Introduction
Design control processes play a crucial role in medical device development, ensuring that products meet quality standards, regulatory requirements, and deliver safe and effective results. These processes involve a systematic approach to managing the design and development life cycle, mitigating risks, and upholding the highest quality. Real-world applications of design controls, such as technologies developed for critical situations like in Ukraine, demonstrate their importance.
Incorporating design controls early in the process can also influence cost-effectiveness and market competitiveness. Quality assurance in MedTech extends across the entire product lifecycle, emphasizing the importance of design control and process validation. The versatility of electronic height gages and the relevance of design controls in Agile methodologies are also noteworthy.
The pragmatic approach to engineering and quality assurance advocated by experts underscores the significance of design controls in medical device production. Overall, design control processes are essential for successful, compliant, and beneficial medical device manufacturing.
Understanding Design Control Processes
Design control processes are crucial in medical device development, providing a framework to ensure that products not only meet quality standards but also comply with regulatory requirements, and ultimately, deliver safe and effective results. A methodical approach to managing the development life cycle, from conception to final production, these processes aim to mitigate risks and uphold the highest quality.
Practical applications of control systems are demonstrated by technologies developed with the US Marine Corps and deployed in critical situations, such as in Ukraine. One such innovation can adapt to any ventilator type and has been evaluated for field use by prestigious bodies like the US Army Aeromedical Research Laboratory.
Integrating controls early can also impact the cost-effectiveness and market competitiveness of a medical device. By taking into account the ultimate costs from the beginning, manufacturers can guarantee affordability and accessibility, which are crucial for widespread adoption. For instance, adopting a platform approach to oligonucleotide process development, as approved by the US FDA, can expedite process development, enabling quicker delivery of drugs to patients without sacrificing quality.
Ensuring high standards in MedTech is crucial, encompassing the entire lifecycle of the offering, including control of the development and validation of the procedure. It's not just about compliance; it's about delivering items that enhance patient outcomes. The significance of quality assurance and control is emphasized by the modifications in regulatory documents for drug-device combinations, which outline the lifecycle management of these products based on real-world experiences.
In addition, the adaptability of electronic height gages supported by Mahr emphasizes the precision and efficiency that can be achieved through strong control measures. The incorporation of such tools in the planning stage can result in high-quality outcomes in medical device manufacturing.
The importance of control measures in the context of Agile methodologies is also worth mentioning. Inquiries regarding utilizing documentation from Application Lifecycle Management (ALM) tools to meet regulatory control requirements are answered affirmatively; with proper understanding and application, Agile can be compatible with regulatory compliance.
Kevin Becker's quotes highlight a pragmatic approach to engineering and quality assurance, advocating for action over paralysis by analysis in development. This perspective is crucial in the ever-changing realm of medical equipment creation and emphasizes the significance of controlling the development process for prosperous, compliant, and advantageous medical tool manufacturing.
