Introduction
The International Council for Harmonisation (ICH) plays a crucial role in standardizing guidelines for the pharmaceutical industry. With the aim of streamlining regulatory processes globally, the ICH brings together regulatory bodies and the pharmaceutical industry to ensure the development and availability of safe and effective medicines. This article explores the history, key components, importance, development process, implementation, and future directions of the ICH.
From the harmonization of guidelines to the integration of innovative technologies and personalized medicine, the ICH's efforts are shaping the future of healthcare regulation and patient care on a global scale.
What is ICH?
The International Council for Harmonization (ICH) is an umbrella organization that brings together governing bodies and the pharmaceutical industry with the objective of standardizing guidelines for drug development, registration, and post-market surveillance globally. The ultimate goal of the ICH is to streamline the processes across different regions, ensuring that safe, effective, and high-quality medicines are developed and made available to patients in a timely manner. It addresses the need for a consensus in the technical requirements for pharmaceutical product approval, reducing duplication in the drug development process, and minimizing delays in global drug launches.
In a similar vein, the International Regulatory Cooperation for Herbal Medicines (IRCH), initiated in 2006, exemplifies the significance of global collaboration for the regulation of herbal medicines. It emphasizes the significance of protecting public health by improving frameworks for herbal products. With participation from numerous national regulatory authorities and regional entities, the IRCH operates with a shared mission of promoting public health through better regulation of herbal medicines, mirroring the harmonizing efforts of ICH in the broader pharmaceutical sphere.
The ICH's role is also apparent in the creation of multidisciplinary standards like the ICH M14, which are intended to synchronize post-approval studies using real-world data. This initiative highlights the recognition by regulators of the growing availability of real-world evidence and the need for internationally agreed-upon guidelines to improve efficiency for both sponsors and regulators.
As the international healthcare landscape evolves, initiatives like ICH and IRCH demonstrate the ongoing commitment to enhancing global health through cooperation, standardization, and the utilization of data to inform decisions. These efforts contribute to a more unified environment that facilitates the delivery of safe and effective medical treatments to patients around the world.
History and Background of ICH
The formation of the International Council for Harmonization (ICH) in 1990 was a crucial moment in the pharmaceutical sector, propelled by the pressing requirement to establish a consistent set of standards for drug development and approval on a worldwide level. This initiative was driven by the growing globalization of the pharmaceutical sector and the crucial need for consistency across Europe, Japan, and the United States. Through the ICH, regulators and business stakeholders have worked collaboratively to ensure the provision of pharmaceutical products that are not only safe and effective but also of the highest quality, thereby safeguarding public health.
Similar collaborative efforts have been observed in the realm of herbal medicines with the establishment of the International Regulatory Cooperation for Herbal Medicines (IRCH) in 2006. Akin to the ICH, the IRCH serves as a worldwide network dedicated to the enhanced regulation of herbal medicines, underpinning the commitment to public health and safety. With the World Health Organization as a cornerstone in its inception, the IRCH encapsulates the essence of global cooperation among diverse national authorities and regional entities responsible for herbal medicine oversight.
Recent progress in science related to regulations, as demonstrated by the Innovative Medicines Initiative (IMI) project VAC2VAC, shows the industry's move towards non-animal testing methods like the monocyte activation test (MAT). This aligns with the Ich's vision of streamlining the drug development process while adhering to safety standards. Furthermore, reforms underway in the EU further indicate a move towards a more interconnected, digital framework, which is essential for maintaining a competitive edge in the global pharmaceutical landscape.
The ICH's influence goes beyond mere harmonization in regulations; its principles have become a cornerstone for international collaboration, similar to the foundational role of INTERPOL in law enforcement and the ICD in disease classification and global health statistics. It is in such collaborative, harmonized efforts that the future of pharmaceutical innovation and patient-centric care is being shaped, as the industry navigates through the complexities of disease, access to information, and innovation, in pursuit of improved health outcomes for all.
Key Components of ICH
The International Council for Harmonization (ICH) plays an important role in the harmonization of regulations for pharmaceuticals, facilitating the creation of comprehensive instructions that cover various aspects of drug development and oversight. These instructions cover important areas such as quality assurance, nonclinical and clinical study protocols, safety reporting, and pharmacovigilance. Although it is not obligatory to follow these recommendations, their global recognition and adoption by regulatory authorities highlight their importance. The ICH principles serve as a framework for consistent evaluation and control of medicinal products, promoting global uniformity.
