulatory framework facilitate compliance and ensure that any changes, such as material modifications in labeling or advertisements, do not compromise the device's identity or safety and effectiveness. The process of updating or modifying such regulations is meticulous, involving multiple levels of review and approval, as seen in the process for Executive orders or proclamations, which must receive the assent of the Office of Management and Budget and the Attorney General before being presented to the President.
CFR Numbering System
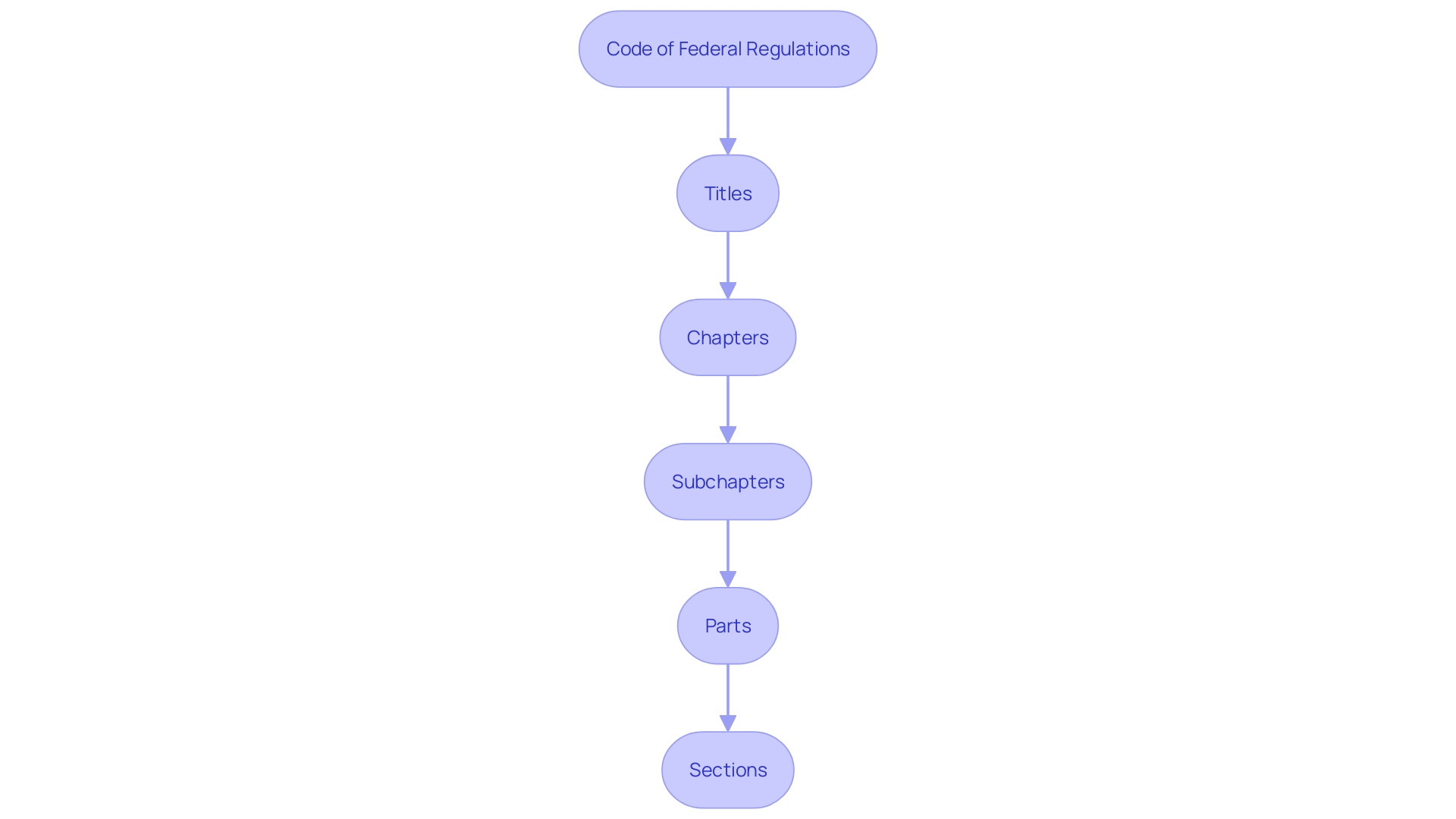
Understanding the intricacies of the Code of Federal Regulations (CFR) is essential for compliance in clinical research. The CFR's organization is based on titles that are subdivided into chapters, subchapters, parts, and sections. For instance, 21 CFR 312.23 denotes Title 21, Part 312, Section 23, which pertains to investigational new drug applications.
