Introduction
The De Novo classification pathway is a crucial regulatory conduit used by the U.S. Food and Drug Administration (FDA) to evaluate novel medical devices that lack a valid predicate. This pathway ensures that new devices meet the necessary criteria for safety and efficacy before they enter the healthcare market. While there are already established processes for low to moderate-risk devices, the De Novo classification is reserved for devices that do not have a comparable product already in circulation.
This article explores the background, eligibility criteria, submission pathways, and review process for De Novo classification, highlighting its importance in introducing innovative medical devices to the market. It also discusses the post-approval implications and challenges faced by manufacturers, emphasizing the need for a collaborative environment between industry stakeholders and regulatory agencies. With the evolving regulatory landscape and advancements in medical technology, understanding the De Novo classification process is crucial for manufacturers seeking to bring their devices efficiently and compliantly to the market.
Background and Purpose of the De Novo Classification
The De Novo classification pathway stands as a pivotal regulatory conduit utilized by the U.S. Food and Drug Administration (FDA) for innovative medical products that lack a valid precedent. Its beginning enables the appropriate evaluation of new equipment, ensuring they fulfill the necessary standards for safety and effectiveness prior to entering the healthcare market. This categorization holds significant importance as it relates to products that are inherently low to moderate risk but do not have a comparable item already in circulation, thereby necessitating a distinct review process to determine their appropriate classification.
The FDA categorizes healthcare instruments into three distinct categories based on the level of potential risk they pose, with Class I representing the lowest risk and Class III the highest. Applications that are not suitable for the current clearance process, which relies on comparison to an existing model, known as a predicate, are often the route for those devices. The significance of the new process is highlighted by the reality that it enables the introduction of cutting-edge healthcare products into the market that might otherwise be delayed due to rigorous regulatory demands for high-risk products.
Given the vast range and intricacy of health conditions, including more than 100 forms of cancer as mentioned by the Centers for Disease Control and Prevention, the need for an efficient and all-encompassing regulatory pathway becomes evident. The De Novo process facilitates the emergence of new diagnostic and therapeutic tools, such as the Invitae Common Hereditary Cancers Panel, which assists in identifying inherited cancer causes, albeit not comprehensively examining all genes associated with cancer predisposition.