A recent project, highlighted in the journal Drug Safety, underscores the value of ICH's work in addressing data collection for medication safety during pregnancy. The project identified and defined 98 core data elements essential for evaluating the risk of adverse outcomes in pregnant women, fetuses, and infants, as well as long-term childhood outcomes. This initiative demonstrates the importance of standardized data collection methods across different countries and the role of international organizations in achieving this standardization. The framework aims to guide global data collection practices, thereby expediting the determination of medication safety during pregnancy. This is especially crucial given that nearly all pregnant women use medication, yet standardized data on medication safety for this demographic has been lacking.
As the healthcare landscape evolves with technological advancements like artificial intelligence (AI), Ich's guidelines become even more relevant. The healthcare industry is increasingly incorporating AI into clinical research, introducing new challenges in governance. To address these challenges, regulatory agencies are adopting a risk-based approach to AI usage, emphasizing the importance of compliance, privacy, and ethical considerations. This approach aligns with Ich's ethos of balancing innovation with patient safety and maintaining the integrity of data management and governance standards.
In conclusion, the work of ICH is more than just the development of guidelines; it is about ensuring that as medical science progresses, patient safety and the reliability of data remain at the forefront. It's about establishing a shared language and set of expectations that go beyond borders, facilitating a cooperative approach to healthcare regulation that benefits patients, researchers, and the business sector as a whole.
Importance of ICH in the Pharmaceutical Industry
The International Council for Harmonization (ICH) plays a vital role in advancing the efficiency and harmonization of the pharmaceutical industry's landscape. By promoting uniformity in rules and standards, ICH streamlines the intricate drug development process, reduces repetitive endeavors, and simplifies the global registration of pharmaceuticals. The Ich's guidelines are not only scientifically robust but also practical, reflecting the most recent scientific advancements. This global harmonization is instrumental in enhancing efficiency, reducing costs, and ensuring that innovative and safe medicines are promptly accessible to patients.
Moreover, the impact of ICH is exemplified by initiatives such as the International Regulatory Cooperation for Herbal Medicines (IRCH), which echoes a similar mission in the realm of herbal medicine regulation. IRCH, established in 2006, emerged from significant discussions among international delegates, highlighting the need for global networks to safeguard public health through better regulatory frameworks.
Amidst fast technological advancements and changing paradigms, accentuated by the challenges of the pandemic, the significance of ICH has become even more crucial. The Association of the British Pharmaceutical Industry (ABPI) underscores that traditional drug development is both time-intensive and costly, often taking over 12 years and more than £1 billion to bring a new medicine to market. ICH's work aligns with the sector's momentum towards innovative and efficient methodologies that address these challenges.
The ongoing advancement of digital health technologies, new non-animal testing methods like the monocyte activation test (MAT), and the development of recommendations for post-approval non-interventional studies using real-world data are current focal points of ICH efforts. These efforts showcase Ich's dedication to adapting to the needs of the field, guaranteeing that practices in compliance keep up with advancements and still prioritize the safety and availability of treatments for patients.
How ICH Guidelines are Developed and Updated
The ICH recommendations are the result of a collaborative effort among specialists from different sectors, including regulatory authorities, the pharmaceutical industry, and academic institutions. These professionals come together in teams with the aim of assessing and enhancing current directives, pinpointing areas that require direction, and formulating suggestions for novel or revised instructions. This process is thorough and includes open consultations with the public to ensure that the instructions are comprehensive and up to date. Once the ICH assembly gives its approval, the recommendations are adopted and periodically revised to reflect the latest scientific, technological, and regulatory advancements.
One of the most profound examples of collaborative revision and development of medical standards is the International Classification of Diseases (ICD). The eleventh revision of the ICD (ICD-11) was completed after nearly three decades of development, involving a global network of approximately 15,000 clinicians from 155 countries. This extensive process led to significant changes, such as the removal of arbitrary cutoffs for diagnostic symptoms in favor of descriptions that align more closely with clinical practice. The ICD-11, which came into effect in January 2022, has been adopted by 35 countries and is available in multiple translations.
