Introduction
Design controls in medical device manufacturing are essential for ensuring that devices meet their intended purpose and address the needs of a wide range of users within the healthcare ecosystem. This article explores the various phases of design control, emphasizing the importance of user-centered design, compliance with regulations, risk management, and the integration of digital technologies. It also highlights the significance of design history files, traceability, and the integration of design controls into a quality management system.
By delving into these topics, we gain insights into the meticulous processes involved in developing safe and effective medical devices that enhance patient care.
What Are Design Controls?
Establishing control procedures in the manufacturing of medical products is vital to guarantee that items not only fulfill their intended function but also cater to the requirements of a diverse range of stakeholders within the healthcare system, including patients, clinicians, and support personnel. The notion of user-centric approach is vital, underscoring the significance of crafting tools that enhance the interaction between individuals and the product across all touchpoints. This strategy is supported by careful user research, usability testing, and iterative processes that improve the product based on the feedback from its real users.
In the present scenario, the incorporation of digital technologies into healthcare apparatuses has greatly revolutionized the experience. Devices that were once purely mechanical, such as surgical instruments, are now becoming advanced with digital capabilities, augmenting the physical world with digital enhancements. This transformation requires a design approach that embraces the merging of digital and physical encounters, guaranteeing that healthcare equipment is not only secure and efficient but also seamlessly assimilates into the healthcare setting.
Design controls also serve to manage the lifecycle of an instrument, from conception through development and into the market. As emphasized by the vice-president of UL Solutions mobility and critical systems group, Mary Joyce, the state of Michigan demonstrates this with its flourishing technology sector for healthcare instruments, providing strong testing and compliance facilities. These controls align with application lifecycle management (ALM) practices and are instrumental in maintaining high standards of quality and safety.