Furthermore, the regulatory landscape is in constant flux, as evidenced by the Medicines and Healthcare products Regulatory Agency's (MHRA) recent policy intent statement. This statement reflects an ambition to align international approval standards for healthcare equipment, suggesting a future where cross-border regulatory recognition could streamline market entry for manufacturers and expedite the availability of cutting-edge tools to patients.
The strategic direction of the FDA and international regulatory agencies, coupled with advancements in fields like digital health and personalized medicine, has catalyzed the push towards more integrated and efficient regulatory processes. This evolution is crucial for keeping pace with technological progress and meeting the urgent healthcare needs of populations worldwide.
Eligibility Criteria for De Novo Classification
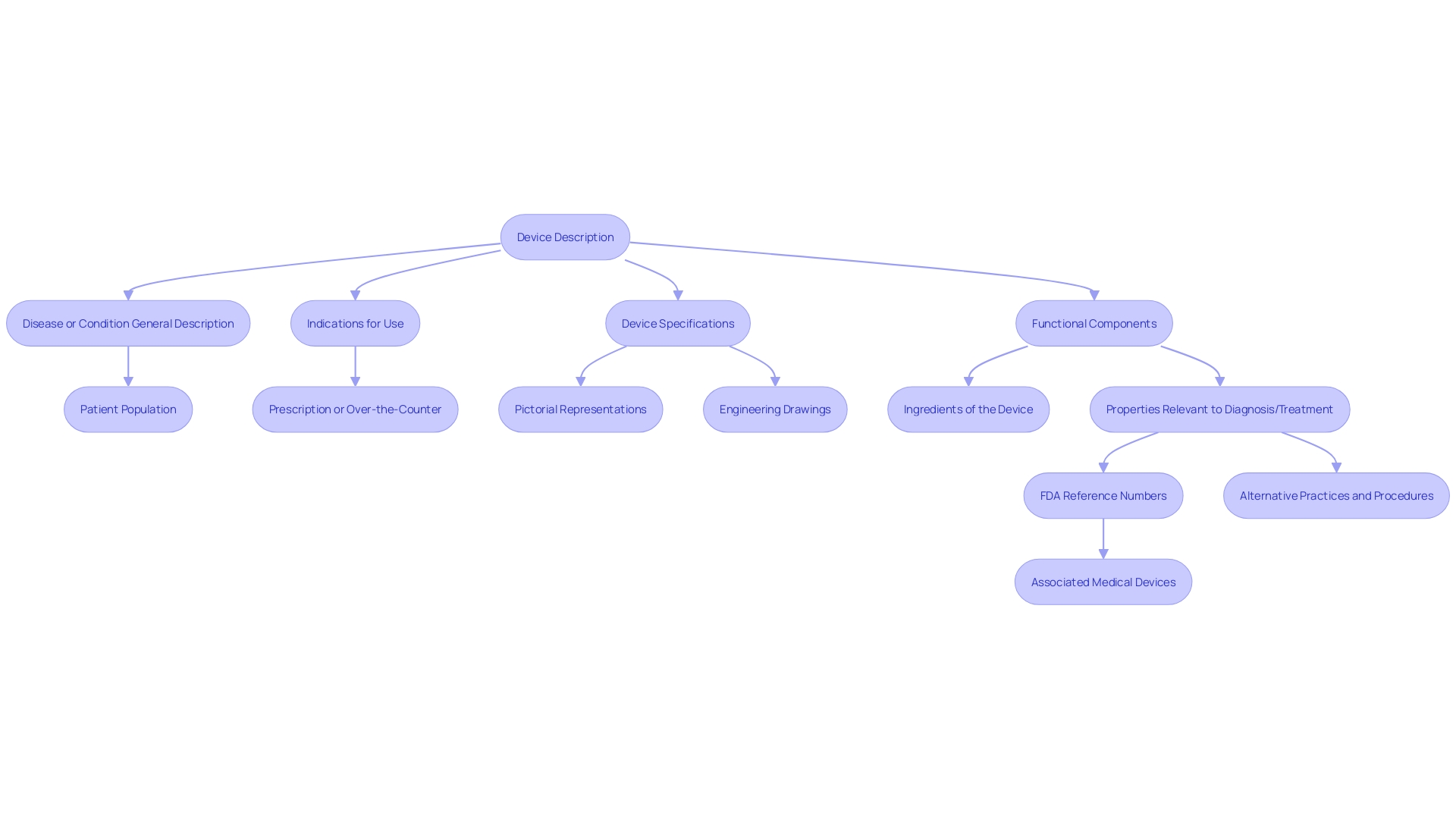
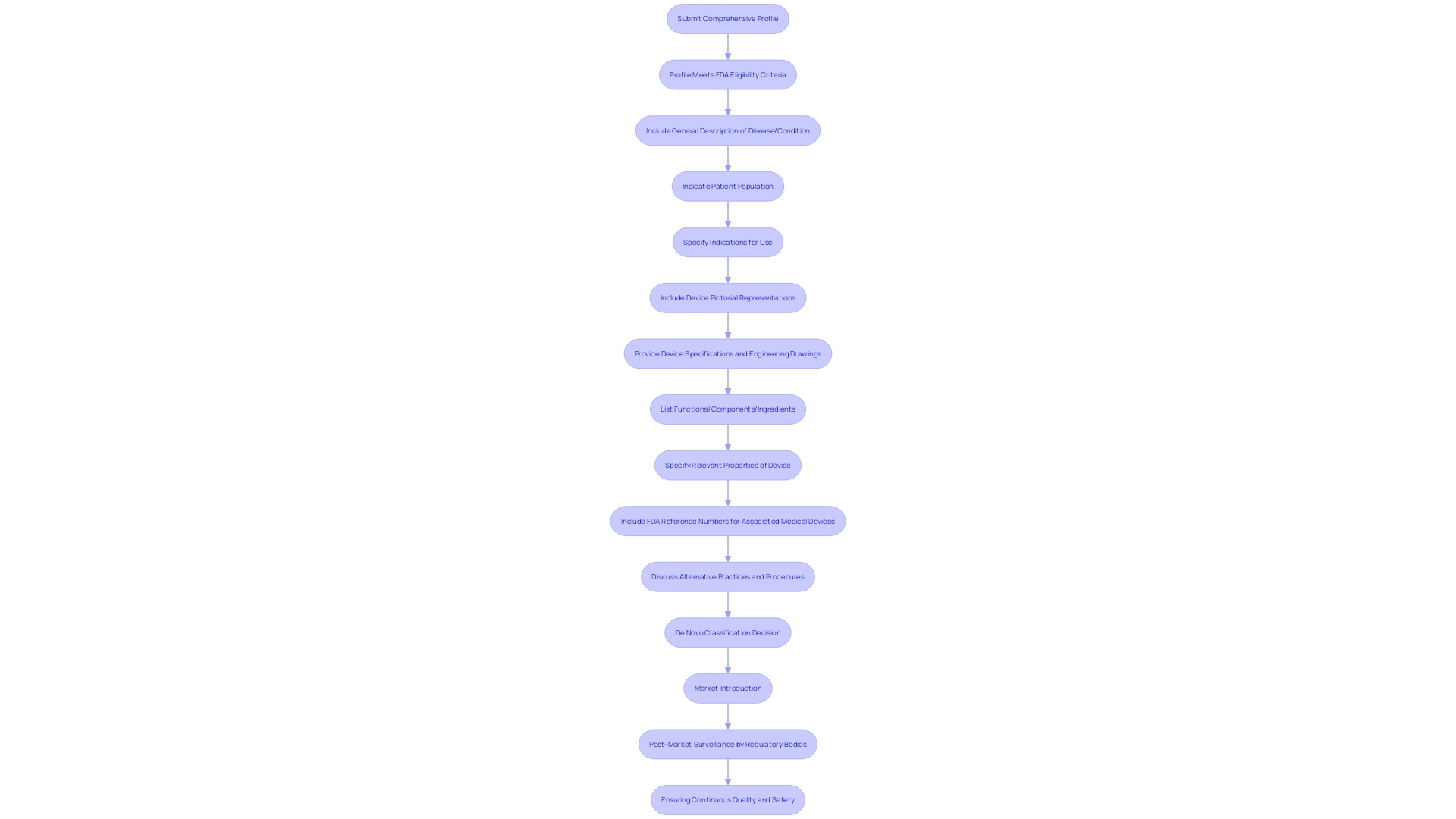
For a medical instrument to be considered for De Novo classification, it must present a comprehensive profile that meets the eligibility criteria set by the FDA. The submission must include a detailed description of the condition the equipment aims to diagnose, treat, prevent, cure, or mitigate, as well as the specific patient population it is designed for. This covers all possible uses of the equipment, whether it is meant for prescription or over-the-counter distribution.
The submission should also provide the generic and any proprietary names of the equipment, alongside clear pictorial representations, precise specifications, and engineering drawings. Moreover, it is crucial to depict every part or element if the equipment includes multiple elements. The qualities of the apparatus that are relevant to its intended health purpose or its influence on the body's structure or function should be specified. Furthermore, if the equipment is intended for use with lawfully sold healthcare instruments, the corresponding FDA reference numbers must be included.
It is also crucial to discuss alternative practices and procedures for the condition the mechanism targets, offering insight into existing methods known to the requester. The De Novo process, distinct from the 510(k) submission due to the absence of a predicate product and reserved for cases where general and/or special controls ensure safe and effective use, allows for the market introduction of innovative healthcare instruments. It is a critical pathway for advancing patient care by enabling access to the latest innovations that meet rigorous quality and safety standards as enforced by regulatory bodies like the MHRA.