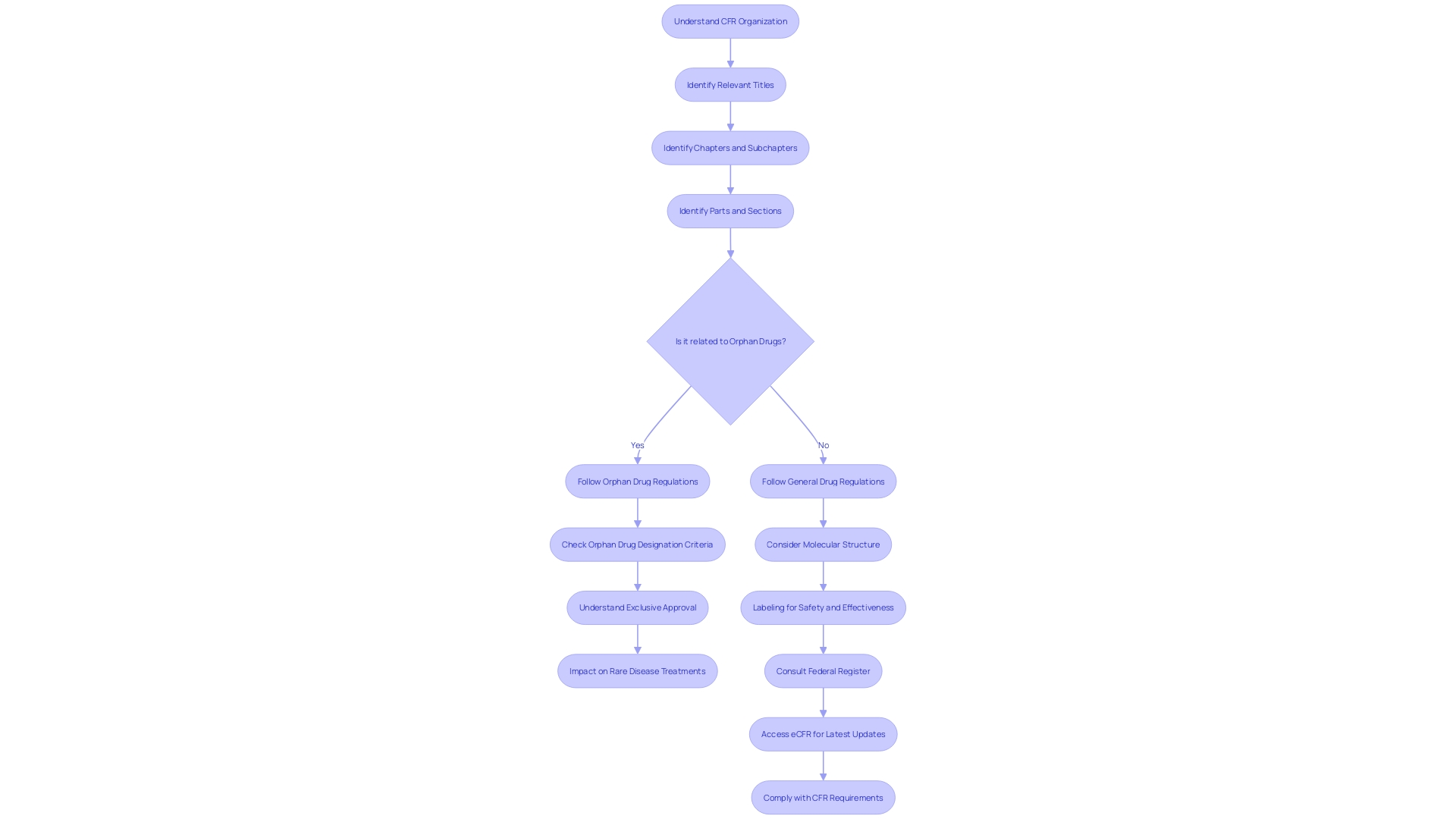
This systematic approach assists in pinpointing specific regulations, like those governing orphan drugs—medications developed for rare conditions. The CFR details the criteria for orphan drug designation, which includes exclusive approval for seven years post-FDA approval, provided no prior approval for the same use exists. This designation is critical for incentivizing the development of treatments for rare diseases, impacting less than 200,000 individuals in the U.S. or meeting specific medical needs.
