Introduction
The De Novo classification request is a crucial pathway for novel medical devices that lack an existing predicate, offering a streamlined route for devices that do not fit into existing classifications. This process requires a comprehensive submission, including a detailed description of the device, its intended use, and the patient population it serves. Visual representations, specifications, and engineering drawings must also be provided.
The submission should elucidate the device's properties and its interaction with the body to diagnose, treat, prevent, cure, or mitigate diseases or conditions. Additionally, a comparative analysis of existing alternative practices or procedures must be included. The De Novo pathway facilitates the introduction of innovative medical devices, ensuring safety and effectiveness while promoting market access and technological advancements in healthcare.
What is a De Novo Classification Request?
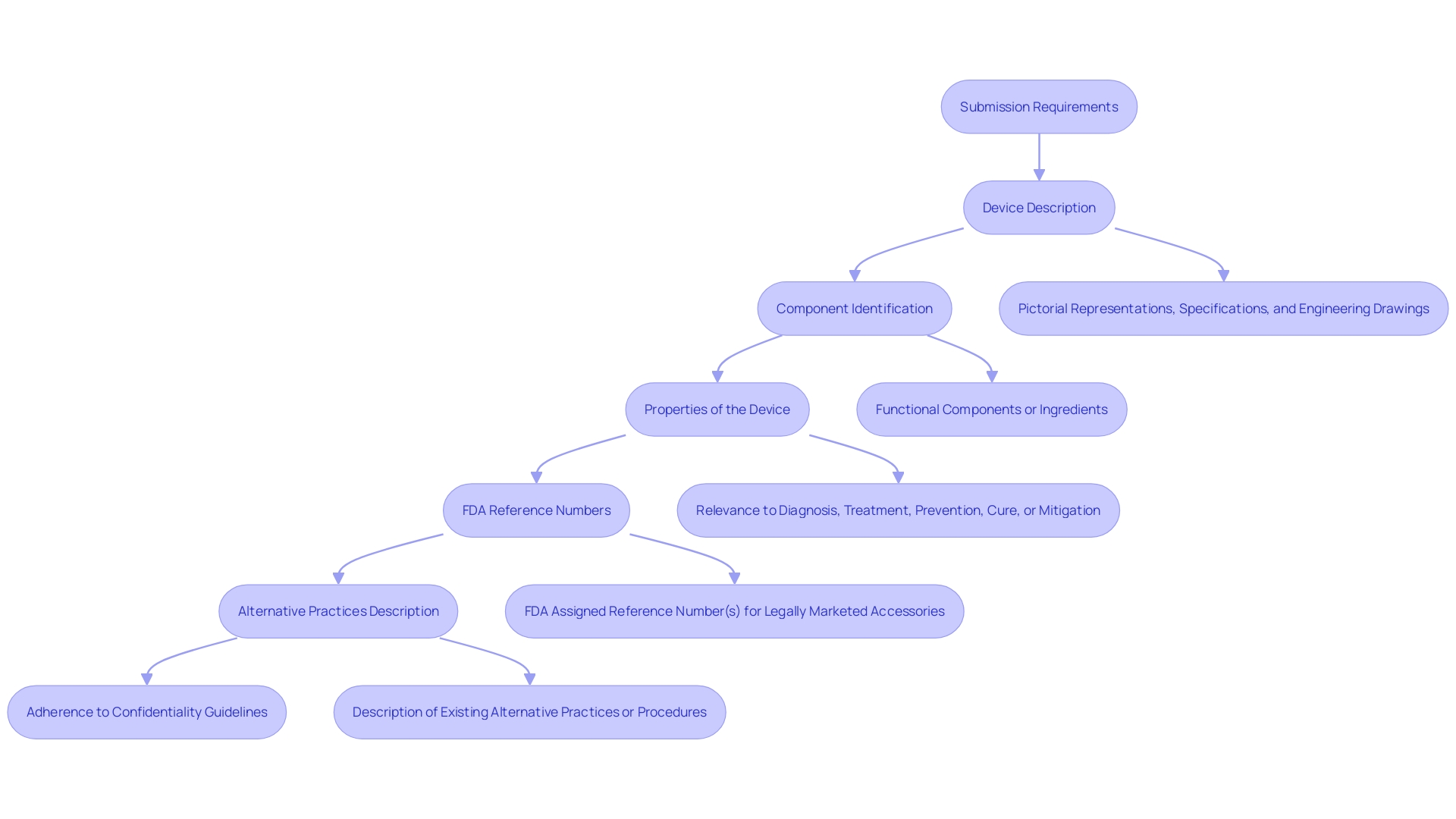
The classification request, as outlined by the FDA, is a crucial pathway for new medical instruments that do not have a preexisting example. This pathway is especially crucial for gadgets that are distinctive and do not neatly fit into an existing classification. De Novo requests require a comprehensive submission, including a detailed description of the equipment, its intended purpose, and the patient population it serves, whether it's for prescription or over-the-counter use. Submissions must also include visual representations of the equipment, specifications, engineering drawings, and details on each of the functional components or ingredients if the apparatus comprises multiple elements.