Submission Pathways for De Novo Requests
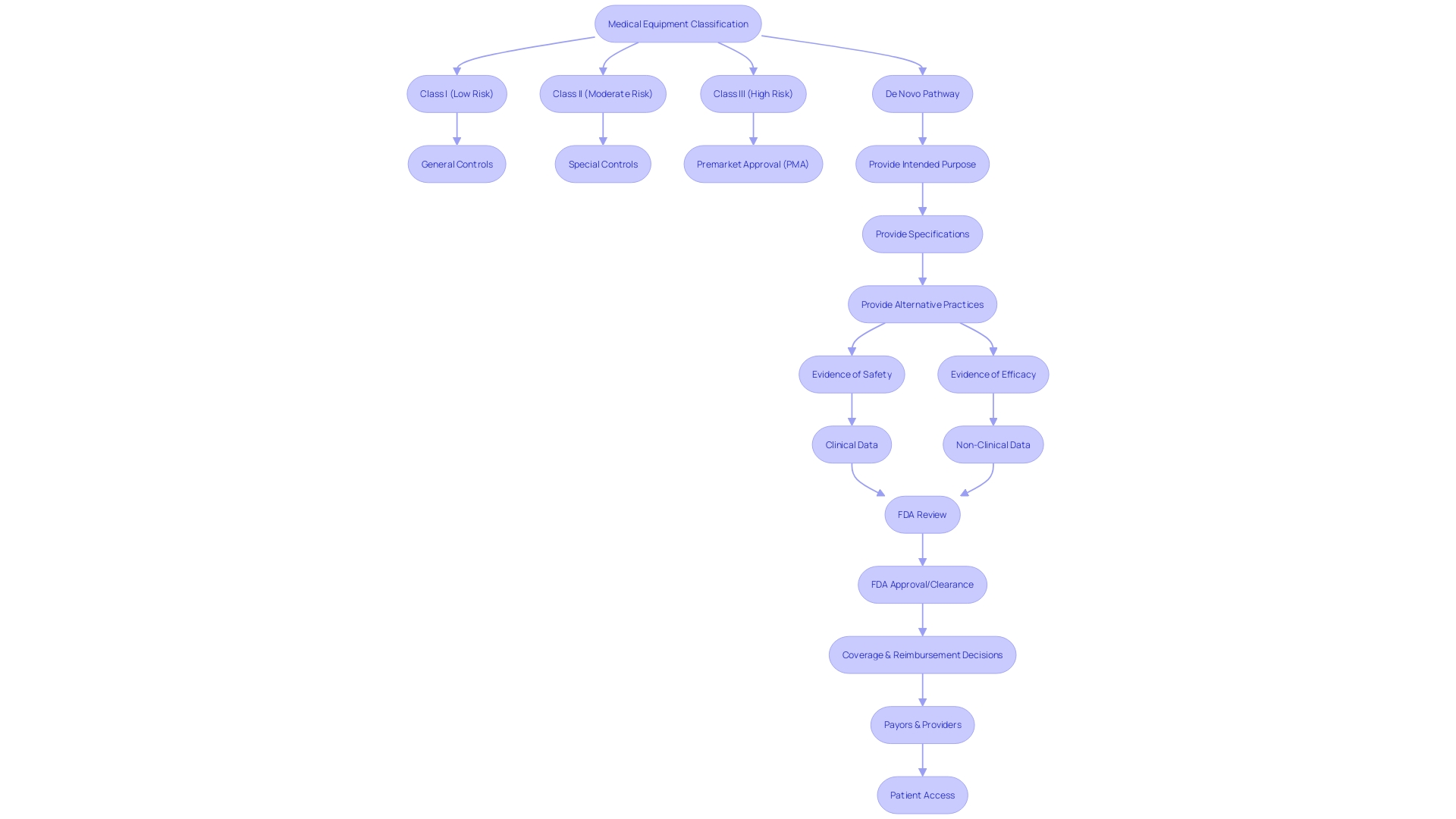
Understanding the classification is crucial when navigating the submission pathways for De Novo requests. The FDA classifies medical equipment into three classes reflecting their risk levels. Class I products present the least amount of danger and frequently necessitate less strict regulatory supervision. Class II products involve greater risk and typically necessitate a 510(k) clearance, which involves demonstrating that the item is substantially equivalent to a legally marketed predicate. Class III products encompass those with the highest risk, such as life-sustaining implantables, and generally necessitate a Pre-Market Approval (PMA), the FDA's most rigorous review process.
For items that lack a precedent but are of low to moderate risk, the De Novo pathway provides a route to the market. The process initiates with a submission that comprises, among other components, a general depiction of the intended purpose, specifications of the apparatus, and, if applicable, existing alternative practices. This is essential information, as it determines the extent of the application of the equipment and the patient demographics it serves.
The request for a new approval must also provide significant evidence of the product's safety and efficacy, which could incorporate clinical or non-clinical data, based on the nature and intended application of the product. Following recent surges in submissions with unreliable data, the FDA has underscored the importance of meticulous data scrutiny and the qualification of third-party test laboratories involved.
Additionally, the updated guidance from the FDA demonstrates a dedication to tackling health disparities and enhancing healthcare accessibility, indicating that instruments falling under these domains might undergo a more streamlined approval process. In view of the COVID-19 pandemic, there has been a joint effort to streamline regulatory pathways to expedite the introduction of innovative healthcare technologies, especially those that address unmet medical needs in the fields of digital health and personalized medicine.
Comprehending these subtleties is crucial for manufacturers to successfully navigate the De Novo classification process and bring their healthcare instruments to the market efficiently and in compliance. With roughly 10% of gadgets falling into the high-risk Class III category and the FDA's growing attention on innovation, the landscape for equipment approvals is evolving to prioritize patient safety while fostering advancements.
Content Requirements for a De Novo Request
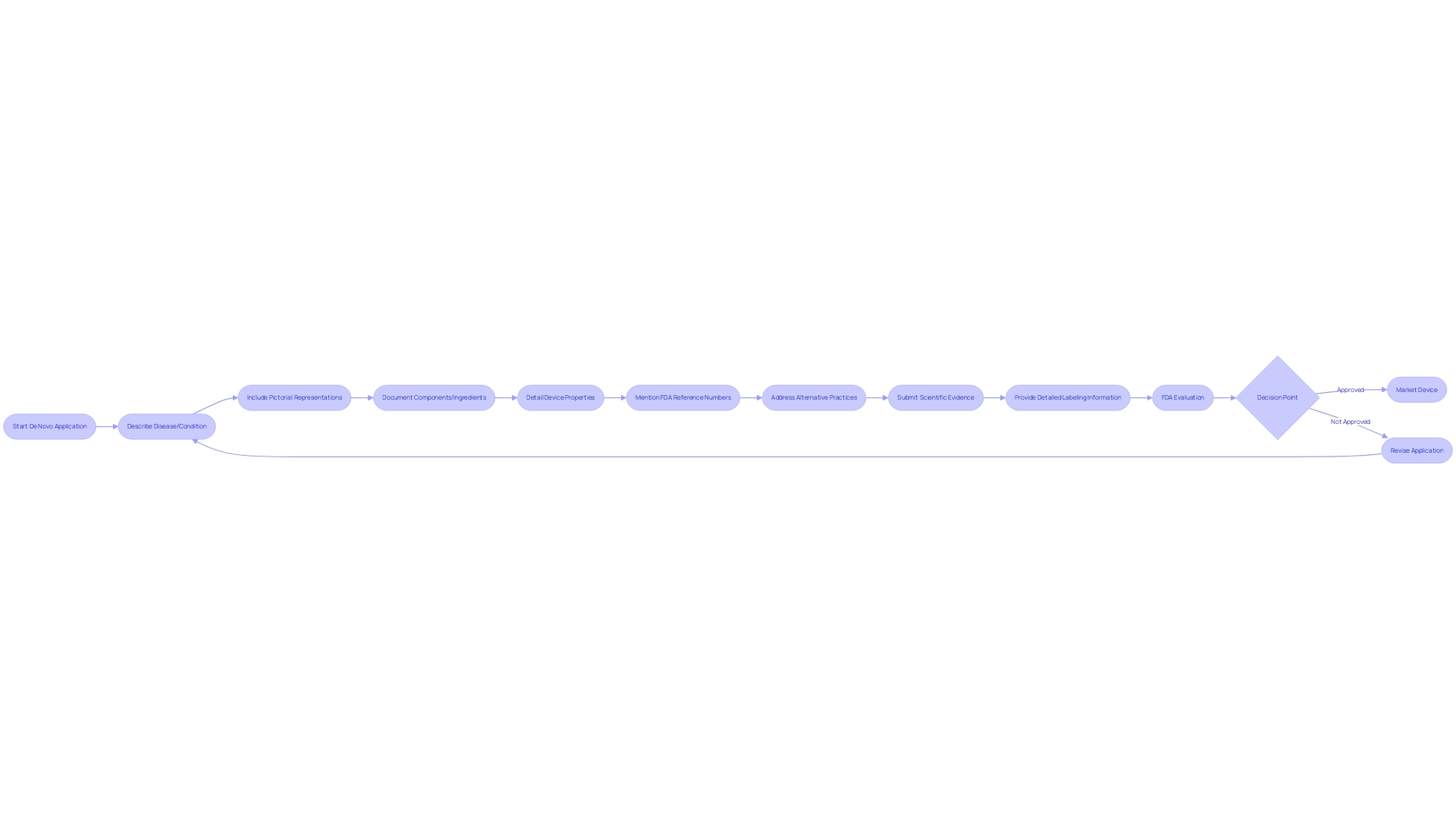
When building a De Novo application for FDA evaluation, it's crucial to incorporate a comprehensive set of data and information that will support the classification determination for the medical instrument in question. The request must elaborate on the intended use of the equipment and provide a detailed description, which includes, but is not limited to, pictorial representations, specifications, and engineering drawings. A clear depiction of the equipment's components or ingredients is necessary if it comprises multiple elements. Furthermore, characteristics of the apparatus that relate to its function in diagnosing, treating, preventing, or alleviating any ailment or state should be exhaustively recorded.