For example, the CFR explains that an orphan subset can be designated for a drug used for a subset of patients with a non-rare disease, where the drug would not be suitable for the broader patient population due to factors like toxicity or unique mechanism of action. This designation reflects the nuanced application of the law, where even the molecular structure of a drug—be it a small molecule or a macromolecule—can influence the regulatory outcomes. Such attention to detail underscores the importance of labeling, which is as critical as the product itself, according to industry experts.
Labeling ensures that products are used safely and effectively, serving a range of users for different purposes.
Recent updates to the Federal Register, with XML renditions of documents, facilitate access to the latest regulatory information, fostering a shared understanding among stakeholders. This shared foundation is vital for financial institutions and market participants, who can refer to a common set of definitions. As the CFR evolves, it remains an indispensable resource for the clinical research community, providing clarity on the regulatory requirements that support the efficacy and safety of healthcare products.
How to Cite CFR Sections
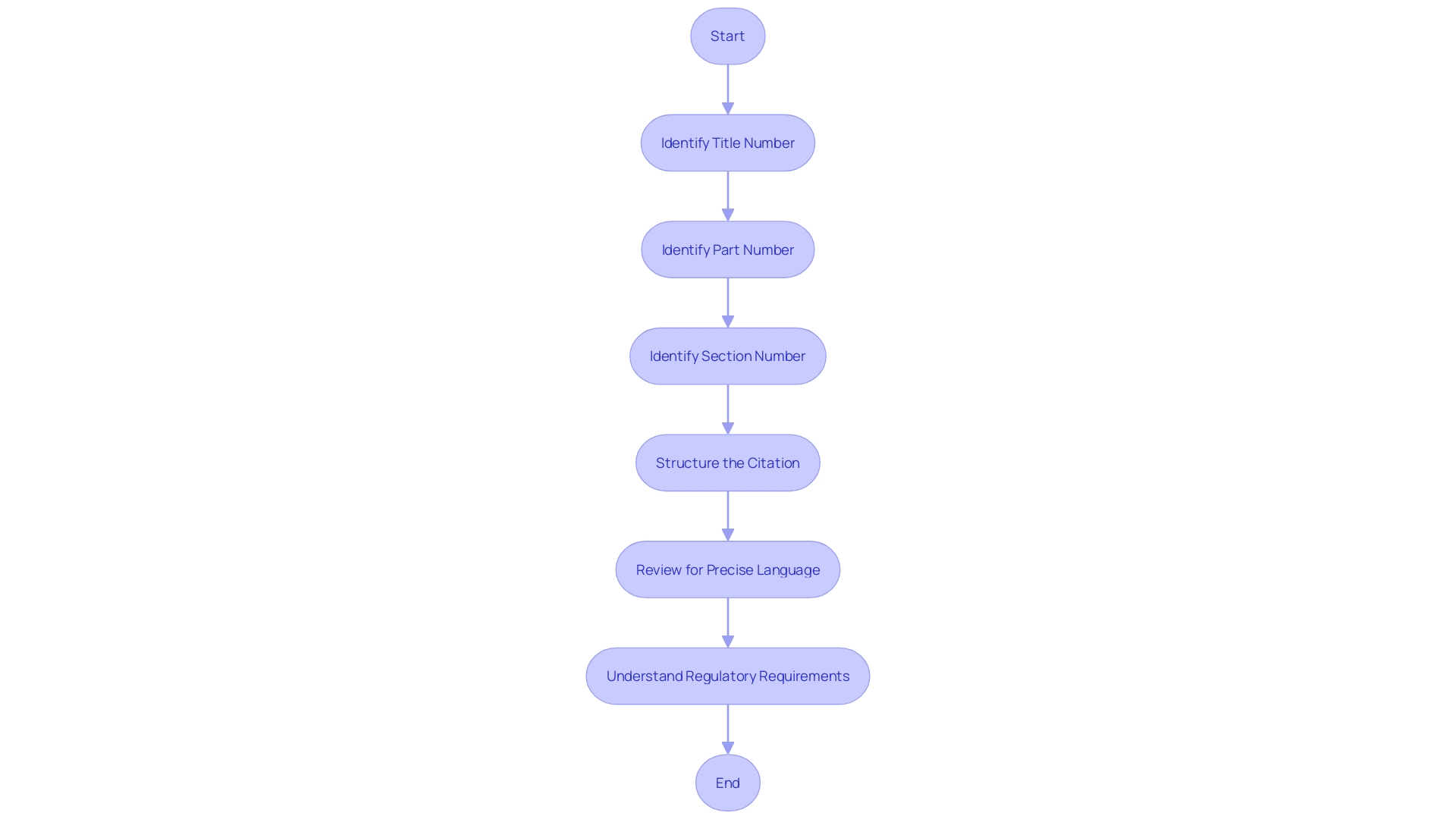
To ensure precision and clarity when referencing regulations, it's essential to correctly cite the Code of Federal Regulations (CFR). The citation must include the title number, part number, and section number in a structured format: 'Title CFR Part.Section.' For example, '21 CFR 312.41' points to Title 21, Part 312, Section 41 of the CFR, which pertains to drug applications and related regulations.
The Electronic Code of Federal Regulations (eCFR) optimizes readability by structuring paragraphs to reflect the document's hierarchy, an automated process to aid users without modifying the original agency intent. Such meticulous citation is crucial, for instance, in understanding the nuances of orphan-drug designation, where precise language outlines the criteria for exclusive approval and clarifies the definitions of an orphan subset of a non-rare disease. This approach ensures that professionals in the field can navigate and comply with the intricate details of regulatory requirements.
Update Cycle of the CFR
The Code of Federal Regulations (CFR) undergoes a meticulous update process each year to ensure that it contains the most up-to-date information and regulatory guidance. The CFR's structure is such that it is revised on a staggered basis, with a quarter of the entire document reviewed and updated over the course of each year. This systematic approach guarantees that the CFR remains an authoritative source for federal regulations, reflecting the latest changes and corrections made by various federal agencies.