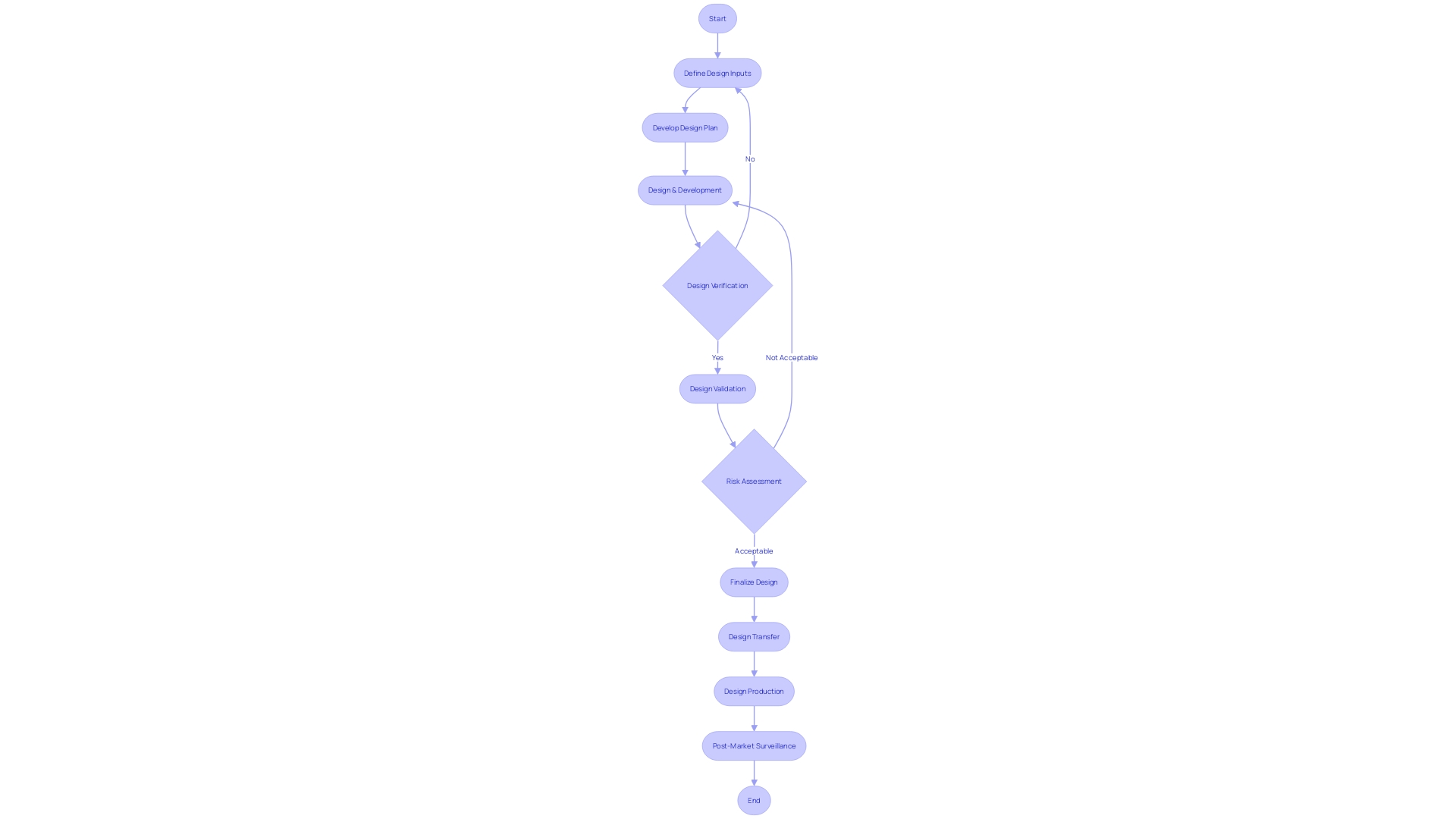
Key Components of Design Controls
Medical device development and manufacturing are regulated by comprehensive control processes that shape the foundation for creating products that meet user requirements and regulatory standards. These processes encompass a structured framework that includes identification of inputs, formulation of outputs, and a series of evaluations through reviews, verification, and validation activities. Furthermore, the transition from creation to production, referred to as transfer of the plan, is carefully controlled to guarantee uniformity and excellence. Following alterations to the structure are meticulously managed to uphold the integrity and adherence of the apparatus.
To ensure resilience in the face of the evolving healthcare landscape, adaptability within these design control components is paramount. This flexibility is demonstrated in the ongoing evaluation and incorporation of clinical data, as highlighted by industry professionals, to tackle the risk of inaccuracies in tools such as In Vitro Diagnostics (IVDs). The Global Medical Device Podcast, powered by Greenlight Guru, underscores the importance of understanding the specific data and performance characteristics that regulatory bodies such as the FDA require for IVDs.