Moreover, the significance of controls on the creation process is emphasized by the direction given for compliance with tools used in healthcare, such as comprehending market reach and regulatory responsibilities. For example, the Conflict Minerals policy of the OECD and the RoHS directive for electronics supply chains are instances of particular regulations that can have an effect on manufacturing of healthcare equipment. Ensuring compliance with these regulations is a crucial element of control processes.
Finally, the importance of managing risks in the design of healthcare equipment cannot be emphasized enough. Experts like Bijan Elahi, with extensive experience in safety risk management for healthcare equipment, advocate for clarity and confidence in practitioners by providing practical guidance for compliance with standards like ISO 14971. This includes establishing risk acceptance criteria and deciding when risk mitigation efforts can be stopped, ensuring that products adhere to the highest standards of safety and quality.
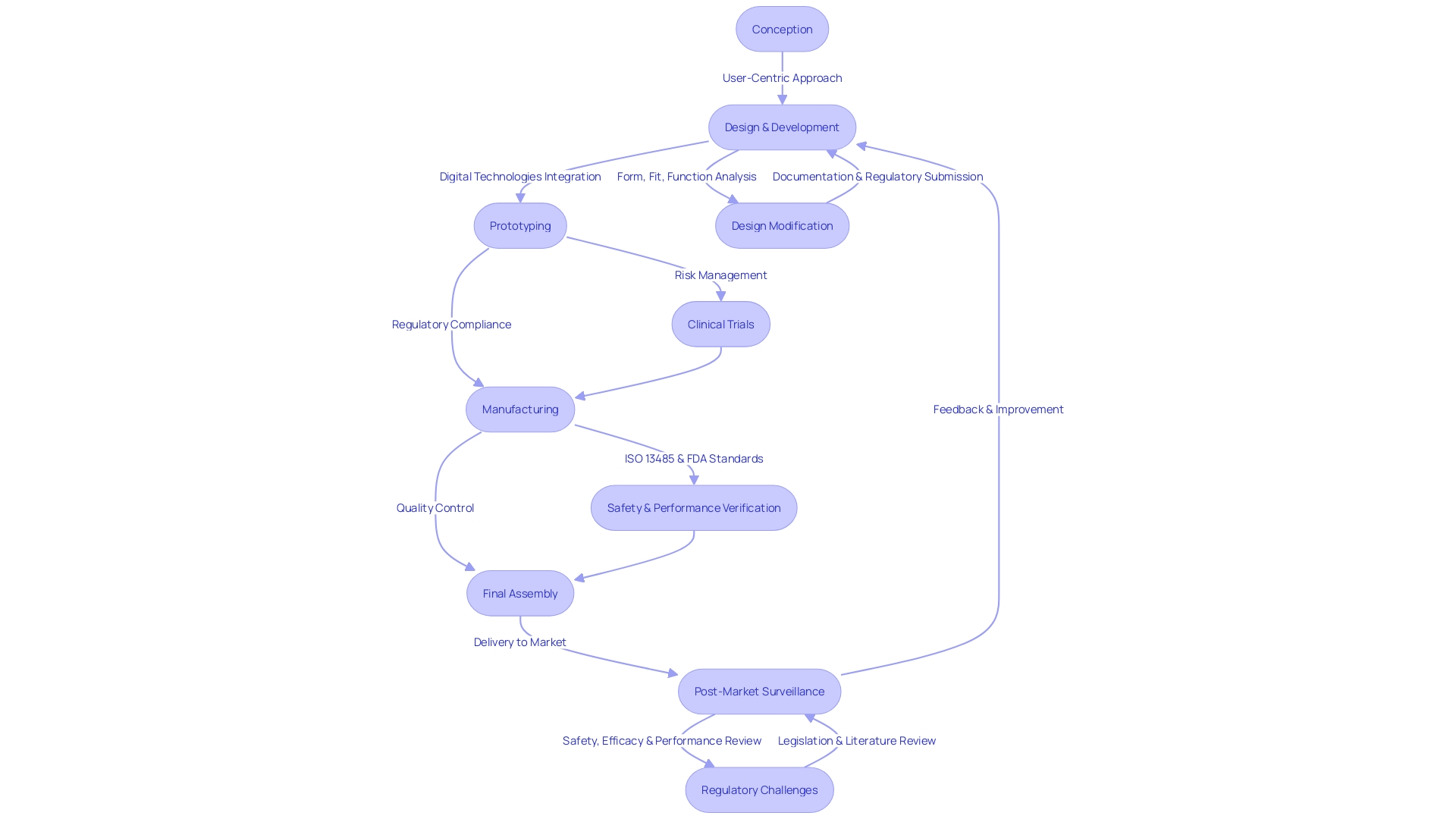
Phases of Design Control
Design control in healthcare instrument development is a multifaceted process that extends from idea conception through to implementation and usage in clinical environments. This iterative process is crucial for ensuring medical equipment not only meet the strict regulatory standards but also align with the nuanced needs of diverse users, including patients, clinicians, and hospital staff. The process emphasizes the significance of constructing gadgets that focus on the unique preferences, behaviors, and experiences of all individuals who interact with them. Service planning, in particular, plays a crucial role in considering the broader context of patient care, acknowledging the impact of nurses, doctors, and support staff on patient outcomes. With the arrival of digital technologies, the distinction between physical and digital experiences is fading, calling for a more integrated approach to healthcare technology creation. Adherence to these principles is not only a marker of quality but also a driver for enhancing safety and effectiveness, as evidenced by recent testing advancements at UL Solutions' Rochester Hills laboratory in Michigan. These efforts emphasize the importance of a focused approach to control that is responsive to both the human and technological aspects of healthcare.
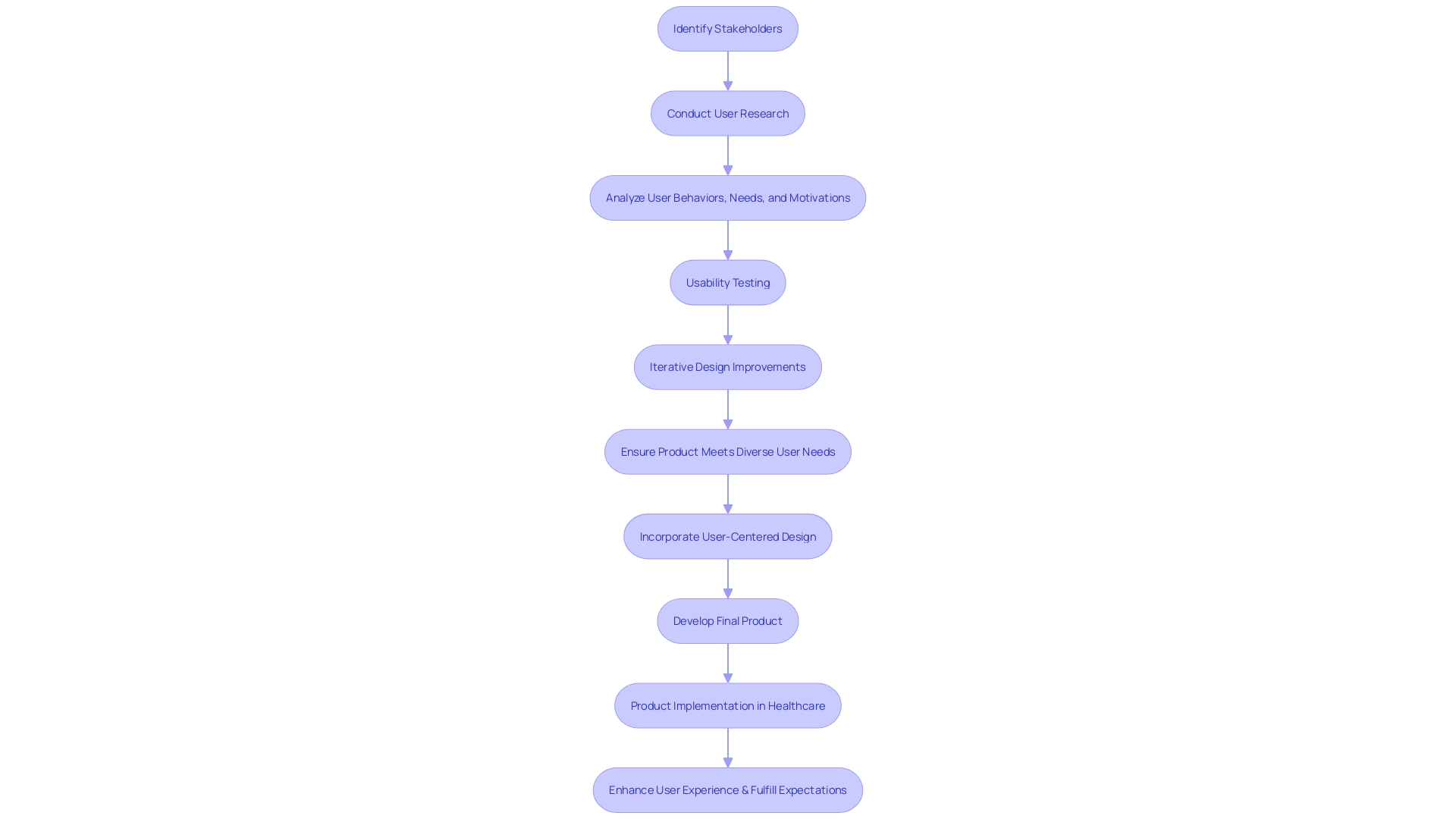
User Needs Phase
In the realm of device development, the 'user needs phase' is paramount. This phase is much more than just an initial step; it's a comprehensive approach to understanding the intricate tapestry of stakeholders involved in a healthcare setting. By focusing on user-centered design, developers aim to address the distinct needs, preferences, and experiences of each user, whether patients, clinicians, or support staff.