For instance, a recent correction in the Federal Register exemplifies the attention to detail in maintaining the CFR. An earlier response concerning the pluralization of terms, which mentioned the unnecessary use of '(s)' in specified provisions, was corrected for consistency and clarity. Furthermore, clarifications were made regarding how Key Data Elements (KDEs) for Critical Tracking Events (CTEs) could be 'linked' within records, emphasizing that such elements can be grouped in various formats like electronic spreadsheets, databases, or printed documents.
These updates are not only technical corrections but also involve substantial regulatory changes that affect numerous industries and stakeholders. As an example, the CFR amendments included revisions on exemptions related to the Food Traceability List, ensuring that changes in food products are adequately recorded or subject to written agreements.
The process of updating the CFR is collaborative and involves input from committees and working groups made up of industry representatives and experts. They play a crucial role in reviewing existing regulations and suggesting improvements, ensuring that the CFR remains relevant and effective.
The significance of these updates is evident in the context of public health and safety, where accurate regulatory text is paramount. The Centers for Disease Control and Prevention (CDC), for example, relies on precise regulations to guide its policies, as seen during the 2022-2023 flu season when influenza activity reached levels reminiscent of the pre-COVID-19 era.
In summary, the annual revision of the CFR is a critical process that upholds the integrity of federal regulations, ensuring they are current, clear, and correctly aligned with ongoing changes in legislation, industry practices, and public health requirements. Stakeholders are encouraged to engage with the process, recognizing that the CFR's content directly impacts compliance and operational activities across a multitude of sectors.
Accessing the CFR
The Code of Federal Regulations (CFR) is not only foundational for legal and regulatory processes but is also readily available to the public in various formats for convenience and transparency. The Government Publishing Office (GPO) ensures that the official print version is accessible, which individuals can purchase or find at select libraries. For those who prefer digital access, the GPO's website hosts an electronic version, known as the eCFR.