The industry is also witnessing a surge in novel regulatory considerations, particularly in the realm of integrated drug-device combinations and companion diagnostics. These regulations require comprehensive guidance encompassing the lifecycle management of these combinations, labeling requirements for co-packaged products, and consultations for products with ancillary medicinal substances. These changing regulatory environments emphasize the crucial importance of control measures in guaranteeing that medical products are not just efficient but also secure and in accordance with existing and upcoming standards.
In the context of cardiovascular diseases (CVD), the importance of strong control measures becomes even more evident. Progress in CVD management and treatment has been facilitated by meticulous control processes that guarantee the creation of groundbreaking diagnostics and therapeutic tools, which are revolutionizing patient outcomes. This progression is a testament to the significance of maintaining stringent design controls that align with the dynamic nature of healthcare technology and regulatory expectations.
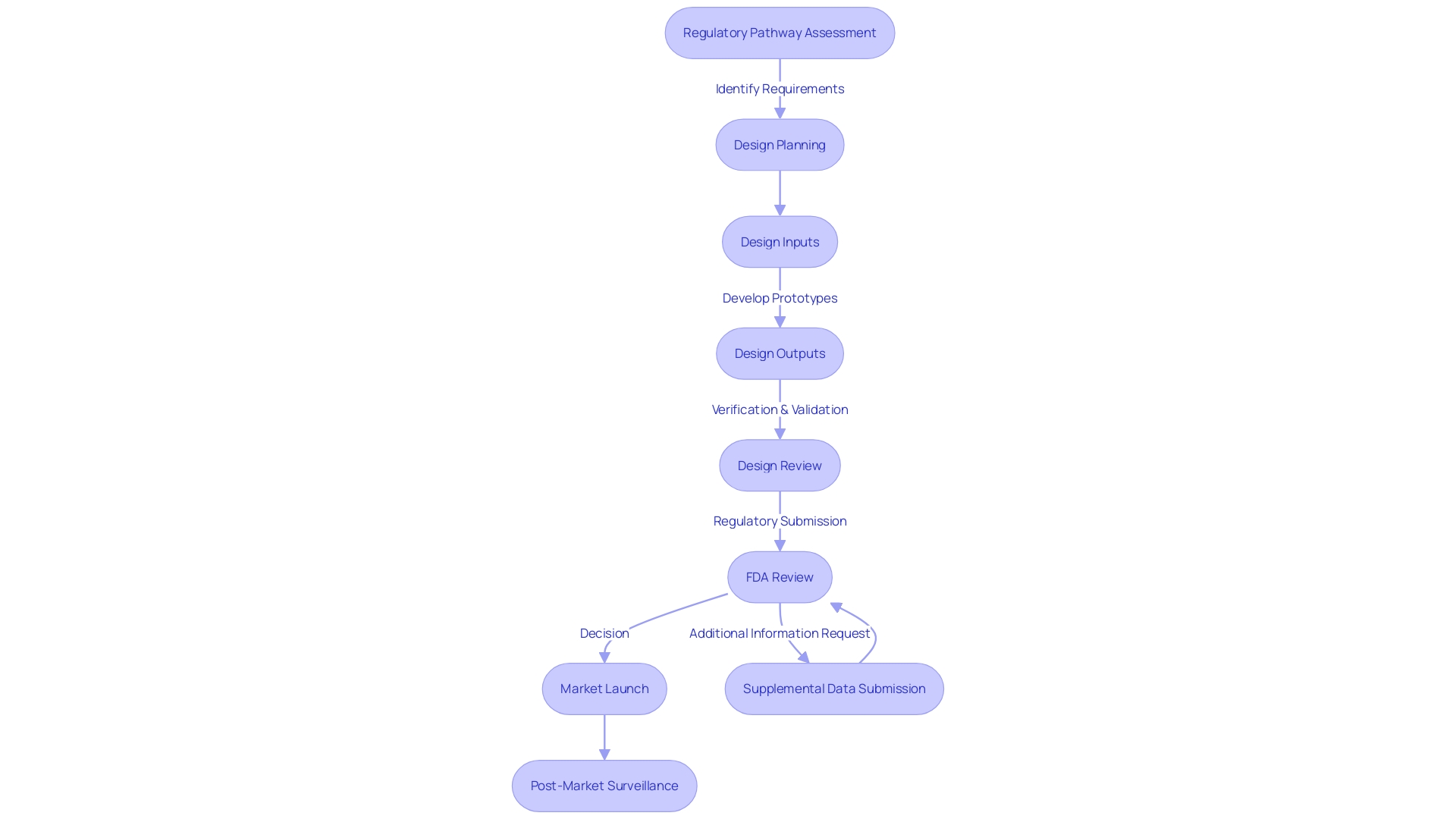
Design Control Process Steps
Design control is an essential element of the development of devices, crucial for ensuring that items are safe and effective. This multifaceted process requires manufacturers to systematically evaluate their product from conception to market launch. As per the insights from UL Solutions, Michigan's thriving medical technology sector underscores the importance of these controls in maintaining a competitive edge in the industry.