In the realm of clinical trial guidance, recent updates to the European good clinical practice (GCP) guidance have introduced a dedicated section on data governance, as highlighted by Silvia Perez of AstraZeneca at the Outsourcing in Clinical Trials Europe 2024 conference. This update underscores the necessity of designing clinical trials that prioritize participant safety, wellbeing, and result reliability, and emphasizes a focused evaluation of trial objectives and associated risks. The updated guidance is expected to be implemented in late 2024, with a draft version available for comment.
Moreover, the work conducted within ICH M14 is evidence of the continuous endeavors to standardize protocols for post-approval studies utilizing real-world data. With the increasing availability of such data, there is a recognized need for international guidance to reduce inefficiencies for sponsors and regulators. This work exemplifies the Ich's commitment to streamlining and standardizing the approaches to pharmaceutical vigilance and safety evaluations.
The ICH's initiatives are instrumental in standardizing clinical practices and data collection, significantly contributing to global health knowledge. By aligning recommendations, the ICH supports extensive research, well-informed decision-making, and ultimately, the enhancement of patient care and outcomes.
Adoption and Implementation of ICH Guidelines
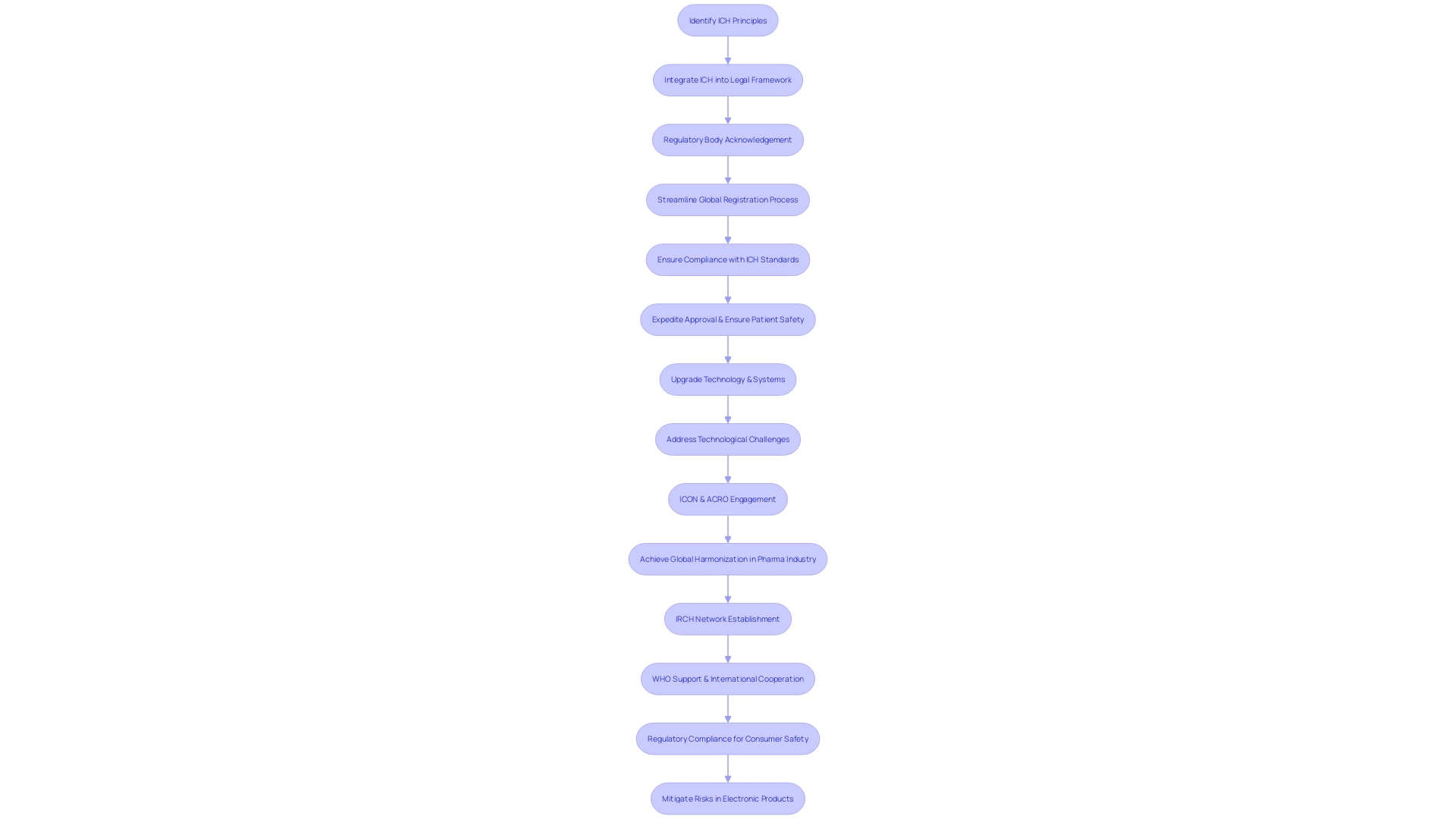
The alignment of requirements for pharmaceutical products is a vital process for ensuring their safe and efficient evaluation and approval globally. Although it is not legally required, the integration of International Council for Harmonization (ICH) principles into the current legal framework has been extensive. Regulatory bodies globally have acknowledged the importance of these principles, and their widespread adoption has resulted in a more efficient procedure for the global registration of pharmaceuticals. Pharmaceutical companies are keenly aware that compliance with ICH standards can significantly expedite the approval process across various regions, which is crucial when balancing the urgency of innovation with the paramount importance of patient safety.
In the ever-changing realm of clinical research, rules and recommendations must be followed diligently, particularly with the growing adoption of advanced technologies such as artificial intelligence and machine learning. The latest EU regulations, including the proposed EU AI Act, underscore the need for a risk-based approach and transparency when employing these technologies.
The application of ICH standards demonstrates a cooperative endeavor among regulatory authorities, business participants, and other important stakeholders. It is a complex endeavor, as highlighted by recent challenges faced by the sector. The European Medicines Agency, for instance, has established a brief six-month duration from the release to the enforceable date of new recommendations, which puts significant pressure on the industry to adjust quickly. The intricacies involved in upgrading technology and systems to meet these new expectations cannot be underestimated. It involves a series of meticulous steps: defining, scoping, planning, piloting, testing, validating, and finally implementing changes.