This version features an automated layout with paragraphs neatly split and indented to maintain the document's hierarchical structure, enhancing readability and user experience. The digital format is not considered an official legal edition of the CFR; however, it mirrors the official formatting and is regularly updated to reflect the most current information, serving as a practical reference for those engaged in federal agency work or requiring up-to-date regulatory information. It's a testament to the commitment of providing resources that are not only legally compliant but also accessible and user-friendly.
Types of CFR Publications
While the CFR provides the official regulations, there are also supplementary materials that can significantly aid in understanding and applying these regulations. Among these are indexes and finding aids, which serve as navigational tools, helping users to identify and locate relevant regulations quickly. Additionally, supplements offer further insight, often elaborating on complex areas and providing examples or interpretations that facilitate compliance.
These additional resources play a critical role in the research and application of federal regulations. For example, detailed indexes can simplify the search for specific topics, mirroring the way an academic researcher might use references and citations to gauge the impact of scientific work. Similarly, finding aids can help to distill the essence of lengthy regulatory texts, akin to how abstracts summarize research papers, making the information more digestible and actionable.
Moreover, supplements to the CFR can be compared to secondary outcomes in research projects—they may not be the primary source of regulatory information but can provide valuable context and clarification, thus enhancing understanding. This is particularly useful in complex fields where regulations may intersect with rapidly evolving technologies, such as the integration of AI in public transport systems or the ethical considerations of autonomous weapon systems.
These supplementary materials, while not legally binding, are instrumental in ensuring that professionals, including those in clinical research, can interpret and adhere to the regulations effectively. They provide the necessary support to navigate the nuanced landscape of federal regulations, ensuring that the adherence to compliance is as accurate and informed as possible.
Online Resources for the CFR
Navigating the vast expanse of regulatory information in the field of clinical research can be daunting. The Code of Federal Regulations (CFR) is critical for ensuring compliance and maintaining standards within the industry. Fortunately, the Electronic Code of Federal Regulations (eCFR) provides an accessible online version of the CFR, which is continuously updated to reflect the latest amendments and changes.
While not an official legal edition, the eCFR offers advanced search functionalities and navigation features that enhance research efficiency. This digital resource simplifies the process of locating pertinent regulations, thus supporting clinical researchers in their quest to uphold ethical and legal standards in their work.
In the context of health information technology (HIT), recent data from the National Health Interview Survey reveals that over 58% of adults have utilized the Internet for health or medical information during the latter half of 2022. This underscores the growing reliance on digital resources for accessing critical data. The eCFR's role in providing regulatory information mirrors this trend, offering a platform where professionals can readily find and interpret regulatory documents essential for clinical research.
Moreover, the eCFR's editorial process ensures that users have access to the most current regulatory guidelines. As the landscape of global health policy-making evolves, resources like the eCFR become invaluable tools for professionals, including family researchers and practitioners, who depend on timely and accurate information to inform their work and advocacy efforts.
The importance of such resources is echoed by leaders in the field. Carlos Correa, Executive Director of the South Center in Geneva, emphasizes the significance of up-to-date reporting on international politics of global health for diplomats and organizations. Similarly, Hyo Yoon Kang from the University of Warwick Law School, recognizes the eCFR as an indispensable public resource that combines accurate news with a deep understanding of the dynamics that shape international negotiations.
Taken together, the eCFR and similar online resources are more than mere repositories of information; they are dynamic platforms that foster collaboration, knowledge sharing, and a more profound impact on health policy decisions, ultimately enabling clinical researchers to better navigate and comply with the regulatory frameworks that govern their crucial work.
Historical and Bulk Data Access
The landscape of research and data analysis is continuously advancing, bringing to light the importance of accessible and well-organized data repositories. To meet this need, the Code of Federal Regulations (CFR) not only provides the most current regulations but also offers historical versions and bulk data for in-depth research and analysis. Historical editions of the CFR are invaluable for researchers who need to track regulatory changes over time or understand the legal context at a specific historical juncture.
Moreover, the availability of bulk data sets serves as a foundational resource for comprehensive data analysis, aiding researchers in discerning patterns and making informed decisions in their fields of study.
Efforts are continually being made to enhance the utility of these resources. Anticipating the release of the annual data file, discussions are underway about providing tools that allow for the conversion of this data into various file formats. This initiative is part of a broader conversation with the research community about potential data file formats that could support a wider range of use cases, reflecting a proactive approach to meet diverse research needs.