In addition to the description of the physical and functional aspects, the request should mention the appropriate FDA reference numbers for any lawfully marketed equipment intended for use with the subject product, such as accessories or components. The submission must also address alternative practices and procedures for the condition the apparatus aims to manage, providing context for the apparatus's necessity and utility.
A De Novo request is unique in that it is utilized for new medical products that do not have a legally marketed precedent. This route, which was not fully utilized in its initial 15 years, permits the categorization of these instruments as either Class I or II. Effective classification not only creates a fresh regulatory classification with necessary controls but also designates the equipment as a precedent for future 510(k) submissions. The inherent distinctiveness of the De Novo process underscores the significance of submitting a request complete with robust scientific evidence and detailed labeling information to demonstrate the product's safety and effectiveness.
Pre-Submission Phase: Importance of FDA Consultation
In the intricate process of obtaining approval for healthcare equipment, the De Novo pathway serves as a critical avenue for introducing innovative and low-to-moderate risk apparatus to the market. Engaging with the FDA prior to submitting a request is not only advised but pivotal. It provides a structured opportunity to clarify regulatory expectations, refine submissions, and navigate the complex landscape of medical equipment governance.
The pathway, while providing an alternative to the more common 510(k) clearance, comes with its own set of challenges and considerations. As regulatory agencies like the FDA work to assure safety and effectiveness, they also cater to the dynamic nature of emerging technologies. The organization's latest draft guidance on the Q-Submission Program highlights the significance of interaction between developers and the FDA, offering clarity on processes such as Pre-Submissions that are crucial during the De Novo request phase.
Case studies in the United States, along with global instances, highlight the diverse governance of healthcare technology, recognizing the achievements and challenges experienced over time. These studies emphasize the importance of addressing ethical, legal, and social issues early on, which can be facilitated through proactive FDA engagement. This early dialogue can inform developers about market incentives, intellectual property rights, and ethical considerations that are integral to the evolution of medical technologies.
The FDA’s commitment to public health is evidenced by new regulations, like the finalized rule for direct-to-consumer drug advertisements, ensuring clarity and transparency. Likewise, for new submissions, effective communication is crucial, and manufacturers are encouraged to participate in consultative discussions with the FDA to guarantee that their devices comply with safety and efficacy standards.
Recent statistics reveal a surge in the posting of decision summaries for fresh requests, signifying a responsive and evolving regulatory framework. This trend highlights the FDA’s efforts towards transparency and timely review processes, aligning with the needs of the industry for efficient pathways to market.
In summary, initial consultations with the FDA provide invaluable insights into the regulatory landscape, assist in addressing any potential issues, and ultimately streamline the submission process. This initial involvement establishes the foundation for a more robust application and promotes a collaborative environment for innovation in the development of healthcare equipment.
Submission and Review Process
The classification process known as De Novo is a pathway for medical instruments that are innovative and not substantially equivalent to any legally marketed product, requiring a thorough review by the U.S. Food and Drug Administration (FDA). A De Novo request must encompass comprehensive information, including a general description of the product and its intended use, which covers all labeled patient uses whether it is prescription or over-the-counter. Thorough specifications, like engineering drawings and pictorial representations, are crucial in illustrating the design and function of the equipment. Additionally, the inquiry should describe every part or element if the apparatus consists of multiple components, and the characteristics pertinent to its intended effect on disease diagnosis, treatment, prevention, cure, or structural or functional alteration of the body. It's also crucial to include any relevant FDA-assigned reference numbers for legally marketed products intended to be used in conjunction with the new equipment. A comparative analysis with existing alternative practices known to the requester is also required to provide a comprehensive backdrop for the FDA's evaluation.
Throughout the evaluation procedure, the FDA's divisions and experts thoroughly evaluate the submission to guarantee the safety, effectiveness, and security of the proposed healthcare equipment. The FDA, a crucial component of the U.S. Department of Health and Human Services, is entrusted with the duty of protecting public health by ensuring the aforementioned attributes of healthcare instruments. They also oversee the food supply, cosmetics, dietary supplements, products emitting electronic radiation, and tobacco regulation. Submissions are open to public comment, although it is the responsibility of the commenter to safeguard confidential information. Such details, if deemed confidential, should be submitted via written paper submissions following specific guidelines.
The review timeline and involved steps are structured to facilitate a thorough assessment while adhering to the goal of timely approvals for innovations in healthcare technology. This thorough procedure highlights the FDA's dedication to upholding stringent criteria for new medical instruments joining the market, guaranteeing they offer substantial advantages to patient populations while minimizing potential hazards.