- It is critical to recognize that in a medical environment, 'user' extends beyond just the patient. Nurses, doctors, and even maintenance and laboratory personnel are essential to the patient care ecosystem, and their interactions with the equipment are just as crucial.
Designing a product that fulfills all specified criteria but overlooks the genuine requirements of its consumers is a frequent mistake. To prevent this, it is crucial to bridge the gap between user needs and verifiable inputs, ensuring that the apparatus not only operates as intended but also enhances the user experience in a meaningful way.
The procedure includes thorough research on the individuals who engage with it, which includes testing for usability and making iterative improvements to construct a product that is not only efficient but also connects with everyone. This could range from caregivers to cleaning staff, each playing a crucial role in the device's lifecycle.
- To enhance this approach, establishing a Human Factors Engineering and Usability Engineering Plan can direct the development process, ensuring that the individual remains central to product development. It's a strategic move to maintain focus on who the individuals are, what the intended uses of the product are, and the environment in which it will be used.
The importance of this phase is further emphasized by case studies that demonstrate the value of a well-governed system. Take, for example, the Diality system, which was developed to enable healthcare professionals to tailor haemodialysis prescriptions across care environments. Its user-friendly interface caters to both specialists and non-nephrology clinicians, showcasing the effectiveness of incorporating diverse individual needs into design considerations.
Furthermore, as emphasized by industry experts, beginning with a broad perspective on research involving individuals can result in the identification of unforeseen requirements, which can subsequently be converted into possible solutions. This comprehensive yet focused strategy guarantees that the ultimate outcome not only fulfills but surpasses user anticipations, thereby enhancing the standard of care provided through healthcare instruments.
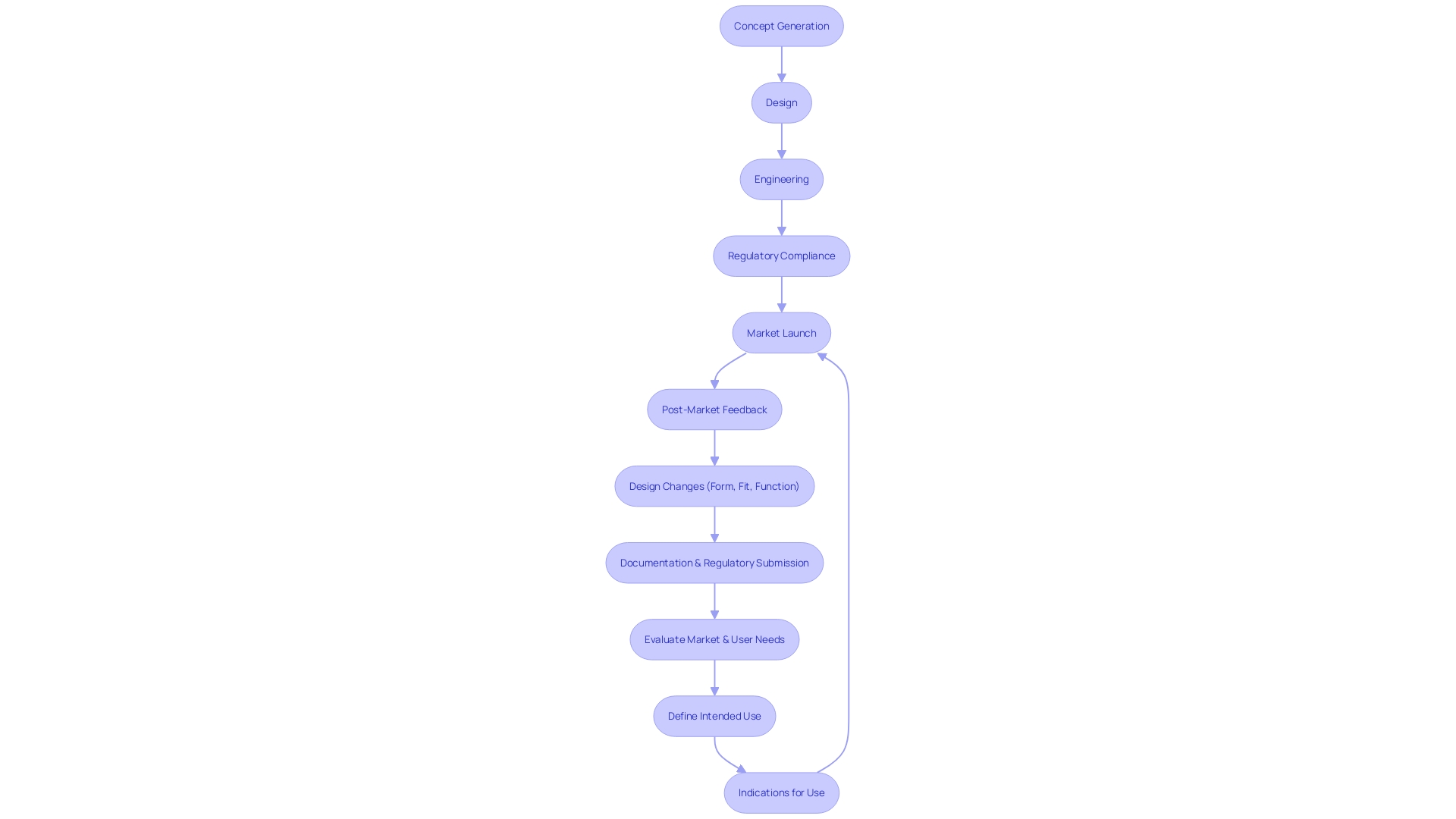
Design and Development Planning Phase
Creating and building a medical instrument is a procedure that starts with conception and extends all the way to its practical implementation in healthcare settings. The crucial initial phase, development planning, necessitates a meticulous strategy that outlines the necessary steps, resources, and projected timelines, ensuring an organized and systematic progression. This stage is important for aligning the product's development with the diverse requirements of its users, varying from individuals to the healthcare professionals who assist them.