The design control process begins with a regulatory pathway assessment, a cornerstone of any sound regulatory strategy. This involves meticulous research into FDA databases to determine the product code, potential submission pathways, and exemptions. Such assessments also guide interactions with the FDA, such as determining the need for Q-submissions and the relevant inquiries to make.
As highlighted by Medtronic's Interstim system's approval in 1997 and recent advances in Crohn’s disease and ulcerative colitis treatments, success in this space requires a clear understanding of regulatory requirements. Manufacturers must integrate digital quality systems effectively, setting clear objectives and realistic goals to align efforts. A phased implementation plan, building on the success of previous stages, ensures a solid foundation for future processes.
Furthermore, the field requires resilience and adaptability, characteristics that prosperous entrepreneurs in the healthcare technology sector exhibit. This resilience is crucial considering the challenges such as supply chain issues post-Brexit, impacting manufacturers of healthcare equipment and professionals alike, as stated in recent manifestos calling for the establishment of a National Stakeholder Forum.
Regulatory guidance documents, such as those for integral drug-device combinations and co-packaged products, provide procedural advice based on the collective experience and actual cases, ensuring products are effectively labeled and managed throughout their lifecycle. These extensive guidelines also encompass consultation procedures for instruments with accompanying therapeutic substances and companion diagnostics.