Ensuring compliance with such guidelines is not without its challenges, as evidenced by the variability of systems used in clinical trials. These range from government and oversight systems to those owned by individual sites, each with its own set of challenges related to access control, functionality, direct access, and audit trails. Entities such as ICON, in partnership with the Association of Clinical Research Organizations (ACRO), are actively interacting with governing bodies like the EMA to tackle concerns arising from current technological limitations.
The adoption of ICH guidelines, while voluntary, has become a cornerstone in the pursuit of global harmonization in the pharmaceutical industry, facilitating not only the approval process but also enhancing the safety and efficacy of products made available to patients worldwide.
Benefits and Challenges of ICH Harmonization
The pursuit of harmonization through the International Council for Harmonization (ICH) is pivotal in aligning drug development practices, requirements, and patient care standards across the globe. This collective effort fosters a unified approach to pharmaceutical control, which in turn enhances the efficiency of drug development processes, alleviates control redundancies, and facilitates wider access to safe and efficacious medications, ultimately serving to bolster patient safety.
In this landscape, stakeholders from different governing environments must address and reconcile disparate practices and requirements, adapt to regional variations, and ensure adherence to dynamic guidelines. Such challenges demand ongoing dialogue and partnership among industry experts, healthcare providers, and policymakers. For instance, the integration of artificial intelligence (AI) in cardiology exemplifies the transformation of medical fields through technology, thus necessitating stringent regulatory oversight to ensure patient safety while fostering innovation.
The European Union's AI Act, emphasizing a 'risk-based approach' to AI usage, reflects the proactive measures taken by regulators to govern the burgeoning integration of AI and machine learning in clinical research. It is a testament to the evolving nature of clinical practice and the need for harmonization in the face of technological advancement. In the same way, efforts such as the research team of Alzheimer Europe's contribution to public engagement in research and projects like EPAD and AMYPAD provide priceless knowledge about disease development and effectiveness of treatment, further emphasizing the significance of synchronized principles for advancing medical science and patient care.