Prioritizing the concepts of user-centered approach is crucial, as it focuses on developing tools that meet the particular requirements, behaviors, and situations of the end-users. It's not just patients who are impacted by the construction of a healthcare apparatus; clinicians, support staff, and even maintenance personnel are essential to the patient care ecosystem and must be taken into account during the development process. As pointed out in a presentation to the Industrial Designers Society of America, the intersection of digital and physical realms is reshaping how healthcare products and services are conceived, demanding a holistic approach that accounts for the tangible and intangible aspects of user interaction.
For instance, the Diality system, although not yet approved for sale, exemplifies innovation with its user-friendly interface designed for both nephrology specialists and non-specialists. This system underscores the importance of versatility and ease of use in haemodialysis equipment development, enabling healthcare professionals to tailor treatments to individual patient needs.
When planning the creation of a healthcare instrument, it is crucial to begin with the end in mind—comprehending who the instrument will assist and the effect it will have. This progressive approach should encompass the entire lifecycle of the product, from envisioning the final creation to considering how it will reach the market, the role of the supply chain, and key milestones.
Furthermore, the triumph of a healthcare equipment endeavor is influenced by a blend of determination, persistence, and tactical business choices, as mentioned by an experienced business development leader. Various exit strategies, such as acquisitions, IPOs, licensing agreements, and strategic alliances, are indicators of a successful venture.
In conclusion, the planning and development phase encompasses more than just a preparatory measure; it is a holistic approach that incorporates service creation, individual investigation, usability assessment, and iterative creation to guarantee that healthcare instruments fulfill the complete range of individual requirements, ultimately resulting in successful market acceptance and favorable patient results.
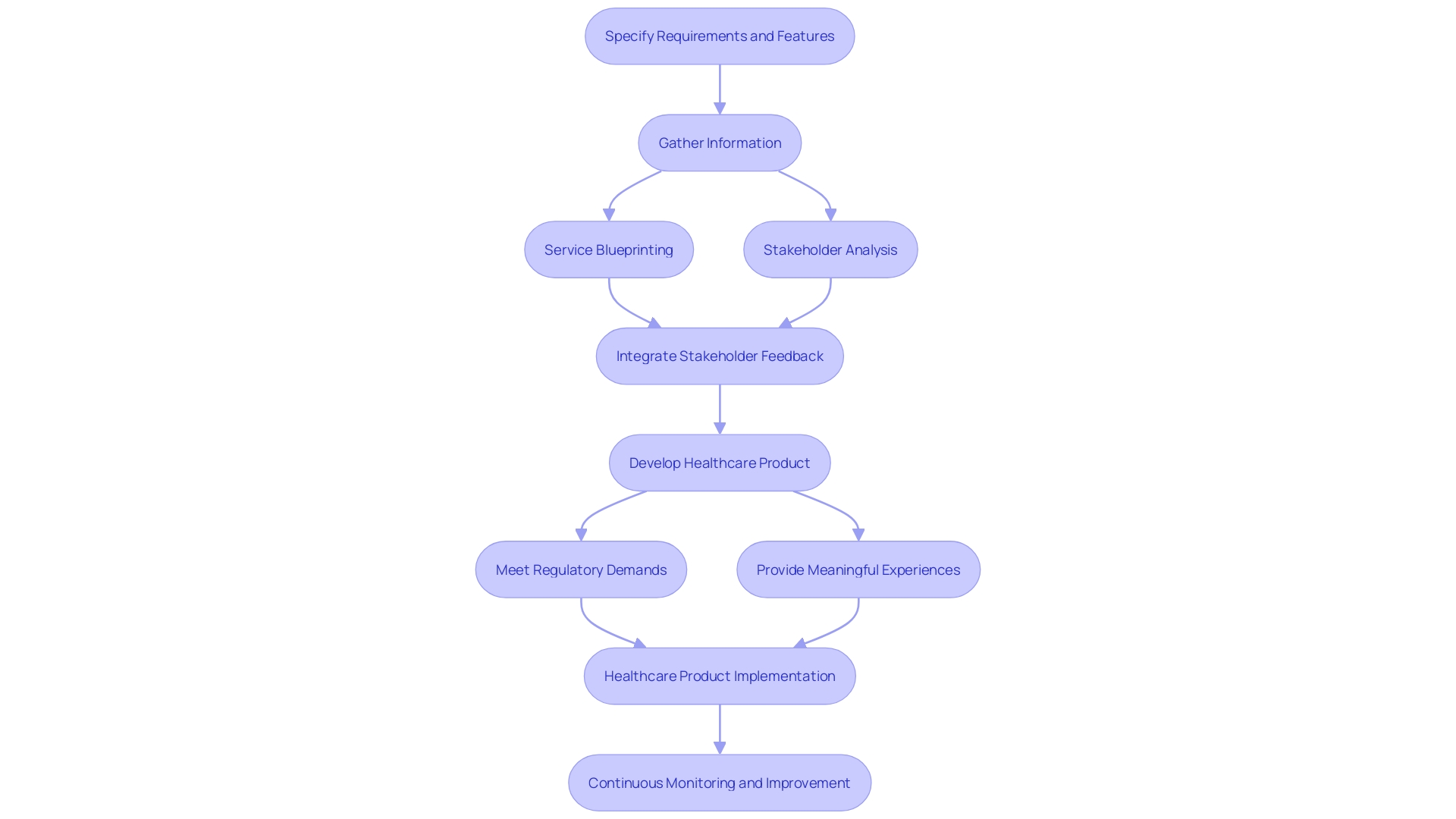
Design Input Phase
The input stage is a crucial step in the development of healthcare products, where the goal is to precisely specify the requirements and features that will guide the whole creation procedure. This stage establishes the foundation, gathering from an extensive range of sources such as feedback from individuals, regulatory standards, and the changing landscape of healthcare technology. In our increasingly digital world, the intersection of physical and digital experiences significantly shapes healthcare products and services. As described in a recent presentation to the Industrial Designers Society of America, the development of medical devices now goes beyond traditional hardware, incorporating digital technologies to enhance user engagement and functionality.