The submission should clarify the properties of the apparatus and how it interacts with the body to diagnose, treat, prevent, cure, or mitigate any disease or condition. In addition, it should include the FDA's assigned reference numbers for any legally marketed accessories or components intended for use with the equipment. Furthermore, manufacturers are required to offer a comparative analysis of current alternative practices or procedures for the circumstance the equipment deals with. The alternative pathway simplifies the procedure for introducing low to moderate risk healthcare products to the market, providing another option to the more stringent PMA pathway, thereby promoting innovation while ensuring the safety and efficacy of the products.
Key Characteristics of De Novo Submissions
Comprehending the De Novo classification procedure is vital for medical product producers when a reference item is absent. Devices undergoing this process are considered novel yet pose a low to moderate risk profile. A comprehensive submission should include a detailed description of the apparatus, such as its intended use in diagnosing, treating, preventing, curing, or mitigating a disease, as well as its effects on the body structure or function, including a clear depiction of the patient population it's designed for. The submission must also contain the generic and trade names, pictorial representations, specifications, and engineering drawings of the equipment. In addition, it is necessary to identify each component and ingredient of the equipment, particularly when it is composed of more than one. The De Novo submission must describe the properties of the apparatus and how they pertain to its intended healthcare purpose. If the equipment is meant to be used with other legally sold medical products, the appropriate FDA reference numbers must be included. It's also beneficial to describe alternative practices and procedures for the condition that is being addressed. Recent feedback indicates a strong interest in the process, with fifteen new decision summaries posted, highlighting the diversity and ongoing developments in this area. This serves as a reminder that all communications with the FDA, including these submissions, must adhere to strict confidentiality guidelines to protect sensitive information.
When to Use the De Novo Pathway
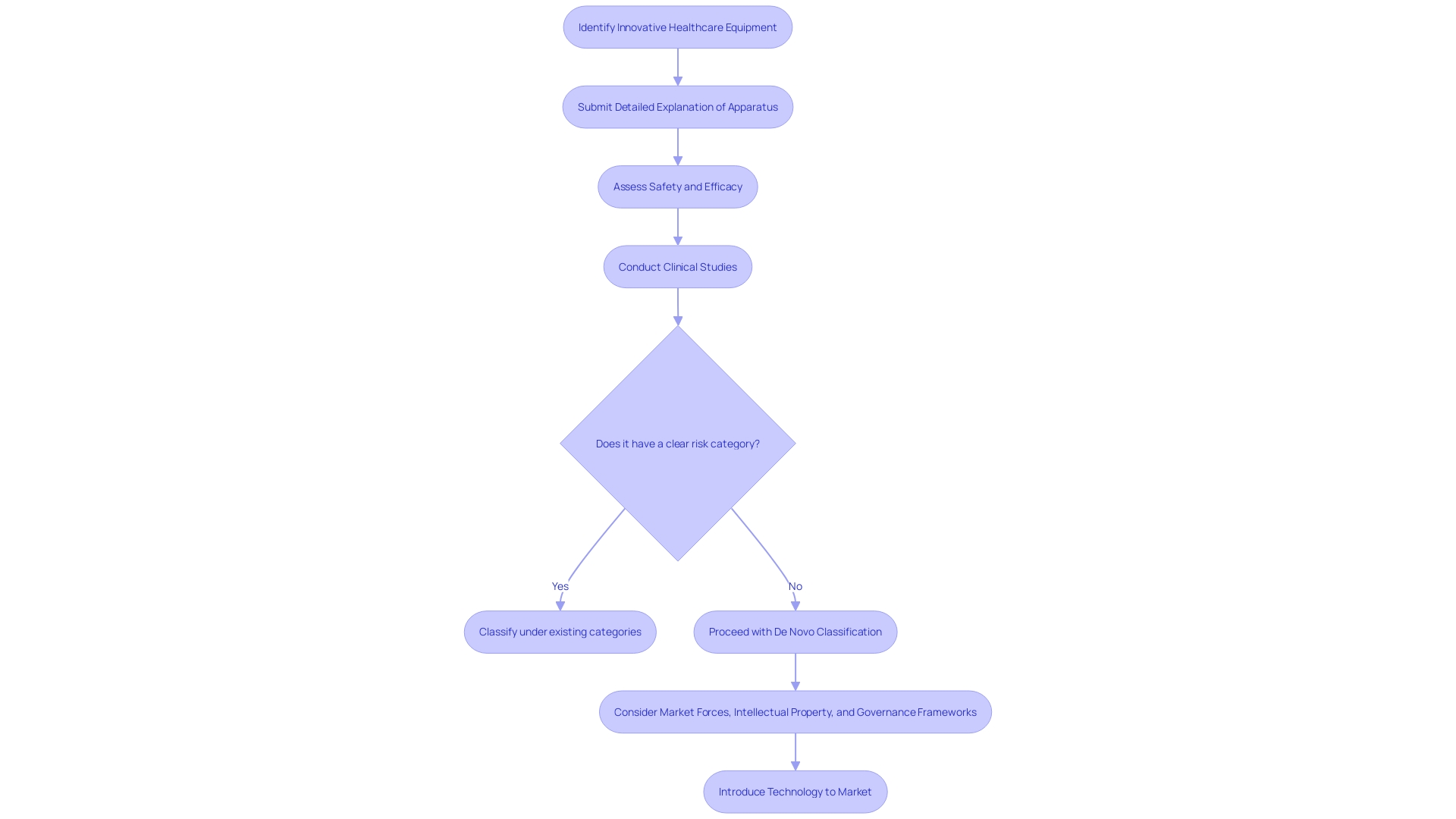