The importance of such resources is echoed in recent trends, where research data services have become a focal point for stakeholders within academic and research institutions. As noted by Ithaka S+R's report on the state of research data services, these services have historically developed in an ad hoc fashion, resulting in a fragmented landscape that can be challenging for researchers to navigate. There is a pressing need for a more cohesive and strategic approach that can streamline access to data services and adapt to evolving research requirements.
In a notable development, the Retraction Watch Database, a comprehensive resource for tracking retractions of academic papers, has been acquired by CrossRef. This acquisition underscores the importance of making research objects, such as datasets and tools, freely and widely available to enhance the efficiency of scholarly communication. CrossRef's commitment to this goal aligns with the broader trend of improving research infrastructure and support services.
It is clear that the provision of historical versions, bulk data, and improved data service tools for the CFR are more than just administrative conveniences; they are strategic assets in the global effort to foster transparent, efficient, and innovative research practices. As these resources evolve and expand, they promise to significantly bolster the research community's capacity to engage with complex data and contribute to meaningful economic, societal, and policy developments.
Common Terms and Usage in the CFR
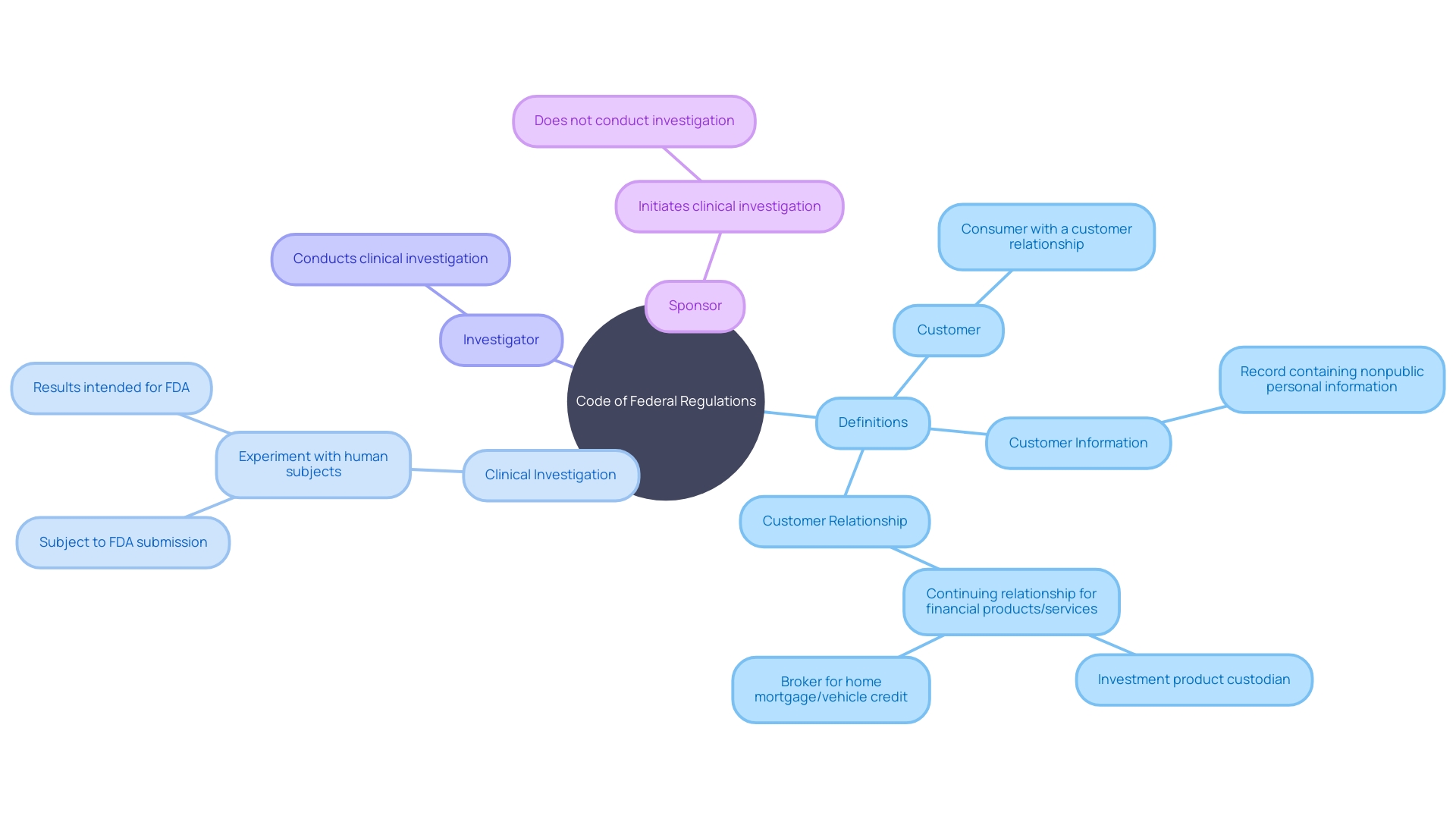
The Code of Federal Regulations (CFR) serves as the codified source of rules and regulations that have an immense impact on various facets of federal governance and public administration. Interpreting the CFR requires a nuanced understanding of specific terms and their unique definitions within the regulatory context. For instance, the term 'customer' in the CFR is not merely a reference to any consumer but is defined as one having a customer relationship with a financial institution, a relationship characterized by the consumer's usage of financial products or services primarily for personal, family, or household purposes.
In practical terms, the implications of these definitions are far-reaching. Take, for example, the Paperwork Reduction Act of 1995, which mandates that federal agencies obtain approval from the Office of Management and Budget (OMB) prior to collecting information from the public. This act underscores the importance of ensuring that data collected is accurate, relevant, and serves its intended purpose effectively.
Therefore, understanding the CFR's definitions is not only about regulatory compliance but also about upholding the quality and integrity of information that underpins public policy.