Comprehending the behaviors, needs, and motivations of individuals is crucial, and the extent of 'user' goes beyond clinicians and patients. It encompasses the entire healthcare ecosystem, including caregivers, support staff, and laboratory technicians. The inputs must reflect this comprehensive approach, integrating service blueprinting and stakeholder analysis to ensure a seamless experience for all user groups. The ultimate objective is to create healthcare tools that not only fulfill regulatory demands but also provide significant and relevant experiences to all participants in the healthcare process.
Design Output Phase
In the field of healthcare equipment development, the design output phase is not just a conversion of design inputs into tangible formats; it serves as a crucial link between concept and reality. It involves the development of comprehensive drawings, models, and prototypes—all crucial in shaping the apparatus that will ultimately be produced. However, this phase is marked by an acute awareness that the success of a medical product is not fully known until it reaches the market. Therefore, the emphasis is on distilling the features of the product to what is most essential, aiming for an initial launch that is effective yet not overly complex. This process can be observed in the careful work put into improving tooling and developing a manufacturing process strong enough to deliver a product that is both secure and efficient for patient use.
A poignant example of this nuanced process comes from Lucid Motors' experience in creating the Lucid Air luxury sedan. They required a digital display that could accurately showcase their vehicle's creation at every stage, from initial sketches to the final design freeze. Just like in the creation of healthcare equipment, they encountered the obstacle of representing intricate elements digitally—elements that are vital for capturing the product's groundbreaking characteristics.
When designing medical devices, it is crucial to take into account the entire range of individuals involved, including clinicians, patients, as well as support staff and technicians. The creation must consider their behaviors, needs, and motivations to ensure the product delivers a meaningful and user-optimized experience. This comprehensive approach is captured by the practice of human-centered development, which includes extensive user investigation, usability evaluation, and iterative creation procedures.
For example, Dr. Liz Kwo of Everly Health Solutions emphasizes the significance of early identification in chronic kidney disease, emphasizing the crucial role technology plays in enhancing patient outcomes. Similarly, when designing healthcare solutions at the intersection of digital and physical realms, it is clear that digital technologies are amplifying our interaction with the physical world, as evidenced by the evolution of surgical tools.
Effective healthcare equipment development, thus, is not a straightforward process but a continuous loop of improvement and adjustment, constantly considering the tangible effect it will have on all individuals in the healthcare system.
Design Review Phase
During the design review phase of medical equipment development, the focus is not only on evaluating design outputs but also on identifying potential issues and areas for enhancement. This methodical assessment is essential for meeting quality and compliance criteria, but furthermore, it plays a central role in ensuring that the tool caters to the practical requirements of all individuals involved in patient care. The review process must consider the broad spectrum of users—from patients to clinicians and support staff—since each group's interaction with the equipment can impact patient outcomes.
In-depth user research, usability testing, and iterative development are key methodologies for aligning the device with user requirements. These approaches allow designers to understand behaviors, needs, and motivations, creating products that deliver impactful experiences. As an example, the Quality system, still awaiting approval, created its interface to be user-friendly for both specialists and non-nephrology clinicians, showcasing the significance of inclusive development. Similarly, Cardiawave's non-invasive ultrasound technology for aortic valve treatment is progressing towards marketing in Europe and clinical studies in the US, showcasing the successful application of user-centered principles that resonate with both regulators and end-users.
Furthermore, the FDA's high-priority evaluation of Humacyte's ATEV—an unprecedented product—emphasizes the importance of inventive development that satisfies rigorous regulatory criteria, thus expediting the accessibility of new solutions to patients. In their pursuit of developing secure and efficient solutions, healthcare companies find it necessary to incorporate a comprehensive approach to the healthcare ecosystem that encompasses service and user experience. This wider viewpoint guarantees the smooth functioning of all service aspects, which in consequence, improves the overall effectiveness and efficiency of healthcare tools within the intricate network of healthcare interactions.