The classification procedure called De Novo is a regulatory pathway for innovative healthcare equipment that does not have a similar product available, providing an efficient route for items considered to have minimal to moderate risk. This pathway circumvents the traditional classification categories, enabling manufacturers to navigate the regulatory landscape when their products do not align with existing categories. Every entry for classification must contain a detailed explanation of the apparatus, covering all aspects from its intended purpose, specifications, parts, to its operational characteristics and any related FDA reference numbers for legally sold attachments or parts intended for use with the apparatus.
Case studies on the governance of emerging technologies in health and medicine, including those that are part of a rapidly evolving landscape, have underscored the complexity of navigating ethical, legal, and social issues. For instance, the Recent System, intended for metabolic function improvement and blood glucose regulation, underwent a series of feasibility clinical studies, like REGENT-1 and EMINENT, to assess safety and efficacy. These studies serve to illustrate the critical role of ethical considerations in the development and approval of new healthcare technologies.
Furthermore, the importance of the New process is additionally emphasized by the reality that the FDA classifies healthcare instruments into three categories based on the danger they present to patients, with category three instruments necessitating the most rigorous approval processes. The pathway thus offers an alternative for tools that do not have a clear risk category, enabling access to innovative healthcare solutions. For example, Cardiawave, a company developing non-invasive ultrasound technology for aortic valve treatment, has been preparing for Series B financing to support clinical studies in the U.S. as part of its strategic approach to obtaining FDA approval.
Additionally, it's important to recognize that FDA approval or clearance does not guarantee immediate coverage or payment from payors. Groups like CMS and private health plans independently determine coverage, which can result in delays in patient access to new healthcare equipment, underscoring the importance of synchronizing regulatory approval and payor coverage determinations.
To summarize, the pathway of New Creation serves as a crucial mechanism for the efficient introduction of innovative healthcare technologies, with a focus on ensuring safety and effectiveness while navigating the complex interplay of market forces, intellectual property, and governance frameworks.