Acceptance and Substantive Review
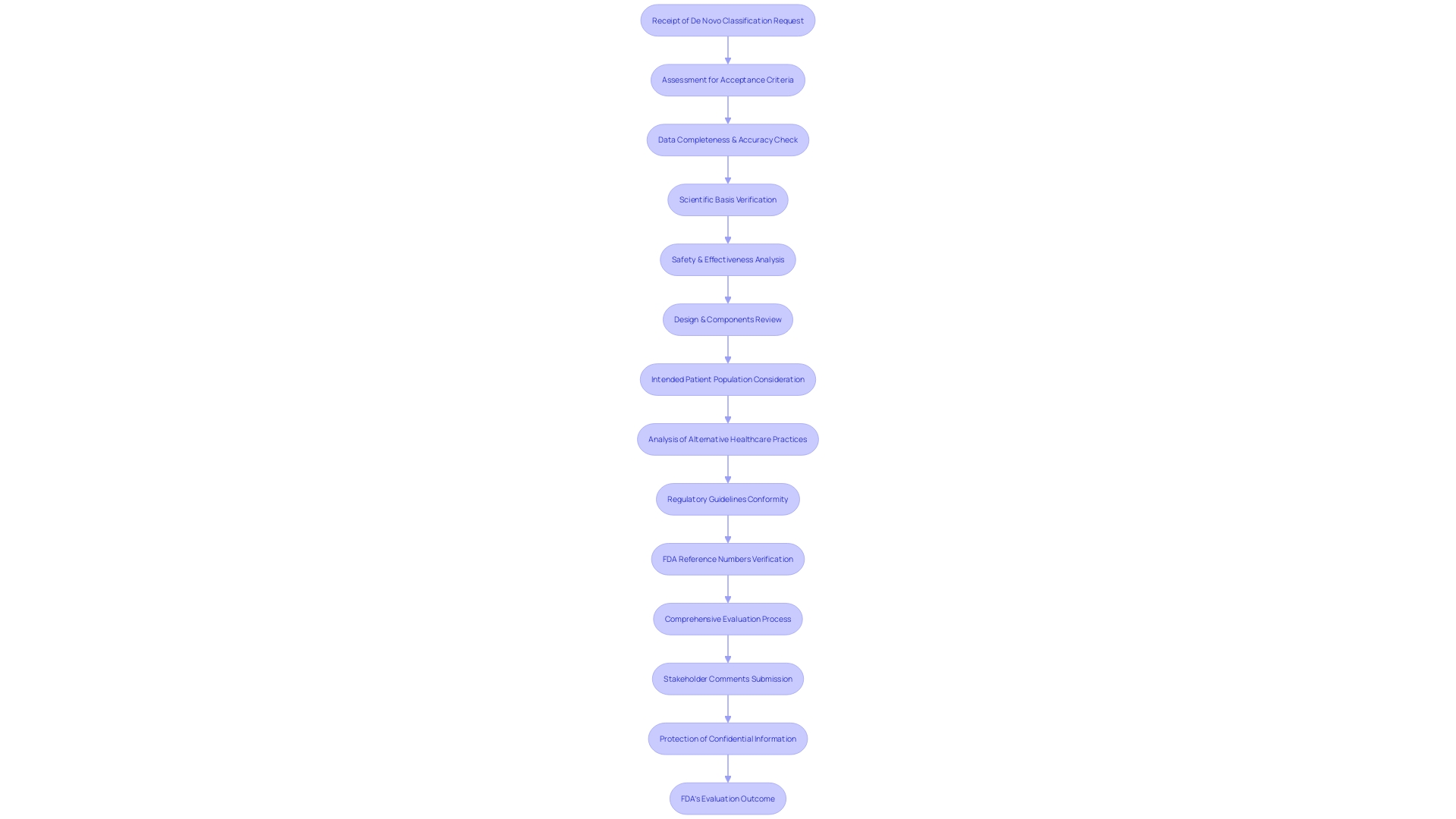
Upon receiving a De Novo classification request, the FDA embarks on a rigorous assessment to ascertain whether the submission meets specific criteria for acceptance. This evaluation scrutinizes the provided data for completeness and accuracy, ensuring it presents a solid scientific basis. The FDA's significant evaluation process is crucial, concentrating on thorough analysis of the equipment's safety and effectiveness. It involves a thorough consideration of the design of the apparatus, including pictorial representations and engineering specifications, along with a comprehensive description of its components, properties, and intended patient population. The evaluation also includes an analysis of alternative healthcare practices and procedures, thereby placing the tool within the wider framework of available healthcare options. Furthermore, the FDA confirms the product's conformity with applicable regulatory guidelines, including adherence to any FDA-assigned reference numbers for legally marketed tools designed for use with the new submission. This process not only ensures the integrity of the approval process but also addresses the ethical, legal, and social implications associated with the introduction of new healthcare technology into the market. Stakeholders are encouraged to submit comments during this process, with caution advised to protect confidential information.
Clinical and Non-Clinical Data Requirements
The classification pathway represents a critical avenue for new medical products that do not have a comparable reference but have a low to moderate risk profile. The submission of robust clinical and non-clinical data is crucial to the process, highlighting the requirement for substantial evidence that establishes the safety and effectiveness of the device. This encompasses a variety of studies, which must account for market incentives, intellectual property rights, and other factors that influence the development of emerging technologies.
Clinical prediction models are emblematic of the scientific rigor required for new submissions. These models, encompassing statistical, machine learning, or artificial intelligence methodologies, aim to predict clinically relevant outcomes and ultimately enhance patient care. However, the meaningful translation of these models from academic research to clinical application is complex, as highlighted by the limited clinical adoption of various cancer subtyping schemes despite their academic recognition.
The integrity and quality of data submitted for De Novo requests are paramount. Real-world data (RWD), for instance, must be scrutinized for relevance and reliability to ensure it can support the scientific inferences necessary for regulatory decisions. In line with this, research into the common themes across different quality frameworks for RWD emphasizes the need for data that is pertinent and trustworthy for the specific application at hand.
It is also informative to take into account the wider regulatory framework of approvals for healthcare equipment. In the United States, the FDA categorizes healthcare equipment into three categories based on risk, with class three instruments subject to the most stringent regulatory scrutiny. The FDA supervises approximately 10% of high-risk products, including implantable devices that sustain life. The approval times for these instruments can be lengthy, underscoring the importance of efficient regulatory processes.
Recent movements toward more efficient regulatory pathways and enhanced collaboration between regulatory agencies and industry stakeholders are noteworthy. These actions, expedited by the demands of the COVID-19 pandemic, are aimed at expediting the approval of healthcare tools that fulfill urgent healthcare requirements, with a specific emphasis on digital health and personalized medicine. The duration for approval of a healthcare instrument is affected by the instrument's intricacy, the nature of the health condition it focuses on, and the efficiency of the regulatory system in position.