Design Verification Phase
Throughout the verification phase, it is essential to guarantee that the medical product layout complies with the specified criteria. This is accomplished through extensive testing, inspections, and analytical procedures that verify the functionality of the apparatus aligns with the intended use. Emphasizing a user-centered approach, design verification entails considering the diverse ecosystem of users—from patients to clinicians and support staff. This comprehensive perspective is essential to the creation of medical instruments that provide meaningful and pertinent encounters for all parties involved.
The verification process not only assesses the physical aspects of a device but also evaluates the integration of digital technologies that are becoming increasingly prevalent in healthcare. For example, the shift from conventional surgical instruments to state-of-the-art digital solutions demonstrates the combination of the physical and digital domains, improving the experience of individuals and broadening the capacities of healthcare professionals.
To address the complexities of healthcare services, a strategic Human-Factors Engineering (HFE) and Usability Engineering (UE) Plan is essential. It raises important questions about the individuals, intended uses, and the environments in which the product will be utilized. This includes understanding the level of training required and the user interface design—both physical and digital touchpoints.
As technology for healthcare instruments continues to thrive, facilities like UL Solutions' laboratory in Rochester Hills, Michigan, exemplify the industry's dedication to advancing the safety, security, usability, and interoperability of medical devices. The testing facility's capacity to adjust verification processes to manufacturers' requirements highlights the significance of versatile and responsive validation procedures in fulfilling the increasing request for healthcare equipment.
Design Validation Phase
Throughout the crucial phase of design validation for medical equipment, it is essential to thoroughly evaluate the performance of the apparatus within the actual context of its intended use. This stage goes beyond bench testing, including clinical trials and other types of studies, to validate the safety and effectiveness of the equipment. In a real-world situation, the evaluation entails a holistic approach that takes into account the diverse interests of stakeholders and the practical compromises in the development and deployment of the apparatus. For example, an adaptive trial approach is used to establish the bioequivalence of highly variable drugs, a method that reflects the nuanced understanding of clinical data analysis. The objective is to ensure that the test and reference drugs do not exhibit significant differences in parameters indicative of bioavailability at the site of action, such as the maximum concentration achievement time or the area under the concentration-time curve. This precise, data-driven procedure is crucial for healthcare equipment to attain their ultimate objective of market acceptance, supported by reliable clinical proof and strategic preparation. Moreover, the approach must be user-focused, considering the requirements not just of patients but also of healthcare practitioners who engage with the system, to guarantee it resolves the appropriate issue and enhances patient results.
Design Transfer Phase
In the crucial transfer phase, manufacturers must navigate the intricate transition from the design and development phase to manufacturing. This procedure is different from typical industry methods, especially because the effectiveness of medical instruments is not completely understood until they are available to the public. It is essential to focus on critical elements, streamlining the initial launch to avoid overburdening the manufacturing process. The investment in tooling and creating a manufacturing process that produces a safe and effective product is substantial and requires careful planning. The labor with Contract Manufacturers (CMS) highlights the difficulty of not only producing a single functional prototype but also guaranteeing the consistent quality of mass-produced units, often a process that can take longer than the development of the product itself.
The transfer of the idea is not only about ensuring that the design is reproducible; it is about preserving the device's intended impact on patient care while considering the entire healthcare ecosystem. From the design's origin, design principles centered around the needs and experiences of all individuals involved, including clinicians, support staff, and patients, play a crucial role. Moreover, rather than looking at users in isolation, it is important to understand their role within the complex chain of healthcare delivery. This comprehensive approach ensures that all interactions and experiences around the medical equipment are optimized, which is crucial when considering that the quality of patient care is influenced by a multitude of stakeholders.
As the healthcare sector develops, the significance of a focused approach to transfer becomes more evident. For instance, the Disability system, still awaiting market approval, exemplifies innovation with its user-friendly interface tailored for both specialists and non-specialists, ensuring adaptability in various healthcare settings. These progressions highlight the importance of careful organization and implementation in the transfer phase of creation to uphold a secure and compliant supply chain, which ultimately contributes to the market success and well-being of the product's users.
To summarize, the transfer phase of the product's development necessitates a comprehensive comprehension of user requirements, a strong manufacturing procedure, and a comprehensive plan that aligns with the intended influence on healthcare. By recognizing the wide range of interactions within the healthcare ecosystem and guaranteeing that the product fulfills these various requirements, manufacturers can enhance the chances of a successful transition from development to market-ready solution.
Design Changes Phase
When considering alterations in the development of medical equipment, it is crucial to acknowledge the influence of these modifications on the entire healthcare ecosystem. The alteration phase is not just a procedural step; it is a crucial point that requires thorough evaluation and documentation to guarantee the instrument remains secure and efficient. This stage must consider the intricate sequence of individuals who engage with the apparatus, from patients to clinicians, nurses, and support personnel.
Service principles emphasize the significance of comprehending these connections to create a tool that smoothly integrates into the healthcare process. By prioritizing the complete service ecosystem, modifications can be handled in a manner that improves the experience for all parties involved. This holistic approach also helps to prevent the pitfall of creating a device that meets technical requirements but fails to address the real-world needs of its users.
In the context of a rapidly evolving healthcare landscape, where physical tools are increasingly augmented by digital technologies, managing alterations is more critical than ever. Companies like Medtronic, with a mission to alleviate pain, restore health, and extend life, exemplify the industry's commitment to innovation and patient-centric care.
As design changes are implemented, transparency becomes paramount. Clear communication about modifications, including their intent and impact on the logic and explainability, is essential to maintain trust and compliance with regulatory standards. This transparency ensures that all updates enhance the product's utility and maintain patient safety, affirming the company's track record in successful product launches and reinforcing its competitive edge in the integration of software and hardware components.