De Novo Process Overview
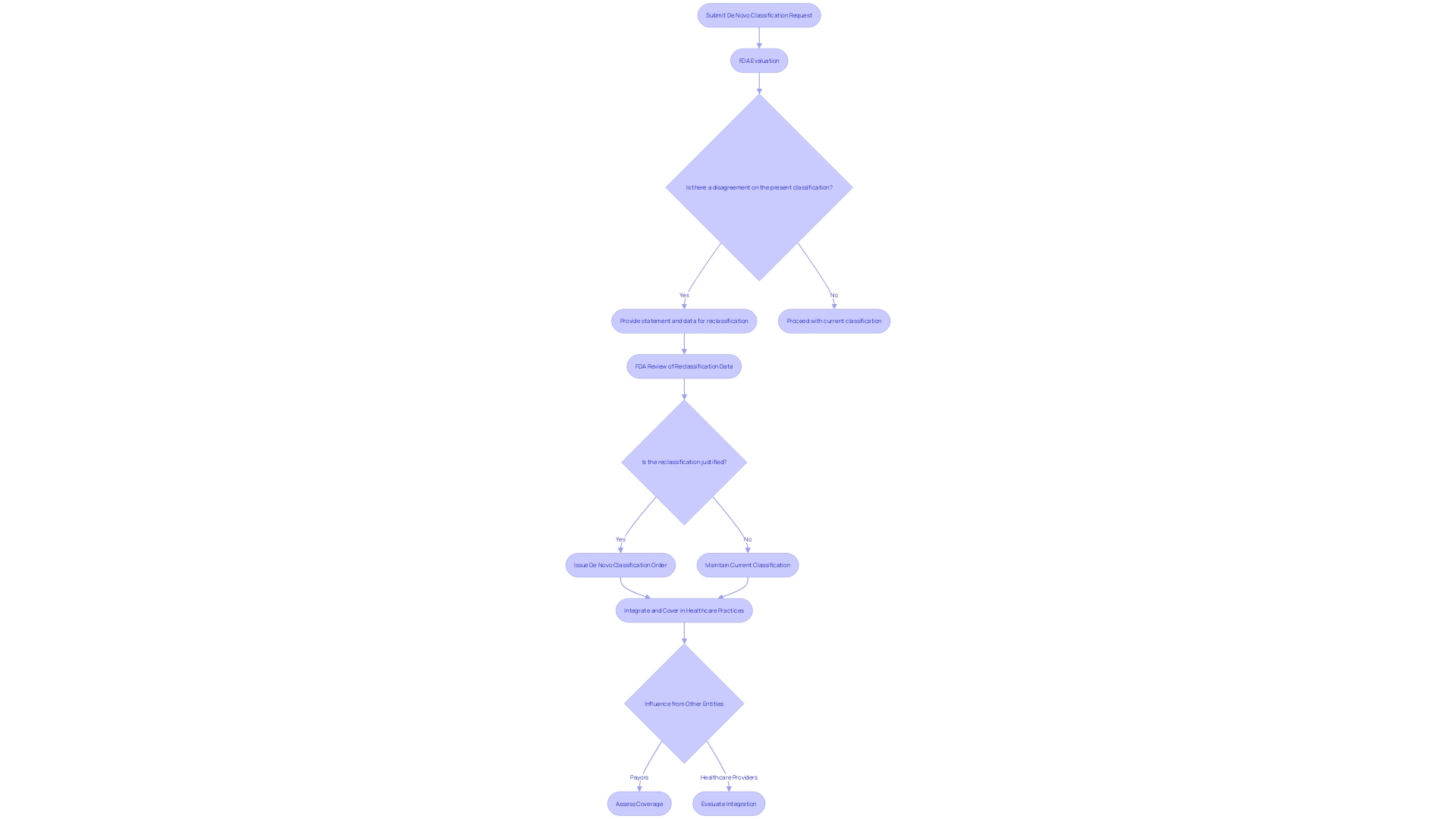
Navigating the FDA's De Novo classification process is a multi-faceted journey that begins with the submission of a comprehensive De Novo request. This request must encapsulate a wealth of detailed information, covering not only the medical equipment's intended function but also its design and composition, including pictorial representations and engineering specifications when applicable. The document should thoroughly describe the usage of the equipment, whether prescription or over-the-counter, and delineate the disease or condition it addresses, including a description of the targeted patient demographic. Crucial to this submission is the inclusion of scientific evidence substantiating the instrument’s safety and efficacy.
Upon receipt, the FDA embarks on a rigorous evaluation, commencing with an acceptance review that ensures the submission is complete and adheres to all pertinent requirements. After this initial phase, there is a comprehensive evaluation, a thorough examination of the properties of the instrument and their significance to the suggested diagnostic, therapeutic, or preventive uses. The FDA's evaluation is not only about the equipment in isolation; it also takes into account any related medical instruments already on the market, indicated by FDA-assigned reference numbers, that may be used in conjunction with the new equipment.
Should the FDA's review conclude affirmatively, it results in the issuance of a De Novo classification order. This significant milestone clears the product for market entry, establishing it as a legally marketable entity. It's important to note, however, that FDA's role extends beyond this point. After the clearance process, other entities, like payors and healthcare providers, have a significant influence on determining the coverage and integration of the equipment into healthcare practices. Even with approval from the FDA, there might be other obstacles when it comes to insurance and payment, which could affect the patient's ability to obtain the new healthcare equipment.