These collaborative efforts underscore the critical balance between innovation and patient safety. By following standardized guidelines, such as the Harmonized Tripartite Guideline for Good Clinical Practice, and the FDA's regulations for Software as a Medical Device, the sector can reduce risks while upholding the integrity of data management and governance. As the frameworks evolve to encompass AI and other technological advancements, maintaining this balance remains paramount to ensuring that new treatments are not only effective but also safely integrated into healthcare systems.
Future Directions in ICH
The International Council for Harmonization (ICH) is resolute in its mission to adjust to the constantly evolving landscape of the pharmaceutical sector and to improve global regulatory harmonization. Anticipating the future, ICH is aiming to establish principles that tackle innovative areas such as personalized medicine, which, similar to the notion of 'your mileage may vary' in the automotive industry, recognizes that treatment effects can vary from person to person. Likewise, the incorporation of digital health technologies and the use of real-world evidence are set to establish recommendations that mirror the present scientific and technological progress.
The development of these new guidelines is grounded in the principles of precision medicine, which seeks to minimize errors and improve accuracy in medical recommendations, transcending traditional disease classifications. This approach aims not only to uphold existing safety standards but also to cater to the unique preferences and needs of individuals, thereby promoting inclusivity and enhancing health equity.
In the realm of digital health, the emergence of AI technologies presents a transformative potential for the sector. Ethical considerations, such as compliance with legal and frameworks, privacy and confidentiality, inclusivity and respect, transparency, and accountability, are vital principles that guide the integration of AI into pharmaceutical operations.
To achieve these ambitions, the ICH is considering a series of actions to enhance the current system, including clarifying criteria for expedited approval and encouraging collaboration among various stakeholders. Challenges such as sourcing fit-for-purpose real-world data (RWD) and safeguarding patient privacy while enabling data access for regulations are also being addressed. Through collaboration with regulators, academia, and the clinical research community, the ICH aims to develop optimal methods for RWD and real-world evidence in areas like data quality, study design, and analysis.
As we embark on this journey, it's essential to remember that behind every prescription picked up at a pharmacy lies a complex ecosystem dedicated to delivering the right medicine safely and responsibly. The pharmaceutical supply chain is a testament to this, ensuring that medicines reach patients after going through meticulous processes of sourcing, manufacturing, distribution, and delivery. As the industry evolves, the Ich's commitment to advancing regulatory harmonization aligns with the broader goal of meeting the demands of a dynamic global health landscape.
Conclusion
In conclusion, the International Council for Harmonisation (ICH) plays a crucial role in standardizing guidelines for the pharmaceutical industry globally. The ICH brings together regulatory bodies and the pharmaceutical industry to ensure the development and availability of safe and effective medicines. By harmonizing guidelines, the ICH facilitates global consistency and promotes the delivery of safe and effective medical treatments to patients around the world.
The history and background of the ICH highlight its pivotal role in establishing uniform standards for drug development and approval on a global scale. Collaborative efforts, such as the International Regulatory Cooperation for Herbal Medicines (IRCH), further exemplify the commitment to public health and safety through enhanced regulation.
The importance of ICH in the pharmaceutical industry cannot be overstated. It advances the efficiency and harmonization of the regulatory landscape, reducing costs and expediting the approval process for innovative and safe medicines. The adoption and implementation of ICH guidelines have become widespread, as regulatory bodies recognize their value in ensuring the safety and efficacy of pharmaceutical products.
Despite the challenges posed by emerging technologies like artificial intelligence, the benefits of ICH harmonization are significant. It fosters a unified approach to pharmaceutical regulation, enhances patient safety, and facilitates wider access to safe and efficacious medications.
Looking to the future, the ICH aims to address innovative domains such as personalized medicine and digital health technologies. By maintaining a balance between innovation and patient safety, the ICH is shaping the future of healthcare regulation and patient care on a global scale.
In conclusion, the work of the International Council for Harmonisation is about ensuring patient safety, reliability of data, and the delivery of safe and effective medical treatments. Through collaboration, harmonization, and the utilization of advanced technologies, the ICH is shaping the future of healthcare regulation and patient care on a global scale.
Learn more about how ICH guidelines ensure the safety and efficacy of pharmaceutical products.