Benefit-Risk Assessment and Special Controls
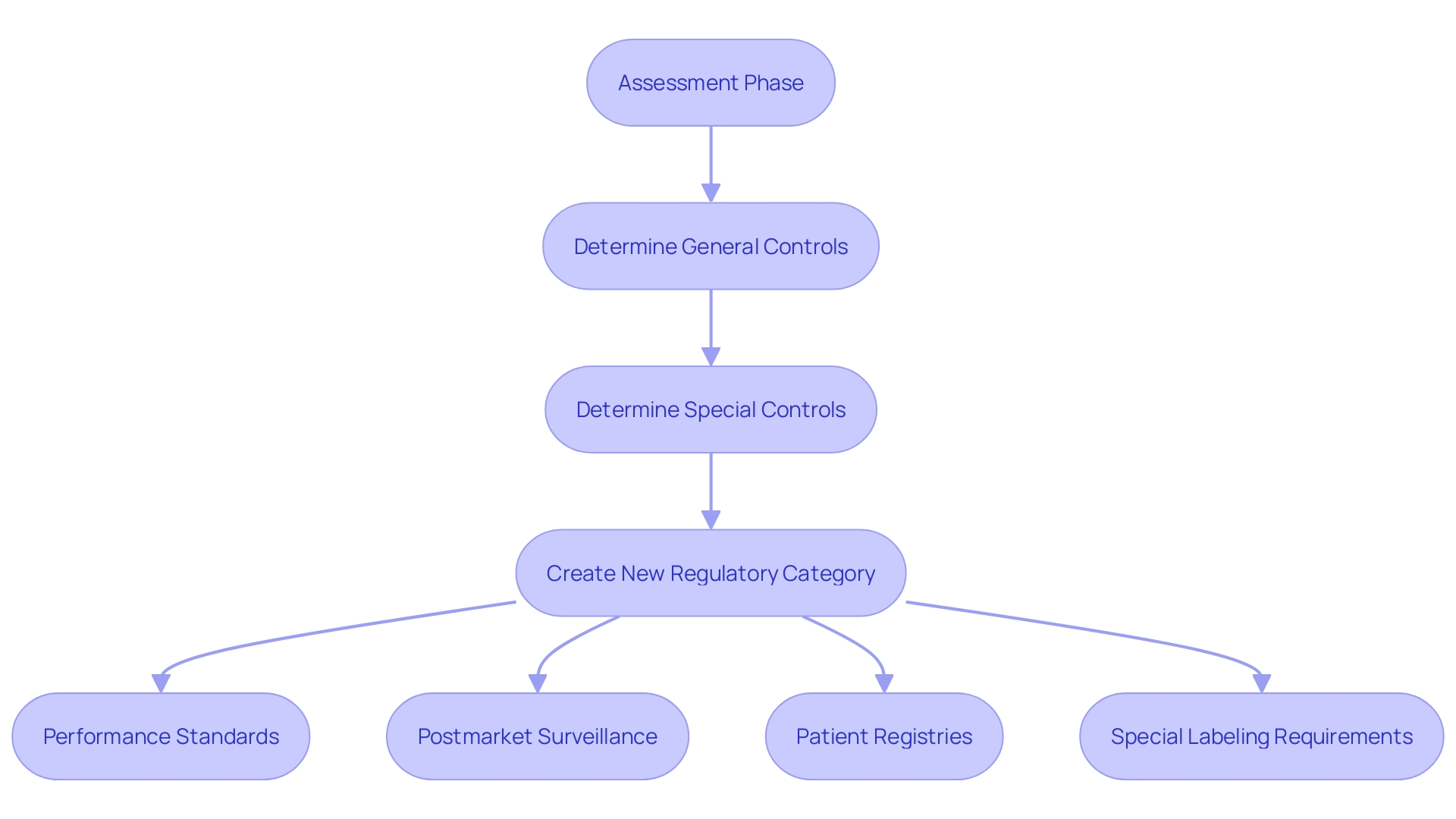
The classification process is a critical pathway for novel medical products that lack a comparable legally marketed precedent. It permits such equipment to be categorized into class I or II, depending on whether general controls or a combination of general and special controls are sufficient to ensure safety and effectiveness. A successful De Novo classification results in the creation of a new regulatory category, including a product code that details the required controls for the equipment type.
During the assessment phase, the FDA undertakes a meticulous benefit-risk analysis to determine if the equipment provides a reasonable assurance of safety and efficacy. In situations where the risks of the equipment outweigh general controls, specific controls may be designated. These are regulatory requisites, such as performance standards, postmarket surveillance, patient registries, and special labeling requirements, which are essential in mitigating potential risks.
One instance of special controls is the necessity for electromagnetic compatibility and electrical safety testing for objects with electrical components. Likewise, for gadgets with software, confirmation, validation, and hazard analysis are required. These controls are designed to be overarching principles that direct manufacturers on the key areas of focus to ensure safety and efficacy. Nevertheless, they frequently allow space for understanding, motivating manufacturers to pursue additional information through decision summaries and classification orders published by the FDA for previously categorized products.
The regulatory landscape continues to evolve, as seen with the Medicines and Healthcare products Regulatory Agency's (MHRA) recent policy intent statement. It aims for international recognition of medical equipment approvals, indicating a move towards reducing redundant assessments and fostering innovation. Similarly, the EU has updated its regulations for In Vitro Diagnostic Medical Devices to enhance patient safety and maintain its leading role in the sector.
In light of these regulatory changes and the process's complexities, manufacturers must stay informed and agile. By utilizing existing regulatory data and comprehending the importance of special controls, companies can effectively navigate the approval process, ultimately introducing innovative products to the market and improving patient care.
Post-Approval Implications and Predicate Device Establishment
Attaining a fresh classification for a healthcare instrument is a crucial moment in the life cycle of a product. However, it also marks the beginning of a new phase of regulatory requirements. After De Novo classification, manufacturers must participate in active postmarket surveillance to ensure ongoing safety and effectiveness of the product. This involves meticulous analysis of data from various sources such as electronic health records and billing claims, to promptly identify and address safety issues that may arise. For example, the FDA has been improving its supervision of medical equipment safety, a crucial issue highlighted by a 2018 research connecting over 1.7 million injuries and 83,000 fatalities to medical tools throughout a ten-year period.
Post-approval, manufacturers must adhere to stringent labeling requirements. Labeling must provide a comprehensive description of the equipment, encompassing indications for use, specifications, and engineering drawings. It should also include the generic and proprietary names, as well as delineate the functional components or ingredients, particularly if the apparatus is comprised of multiple elements. This information serves a dual purpose: it informs healthcare providers and patients about the intended use of the equipment and it establishes the basis for comparison with future devices.