Steps in Preparing a De Novo Request
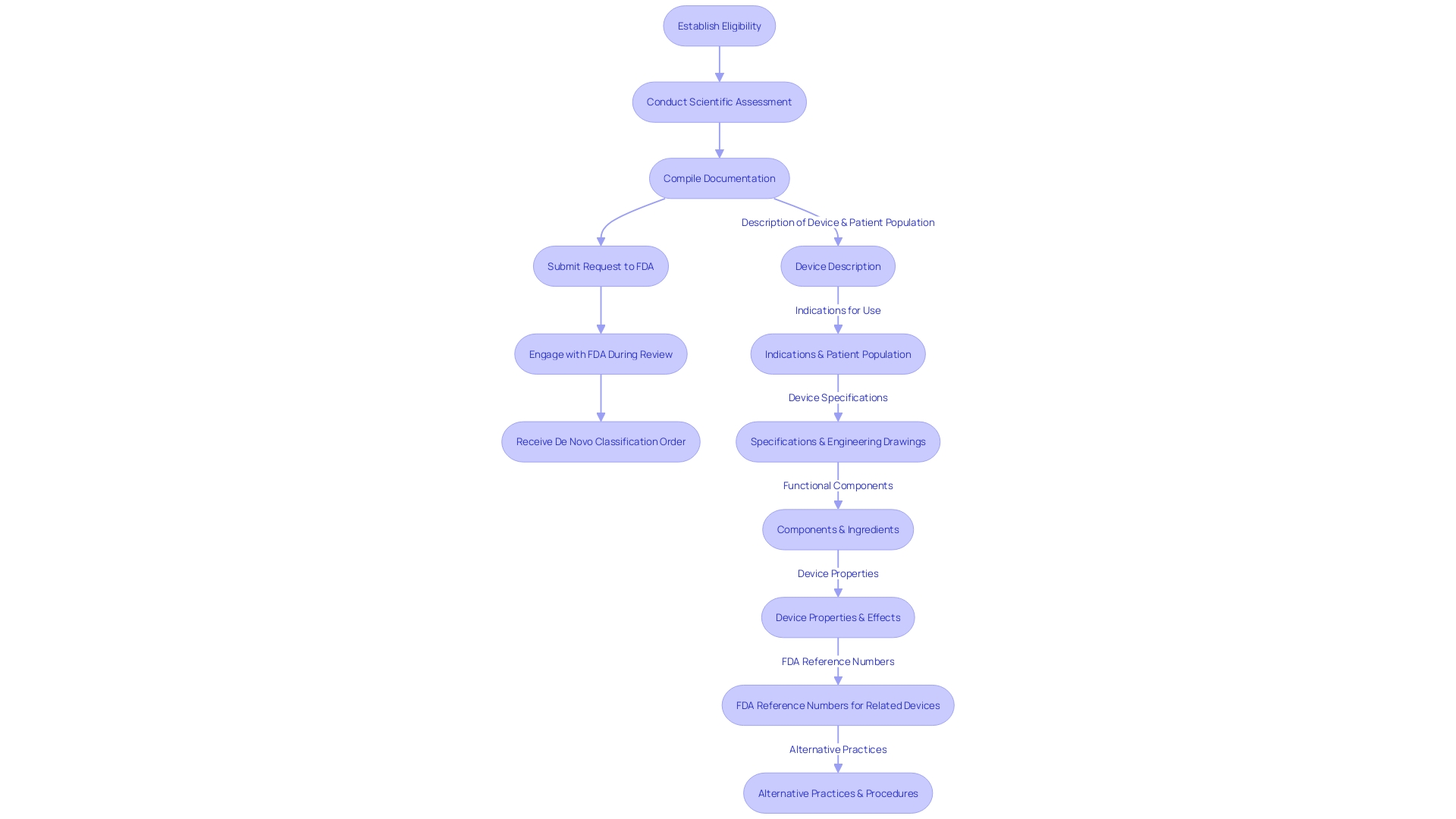
The process of preparing a request for the FDA involves a series of strategic steps, each requiring meticulous attention to detail and compliance with FDA guidelines. At first, it is important to establish if your healthcare apparatus qualifies for a De Novo submission, which entails a comprehensive assessment against the particular standards set by the FDA.
Once eligibility is confirmed, a comprehensive scientific assessment is paramount. This evaluation must compile strong scientific evidence that highlights the safety and effectiveness of your medical product. It includes a comprehensive presentation of the intended use of the apparatus, including the patient population it serves and the conditions it aims to address, whether through diagnosis, treatment, prevention, or cure. The evaluation should also encompass the specifications, engineering drawings, and any other relevant details that capture the functional components and properties of the apparatus.
When preparing the request, it is crucial to include a comprehensive dossier of documentation. This collection should not only cover the technical aspects of the product, such as pictorial representations and engineering specifics, but also the regulatory aspects, including any FDA-assigned reference numbers for legally marketed accessories or components intended for use with your item.