In the healthcare instrument sector, understanding is crucial. Staying informed through resources like the Global Medical Device Podcast can provide valuable insights into FDA expectations for IVDs, emphasizing the importance of accurate clinical data in regulatory submissions. This information not only informs manufacturers but also equips them to navigate the complexities of bringing innovative healthcare solutions to market.
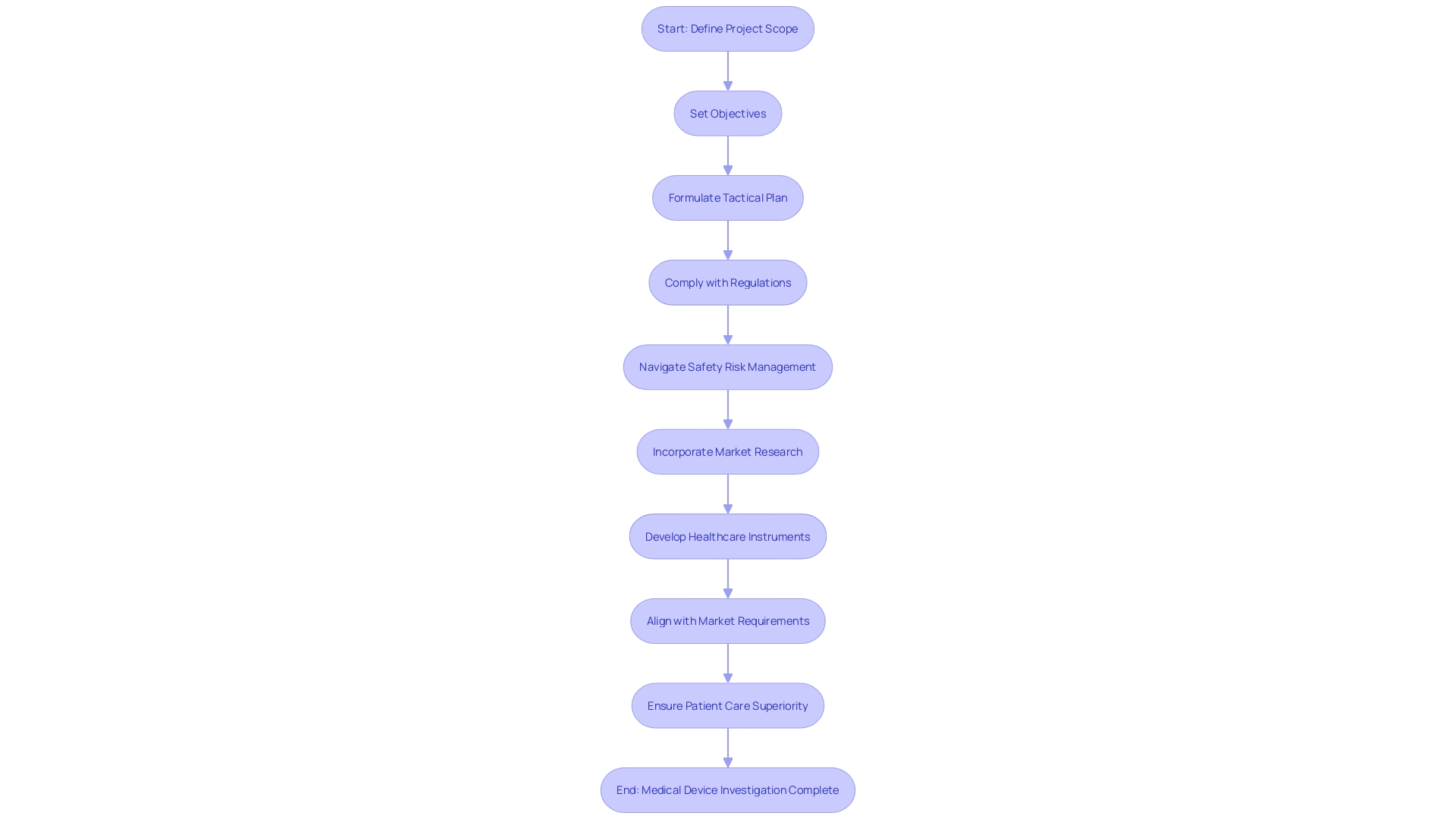
Design and Development Planning
As we begin the control process for medical device investigations and manufacturing, it's crucial to comprehend that planning for development and creation is more than just the groundwork; it's the blueprint for success. The process commences with a robust definition of the project's scope, where clear objectives for design and development are set. A tactical plan is then formulated, outlining the necessary resources, delineating timelines, and specifying deliverables to achieve the set goals.
It's crucial to recognize, as highlighted by Perry Parendo in his analysis of product development, that the focus on compliance and patient health risks must be balanced with a keen awareness of project risk. Truly, it's the synchronization of methodologies—whether waterfall, agile, or set-based design—that customizes the approach to the distinct requirements of healthcare equipment development. The wisdom shared by seasoned entrepreneurs in the healthcare device industry emphasizes qualities such as resilience and adaptability as vital in navigating the intricate journey of bringing a medical instrument to the market.
Furthermore, the growing incorporation of wireless technologies in medical instruments calls for compliance with developing technical standards and regulatory prerequisites. National regulations for radio equipment, for example, must be meticulously complied with to ensure market access. Keeping abreast of market trends, such as the predicted growth of the corneal implant market to nearly $600 million by 2033, provides a backdrop for strategic planning.