Furthermore, a newly classified product establishes a precedent as a basis for future regulatory submissions. This is crucial for other manufacturers who may want to utilize the established safety and effectiveness profile of a newly developed product for their own items through the 510(k) clearance pathway. The significance of this pathway is demonstrated by the FDA's stringent classification system that categorizes instruments based on risk, and requires the proper registration pathwayâwhether it be 510(k), Pre-Market Approval (PMA), or De Novoâto lawfully market a product in the U.S.
To conclude, the process of a healthcare apparatus does not conclude with De Novo classification. It introduces a stage of watchful postmarket monitoring, careful labeling, and the creation of a fresh benchmark for future products, all of which emphasize the FDA's dedication to protecting public health while promoting innovation in the field.
Case Study: Successful De Novo Classification Example
The De Novo classification pathway serves as a crucial regulatory mechanism for introducing innovative healthcare apparatus to the market, particularly when no existing precedent apparatus exists. An impressive illustration of this is a medical instrument that underwent thorough testing and evaluation to meet the FDA's strict requirements. This apparatus, which underwent independent laboratory analysis, discharged specific chemicals from its foam components, an attribute meticulously recorded over a significant period. A comprehensive review of adverse event reports submitted to the FDA's Manufacturer and User Facility Device Experience (MAUDE) database was also conducted, utilizing Device Events, a sophisticated search tool developed by a former FDA analyst. These efforts culminated in more than 100,000 reports related to the apparatus being scrutinized since 2010, a testament to the thoroughness of the review process.
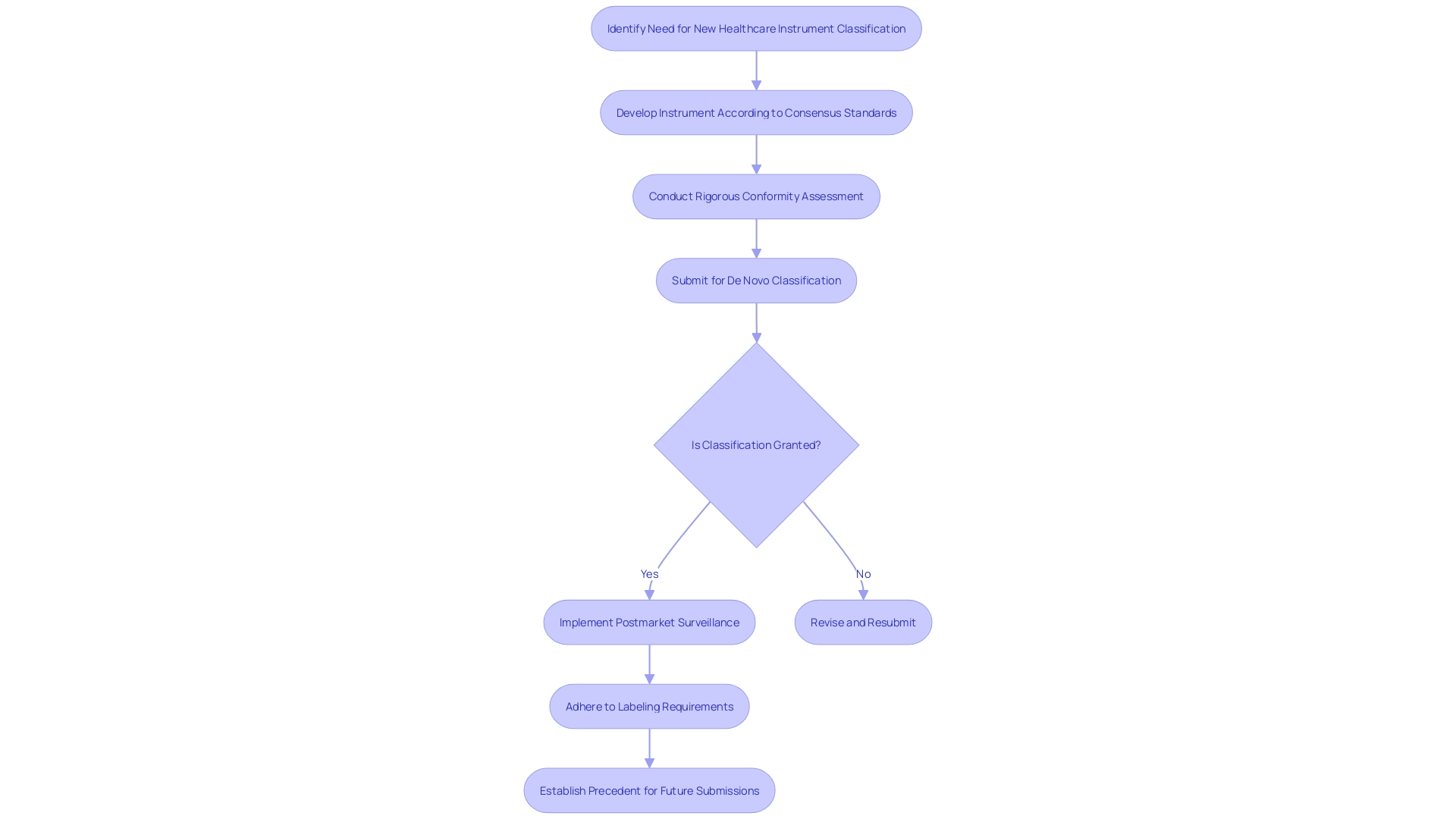
The De Novo pathway, although initially not widely used, has developed to enable the categorization of such distinct instruments, either as class I or class II, based on a risk-based analysis. The procedure not just categorizes the equipment but also creates a fresh regulatory classification and product code, establishing a standard for forthcoming equipment of a comparable kind. The object in question successfully navigated these requirements, thereby becoming the benchmark for subsequent 510(k) submissions and serving as a reference for future market entrants. This accomplishment is especially important considering that, historically, manufacturers were required to initially obtain a 510(k) clearance before pursuing a classification in cases of Non-Significant Risk (NSR) determinations.
Furthermore, the FDA offers decision summaries and classification orders for devices approved through the De Novo method. These documents are priceless resources, offering insights into the specific requirements fulfilled by the recipient. For instance, detailed special controls such as electromagnetic compatibility and software validation are often outlined, though they may leave room for interpretation regarding implementation specifics.
The De Novo process is not only a pathway but also an educational tool for the industry. It offers a plan for adherence and advancement, allowing healthcare equipment to efficiently enter the market while upholding stringent safety criteria. As regulatory agencies continue to refine their processes, the healthcare equipment industry is equipped with clearer guidance and a streamlined approach to bring pioneering products to those in need.