Design History File (DHF)
The Design History File (DHF) acts as a comprehensive record that documents the complete process of creating and enhancing a medical instrument. This document is not only a storage of documentation; it is a representation of the evolution of the product, recording inputs, stages of development, results, important evaluations, activities of verification and validation, as well as any changes made during the procedure.
The creation of the DHF is not a solitary task; it's a collaborative effort that integrates insights from a spectrum of users, encompassing not only patients but also clinicians, maintenance crews, and hospital staff. By taking into account the needs and experiences of this diverse group, the development process adopts a holistic service creation approach, customizing the equipment to not just one kind of end-user but to the entire healthcare ecosystem. This ecosystem-centric design approach guarantees that each stakeholder's specific interactions and relationships are considered, resulting in healthcare tools that are smoothly incorporated into the workflow of healthcare delivery.
In the landscape of healthcare technology, companies like Medtronic are at the forefront, embracing the intersection of digital and physical realms to engineer transformative solutions. As they endeavor to tackle the most formidable health challenges, their mission is to enhance patient care, which is embodied in every piece of technology they develop. Their dedication to innovation is apparent in the digital integration within their instruments, from cardiac tools to patient monitoring systems. These advancements are not merely about digitizing existing documents but about understanding the critical data and leveraging it to drive smarter, more efficient, and more effective healthcare solutions.
The importance of a well-maintained DHF is underscored by the potential risks associated with nonconformance. With patient safety as the priority, any deviation from specified requirements could lead to significant consequences including costly recalls and regulatory penalties. By adopting a data-driven approach, organizations can mitigate these risks, ensuring that every step of the manufacturing and quality control process is documented, traceable, and in compliance with stringent industry standards. Maintaining this level of accuracy relies on the digital continuity of all company functions, allowing access to timely and trustworthy data that guides decision-making and promotes a culture of ongoing enhancement in the development of devices.
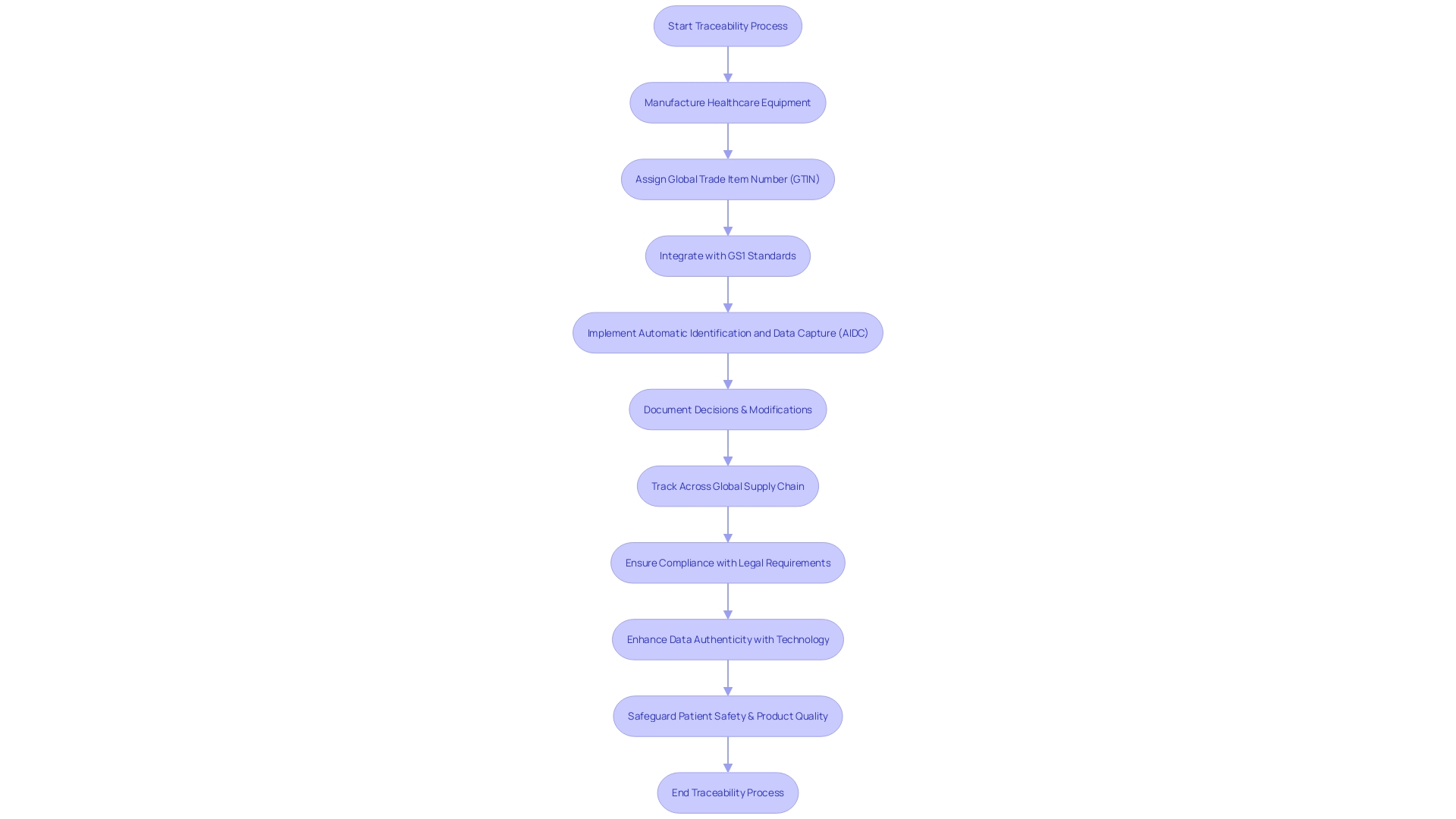
Importance of Traceability in Design Controls
Attaining exceptional performance in control over the creation process is crucial for manufacturers of healthcare equipment, with traceability playing a pivotal role. It ensures that each decision, modification, and activity is meticulously documented and can be traced back to its origin. This level of detail fosters transparency and enables accountability, allowing manufacturers to quickly identify and rectify any issues that may surface, thus safeguarding patient safety and product quality.