Submitting the request to the FDA is the next critical phase. Adhering to the FDA's submission protocols and ensuring that all required information is provided will facilitate a smoother review process. As the FDA evaluates your request, it is essential to engage proactively, responding promptly to any queries or demands for extra information.
Once the review process concludes, a successful submission will result in the FDA issuing a De Novo classification order. This order not only approves market clearance for your healthcare instrument but also confirms that the instrument meets the high standards of safety and effectiveness required for patient care.
Throughout this process, it is essential to acknowledge the FDA's dedication to protecting public health by guaranteeing that healthcare instruments adhere to rigorous safety and efficacy standards. An initial request is a pathway to introducing innovative medical tools to the market, provided they can successfully demonstrate their value in enhancing patient care and outcomes.
FDA Review Process for De Novo Requests
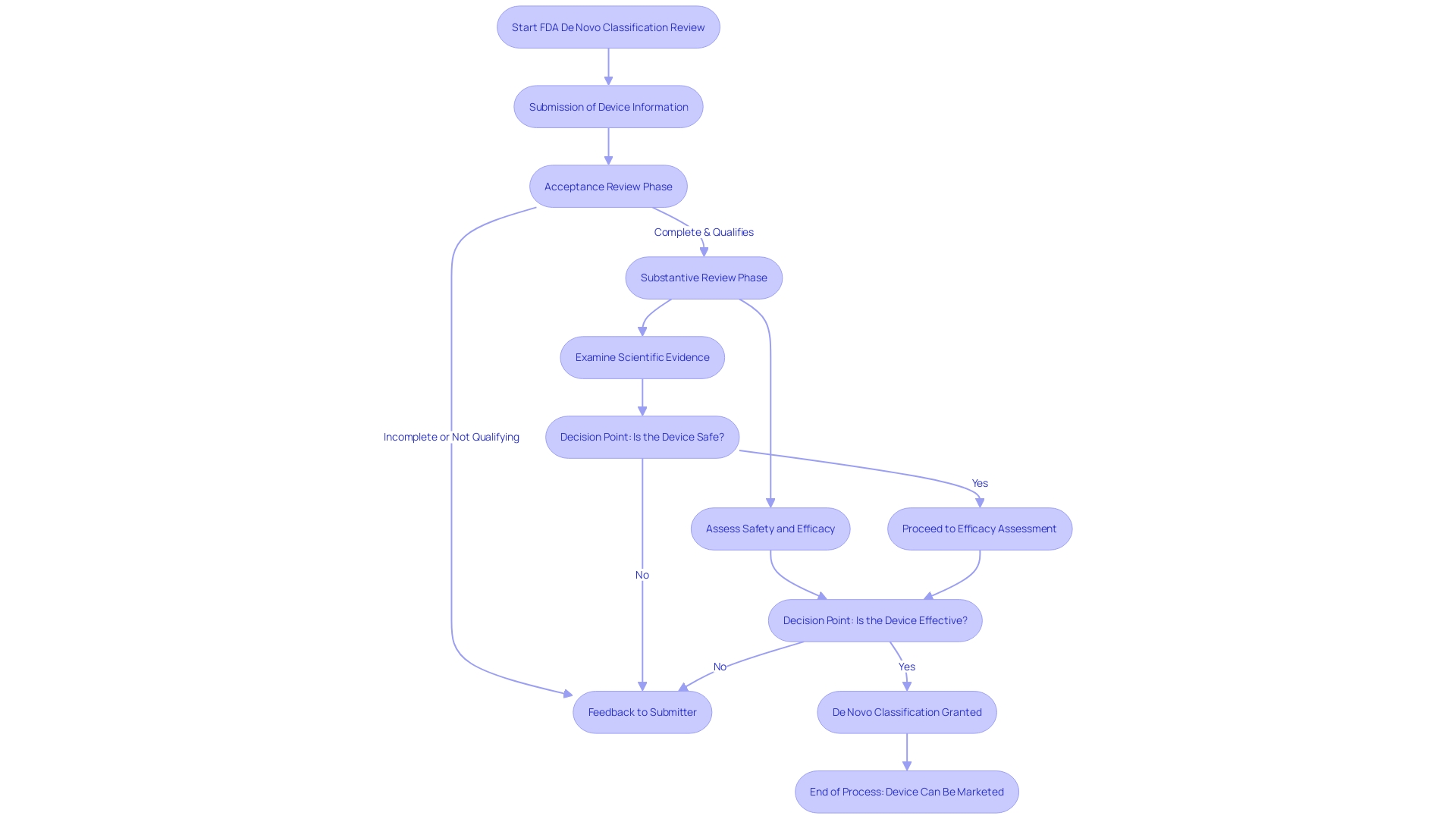
The FDA's De Novo classification process is a rigorous two-phase review that starts with the acceptance review phase, where the FDA confirms that a submission is complete and that the equipment qualifies for the De Novo pathway. This first stage involves a detailed examination of the provided documentation, including a comprehensive description of the instrument's intended use, its specifications, and any pictorial representations. The FDA also examines the components, ingredients, and properties relevant to its function in diagnosing, treating, preventing, or mitigating a disease, as well as any existing alternative practices known to the submitter.