Common Challenges and Best Practices
The classification process, a pathway for innovative medical technology that lacks a comparable predecessor, can present significant challenges for manufacturers. Ensuring a smooth De Novo process involves navigating regulatory complexities and satisfying the rigorous data requirements set forth by the FDA and other regulatory bodies around the globe. For example, class three items, which include high-risk objects like implantable pacemakers, require a meticulous level of scrutiny due to their critical role in patient care. These instruments, comprising approximately 10% of FDA-regulated healthcare equipment, encounter lengthier approval periods because of their intrinsic risks.
To address these hurdles, industry stakeholders have advocated for more streamlined regulatory routes, a push that gained momentum during the COVID-19 pandemic. This movement looks to expedite approvals, particularly for devices that meet previously unaddressed health needs, like those in digital health or personalized medicine sectors. In response to this need for efficiency and collaboration, the MHRA recently announced an intent to acknowledge international regulatory approvals, aiming to reduce redundant assessments and prioritize innovative solutions.
Moreover, adoption of advanced technologies, such as the DeepView diagnostic tool, promises to enhance treatment insights with its predictive capabilities. This innovation, supported by advanced algorithms, demonstrates the move towards more efficient and accurate interventions. However, manufacturers must first understand the specific regulatory landscape for their product, as outlined by the MHRA, to ensure compliance and successful market entry.
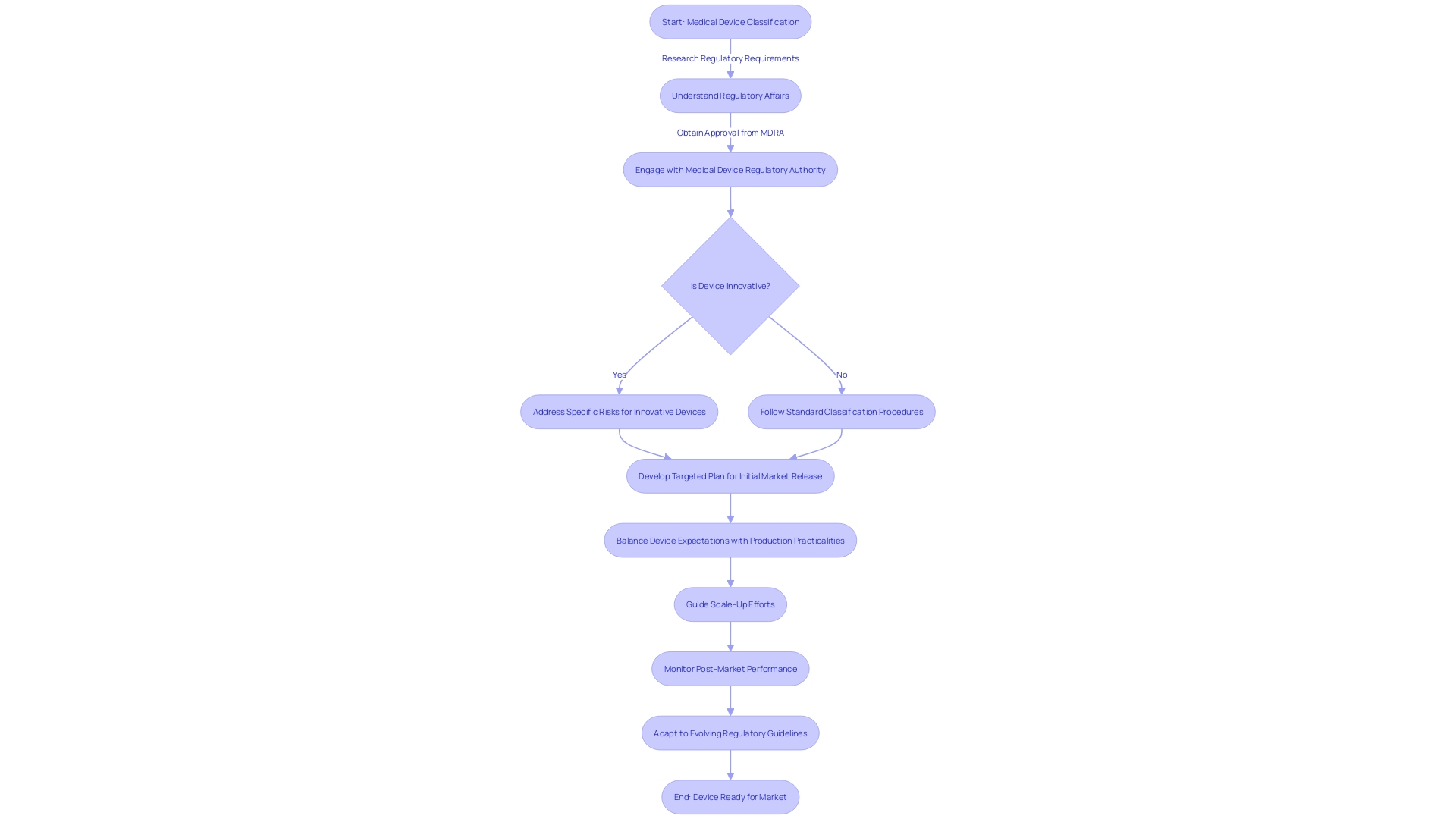
Considering these dynamics, developers of healthcare equipment are recommended to create a targeted plan for their initial market release. It's essential to balance the expectations of the medical device with the practicalities of production, ensuring a safe and effective initial offering. Success in the market will then guide subsequent scale-up efforts.
Conclusion
In conclusion, the De Novo classification pathway is a crucial regulatory conduit used by the U.S. Food and Drug Administration (FDA) to evaluate novel medical devices without a valid predicate. It ensures that new devices meet safety and efficacy criteria before entering the market. The De Novo classification is reserved for devices lacking a comparable product already in circulation, requiring a unique review process.
Manufacturers seeking De Novo classification must meet FDA eligibility criteria, providing a comprehensive profile of the device, including intended use, patient population, and specifications. Understanding the submission pathways is essential, as devices are categorized into three risk-based classes.
The submission and review process involves a thorough FDA assessment to ensure device safety and effectiveness. Robust clinical and non-clinical data must be provided. Engaging with the FDA before submission is advised to clarify expectations and refine applications.
Post-approval, manufacturers must conduct active surveillance and adhere to stringent labeling requirements. De Novo classification establishes the device as a predicate for future submissions, contributing to the regulatory landscape.
Despite challenges, efforts are being made to streamline regulatory routes and enhance collaboration between regulatory agencies and industry stakeholders. Staying informed and agile is crucial for manufacturers in this evolving landscape.
In summary, the De Novo classification process is critical for introducing innovative medical devices. Manufacturers must navigate regulatory complexities, provide robust data, and engage with the FDA for compliance and market entry success. The FDA's commitment to patient safety and innovation is evident throughout the De Novo process.