Recent amendments to Regulation Z, a section of the CFR which pertains to the imposition of credit card penalty fees, illustrate the dynamic nature of these regulations. The Consumer Financial Protection Bureau (CFPB) has updated safe harbor provisions which dictate the maximum allowable penalty fee for credit card violations. The amendments reflect a complex interplay between regulatory definitions, cost analysis, and consumer protection.
In the context of clinical research, terms such as 'clinical investigation' and 'investigator' are defined with precision in the CFR. These definitions delineate the boundaries of what constitutes a clinical investigation and who is considered responsible for conducting it, whether it is an individual or a team of researchers. Such clarity is crucial for the planning and execution of clinical trials, ensuring that all activities are in alignment with FDA requirements.
Considering the variety of terms and their specific usage within the CFR, it is essential for professionals navigating this regulatory landscape to thoroughly grasp these definitions. As elucidated by legal experts, the distinction between 'guidelines' and 'rules' is one such nuance that carries significant legal implications, particularly when it comes to the force and effect of law. The CFR's lexicon, therefore, is not just a collection of definitions but a foundational element for effective policy implementation and legal interpretation.
Conclusion
The Code of Federal Regulations (CFR) is a vital tool for managing complex societal needs and has a significant impact on everyone. Understanding the CFR is crucial for compliance and comprehending its broader implications on efficiency and effectiveness.
The organization and structure of the CFR ensure easy access and navigation. Its numbering system enables precise referencing, promoting accurate interpretation and adherence to the law.
Regular updates to the CFR keep it current and relevant, reflecting the latest changes made by federal agencies.
Accessing the CFR is made easy through print and digital formats. The official print version and the electronic version (eCFR) provide advanced search functionalities and navigation features.
Supplementary materials such as indexes, finding aids, and supplements enhance understanding and application. They simplify the search for specific topics and provide valuable context and clarification.
Historical and bulk data access of the CFR offer invaluable resources for in-depth research and analysis. They allow tracking regulatory changes over time and support comprehensive data analysis.
Understanding unique definitions within the CFR is crucial for accurate interpretation and compliance. Precise definitions ensure clarity and consistency in communication.
In conclusion, the CFR plays a crucial role in shaping an efficient, effective, and trusted government. Its organization, regular updates, accessibility, supplementary materials, historical and bulk data access, and precise definitions ensure compliance, transparency, and efficiency in governance.
Take control of compliance and maximize efficiency with bioaccess™ CRO services in Latin America.