Should a submission pass the acceptance review, it moves on to the substantive review phase. Here, the FDA explores further, examining the scientific evidence and data to assess the safety and efficacy of the product. This can include requests for additional information or clarification from the manufacturer. After completing the thorough evaluation, the FDA will determine whether to issue a classification order based on the equipment meeting all necessary criteria. The objective of the FDA, as a component of the U.S. Department of Health and Human Services, is to protect public health by guaranteeing the safety, efficacy, and security of healthcare equipment and other items within its authority.
Acceptance Review and Substantive Review Phases
Navigating through the process of categorizing new equipment is a complex task that involves multiple critical phases. Initially, the FDA conducts an acceptance review to ensure the submission adheres to the necessary criteria for a comprehensive evaluation. This preliminary assessment analyzes the submission for completeness and confirms the medical product's eligibility for the De Novo pathway, eliminating any potential for missing or incomplete information that could hinder the application's progression.
Once the submission successfully clears the acceptance review, it proceeds to the substantive review phase, where the FDA undertakes a detailed examination of the scientific evidence and data presented. During this stage, the agency may reach out to the manufacturer for additional information, clarifications, or further evidence to methodically assess the product's safety and effectiveness. It is essential for the manufacturer to be prepared for this interaction and ensure all data submitted is clear and devoid of confidential information not intended for the public domain.
In accordance with recent updates, the FDA has shown its dedication to transparency by publishing fifteen new decision summaries on submissions, ranging from devices granted approval in 2020 to those approved in 2023. This provides valuable insights into the evaluation process and outcomes of new submissions, reflecting the agency's rigorous standards for safety and effectiveness.
Furthermore, the FDA's overarching role in safeguarding public health, as evidenced by its recent publication of a final rule for direct-to-consumer prescription drug advertisements, underscores the importance of clear and accessible communication. This principle is equally applicable to the new process, where clarity of information is paramount in the agency's decision-making process. By following these rigorous criteria, manufacturers can better navigate the pathway for new devices, thus contributing to the progress of healthcare technology and patient well-being.
Common Deficiencies and How to Address Them
Navigating the De Novo review process for medical instruments requires meticulous attention to detail and robust evidence to meet the FDA's standards. Common pitfalls manufacturers may encounter include presenting inadequate scientific evidence, which can manifest as either insufficient data or incomplete data sets that fail to comprehensively support the safety and effectiveness of the product. To mitigate such issues, a thorough compilation of clinical and non-clinical data is imperative.
In addition, manufacturers often encounter difficulties with unclear instrument specifications. Accuracy in describing the design, components, and intended use of the apparatus is essential. This includes offering visual representations, detailed specifications, and engineering drawings that provide clarity on each functional component or ingredient of the product. Such meticulous documentation ensures the FDA can thoroughly evaluate the product without ambiguities.
Compliance with regulatory requirements is another critical area where deficiencies can arise. Non-compliance not only hinders the review process but can also lead to legal and ethical complications. As the FDA is vigilant in ensuring public health and safety, adherence to all guidelines is non-negotiable. For instance, the FDA's recent implementation of standards for direct-to-consumer prescription drug advertisements underscores their commitment to clear, conspicuous, and neutral information presentation.
To address these complex challenges, manufacturers are advised to engage in proactive communication with the FDA, leveraging historical governance insights and case study analyses that underscore the evolution of technology governance. This includes considering market incentives, intellectual property rights, and the broader governance ecosystem within the United States, along with pertinent international context when appropriate.
The pathway for new inventions, although demanding, provides a systematic approach for innovative products to enter the market. It requires a thorough account of the apparatus's intended use, its impact on the disease or condition it aims to address, and the patient population it serves. Furthermore, it requires disclosure of any existing alternative practices or procedures, adding layers of consideration to the application.
Considering the changing field of healthcare technology, producers must base their applications in extensive evidence, precise specifications, and consistent regulatory adherence. By doing so, they position themselves to navigate the complex approval process successfully, ultimately contributing to the advancement of healthcare and patient outcomes.