Highlighting the significance of traceability, Mary Joyce of UL Solutions showcases Michigan's dominance in technology for healthcare tools and the requirement for excellence and honesty in this sector. Similarly, Veronica Savu, CEO of Morphotonix, underscores the critical role of traceability and authentication in protecting against the proliferation of counterfeit components which compromise consumer safety and industry standards. Savu's approach involves ensuring that each product's journey, from production to end-use, is traceable and that its authenticity is verifiable, a practice that is increasingly vital in light of complex supply chains and material shortages.
In the broader context, design quality, equated to user value, is a measure of a product's ability to deliver business value, user benefit, and systemic impact. This intricate interaction of elements is mirrored in the variety of healthcare tools, which span from basic consumer products to complex life-sustaining equipment. The technologies involved are extensive and multidisciplinary, necessitating a strong framework for managing diversity in both human factors and equipment factors.
From a regulatory standpoint, the changing environment necessitates that manufacturers of healthcare equipment are skilled at navigating new compliance requirements. A global standard, such as the GS1 system, enables consistent traceability across international borders. Furthermore, the industry is recognizing that standards must be simple and equitable to ensure usability and prevent complexity from favoring certain players in the supply chain.
The consequences of nonconformance, often a result of human error or deviations from work instructions, underscore the necessity for precise traceability. The integration of technology such as blockchain-inspired processes, as seen in the textile industry, provides a new vector for data authenticity, reliability, and availability, principles that are equally applicable to medical device traceability. With the goal of perfect traceability, it is essential to start with credible data sources, a practice that upholds the integrity of the entire supply chain and ultimately contributes to high product quality and user value.
Integrating Design Controls into a Quality Management System (QMS)
The importance of incorporating a well-structured control framework within a quality management system (QMS) cannot be overstated. It's not merely a regulatory checkbox but a strategic approach to ensure medical devices meet the highest standards of safety and efficacy. By integrating design controls into the QMS, organizations can benefit from streamlined documentation practices that foster a clear trail from design inception to final product, supporting efficient change management and updates.
Application Lifecycle Management (ALM) tools and controls often work together to maintain high-quality software applications. These tools are instrumental in upholding comprehensive development cycles, similar to the stringent processes required in medical device development. The intersection of ALM and control strategies is particularly relevant when considering Agile methodologies, which can indeed be compatible with regulatory mandates for control strategies. This synergy is crucial for companies aiming to leverage ALM documentation to satisfy control requirements related to the development of products.
Moreover, the digital transformation within healthcare has further catalyzed the integration of physical and digital realms. The convergence is reshaping healthcare solutions, as evidenced by the enhanced digital capabilities augmenting traditional surgical instruments. The effect on strategy is substantial, as it requires an approach that encompasses both the tangible and intangible aspects of healthcare products.
In the context of medical device investigations and manufacturing, controls serve as the cornerstone for ensuring product integrity. The FDA underscores the significance of creation inputs, advocating for a robust foundation of requirements as the linchpin for all subsequent creation endeavors and validation activities. When executed effectively, design controls not only uphold regulatory compliance but also contribute to the longevity and success of the product in the market.
As the industry evolves, so do the tools and systems at the disposal of medical device companies. The bar for successful product launches continually rises, with the integration of software and hardware becoming a pivotal competitive differentiator. In this digitized age of healthcare, companies proficient in navigating the complex interplay between these components are positioned to excel in an increasingly demanding and innovative market space.
Conclusion
In conclusion, design controls are crucial in medical device manufacturing to ensure devices meet their intended purpose and address the needs of users in the healthcare ecosystem. User-centered design, compliance with regulations, risk management, and the integration of digital technologies are key components of design control processes.
The different phases of design control, including user needs, design and development planning, design input, design output, design review, design verification, design validation, design transfer, design changes, and the creation of a Design History File (DHF), all contribute to the meticulous development of safe and effective medical devices. These phases involve user research, usability testing, iterative design, and the integration of digital technologies.
Risk management plays a vital role in design controls, ensuring devices meet the highest standards of safety and quality. Compliance with regulations, such as the OECD's Conflict Minerals policy and RoHS directive, is also crucial.
Traceability is pivotal in design controls, fostering transparency and accountability. The DHF serves as a comprehensive archive that captures the entire journey of a medical device's design and development.
Integrating design controls into a Quality Management System (QMS) is essential for maintaining high standards of safety and efficacy. This integration streamlines documentation practices, supports efficient change management, and ensures compliance with regulatory requirements.
Overall, design controls are essential for developing safe and effective medical devices that enhance patient care. By considering user needs, complying with regulations, managing risks, ensuring traceability, and integrating design controls into a QMS, medical device manufacturers can create innovative solutions that meet the highest standards of quality and contribute to positive patient outcomes.