Benefits and Challenges of the De Novo Pathway
The pathway, within the FDA's authority, creates a regulatory structure for novel instruments that do not have a comparable, legally marketed predecessor. This procedure not only permits the categorization of these instruments into class I or class II groups but also establishes a new regulatory product code and designates the apparatus as the benchmark standard for future 510(k) submissions. While the New pathway has historically been underutilized due to initial requirements that mandated a prior 510(k) submission, recent years have seen a shift towards more direct access to this pathway, reflecting the FDA's commitment to fostering innovation and addressing unmet medical needs.
The benefits of the De Novo pathway include the ability to clear distinctive products for market entry and a more streamlined regulatory experience compared to the Pre-Market Approval (PMA) process. This stimulates the creation of innovative tools that go beyond the existing categorization system, thus fostering technological progress in the healthcare field.
However, the pathway is not without its challenges. The intricacy and fluctuation of the review process can result in unpredictability, often requiring a strong compilation of scientific data to support the safety and effectiveness of the equipment. Furthermore, the originality of the products falling into the De Novo classification implies that there are fewer previous instances to direct manufacturers, which can make the process of navigating through the regulatory landscape more complex.
Advancement in healthcare technology is crucial for progressive solutions and the evolving policies of the FDA aim to balance rapid technological progress with the assurance of patient safety. The regulatory process, utilizing the De Novo pathway, is an intricate part of this balance, shaping the trajectory of new healthcare instruments from conception to clinical application.
Case Study: Successful De Novo Approvals
The pathway known as De Novo, which is a crucial component of the FDA's regulatory system, offers an essential opportunity for the introduction of groundbreaking healthcare equipment to the market, especially in the absence of any established reference devices. A remarkable example of the successful application of this pathway involves a medical product created by Company XYZ, designed to treat a specific medical condition. Without any reference to predicate devices, Company XYZ started the De Novo path and submitted a comprehensive request to the FDA. Their submission included a plethora of critical information: detailed scientific evidence, robust clinical data, and precise equipment specifications, all of which are essential components as per FDA guidelines.
During the FDA's meticulous review process, Company XYZ responded proactively to all inquiries and provided additional information as requested. Their endeavors reached a climax with the FDA issuing a classification order, effectively enabling the product to enter the market and offering a new therapeutic approach for patients impacted by the medical condition at hand.
This instance showcases the strategic function the Fresh Start route can play for producers, demonstrating not just the route's capability to facilitate market entry for new products but also its wider consequences for healthcare innovation. The De Novo process ensures that products go through a risk-based classification, resulting in either class I or class II categorization, depending on whether general controls or a combination of general and special controls are sufficient to guarantee safety and effectiveness. Furthermore, it establishes a new regulatory category for the device, complete with product codes and required controls, setting a regulatory precedent for future 510(k) submissions.
The evolution of this regulatory pathway reflects a governance ecosystem that balances innovation with patient safety and includes a multitude of factors such as market incentives, intellectual property, and ethical considerations. Through the historical trajectory of such cases, we can observe the dynamic nature of health technology governance, the ethical, legal, and social issues that surface, and the strategic questions that guide the development and approval of emerging healthcare technologies.
In this context, the successful navigation of the De Novo pathway by Company XYZ not only represents a significant achievement for the company but also provides valuable insights into the governance framework that underpins the development and introduction of new medical technologies in the health sector.
Conclusion
In conclusion, the De Novo classification request is a crucial pathway for novel medical devices that lack an existing predicate. It provides a streamlined route for devices that do not fit into existing classifications. The submission process requires a comprehensive description of the device, its intended use, and the patient population it serves.
Visual representations, specifications, and engineering drawings must be included to elucidate the device's properties and its interaction with the body.
The De Novo pathway facilitates the introduction of innovative medical devices, ensuring safety and effectiveness while promoting market access and technological advancements in healthcare. It allows for the classification of unique devices into class I or class II categories, creating a new regulatory product code and establishing the device as the reference standard for future 510(k) submissions.
While the De Novo pathway offers benefits such as clearing unique devices for market entry and providing a more streamlined regulatory experience, it also presents challenges. The review process can be complex and unpredictable, requiring manufacturers to compile robust scientific data. The novelty of devices under the De Novo classification means there are fewer precedents to guide manufacturers, adding complexity to the regulatory landscape.
Overall, the De Novo pathway serves as a critical mechanism for the efficient introduction of innovative medical devices, with a focus on ensuring safety and effectiveness. By adhering to the rigorous requirements and guidelines set by the FDA, manufacturers can successfully navigate the De Novo pathway and contribute to the advancement of healthcare and patient outcomes.