In the field of regulatory navigation, the accurate categorization of products and the choice of the suitable FDA registration pathway are fundamental. As industry veterans like Bijan Elahi articulate, the grasp of safety risk management and the clarity it brings to the development process are non-negotiable. The convergence of application lifecycle management and control reflects a shared ethos of continual improvement and quality assurance.
In the end, the cost of healthcare equipment is affected by a multitude of elements, such as the intricacy of treatments and competitive environments. Insights obtained from market research shed light on the customer's viewpoint, unveiling preferences and satisfaction levels with current products, which are crucial in informing excellent product development. So, as we explore the complexities of control measures, it is this versatile comprehension that will direct us toward developing healthcare instruments that are not only in accordance but also align with market requirements and patient care superiority.
Design Inputs: Identifying User Needs and Product Requirements
The creation of medical instruments starts with a crucial stage: establishing the inputs for the blueprint. This procedure involves a systematic assessment of user requirements and specifications, which are based on a comprehensive comprehension of the intended usage of the equipment, the qualities of the user, and the necessary performance features. These inputs serve as the foundation for all subsequent control activities, directing the development process towards a successful result.
Historically, companies like Philips have demonstrated the significance of patient-centric innovation, which is rooted in a deep understanding of what customers genuinely need. This approach has been integral to their growth and continued success, fostering a culture of partnership and collaboration with clinical partners to drive innovation. Likewise, when creating healthcare instruments, integrating input from those involved at the beginning guarantees that the end outcome is in sync with the real requirements of the healthcare industry.
Furthermore, the recent changes in regulatory guidance for healthcare instruments, such as drug-device combinations and companion diagnostics, emphasize the significance of taking into account the complete lifespan of a product from the beginning. The complexity of regulations emphasizes the necessity for thorough design inputs that account for all aspects of an apparatus's use, from integration with other systems to post-market upgrades and enhancements.
Furthermore, the market success of a medical product is not only determined by its technological innovation but also by its cost-effectiveness and accessibility. Designers must consider the ultimate costs from the start, which can impact the affordability, competitive positioning, and adoption rate of the object. Making sustainable choices not only supports the environment but also adds to the long-term sustainability of the item.
Embracing a philosophy that anticipates regular product changes is crucial, particularly for items that contain software or need to interface with other systems. Quality system procedures should be designed to accommodate updates and ensure continuous alignment with user needs. This approach to design inputs not only ensures regulatory compliance but also promotes innovation and enhances the overall value of devices in the healthcare industry.
Design Outputs: Detailed Specifications and Documentation
Design outputs constitute the foundation of medical equipment development, including a comprehensive set of specifications and documentation that precisely outline the physical and functional characteristics of the product. This critical phase articulates the product's attributes through comprehensive technical drawings, precise schematics, robust software code, and specific packaging and labeling requirements. These outputs are crucial for not only guiding the manufacturing process but also for ensuring the equipment adheres to all necessary regulatory standards and performs reliably in clinical settings.
In the field of healthcare equipment investigations, where compliance with strict regulations is an undeniable necessity, design outcomes serve as a concrete plan that connects the abstract design with the tangible outcome. They provide teams with an ultimate reference that enables a methodical approach to quality assurance - crucial for preserving uniformity, dependability, and adherence throughout the lifecycle of the item, from beginning to post-market monitoring. As such, they are essential in the regulatory scrutiny of drug-device combinations, co-packaged products, and companion diagnostics, ensuring every aspect of the device is examined and validated against the highest standards of device quality and safety.
Furthermore, in the rapidly changing area of medical technology, where the incorporation of advanced systems like artificial intelligence (AI) is becoming more and more common, the outputs must also include the intricacy of software components. As AI expands the capabilities of traditional automation by managing complex, non-fixed inputs and outputs, the documentation must reflect this intricacy, ensuring that every component is accounted for and potential vulnerabilities are preemptively addressed. Therefore, the outputs not only tell the tale of the intended functionality of the apparatus but also establish the foundation for a secure and efficient deployment within the healthcare ecosystem.
Design Reviews: Systematic Assessments and Compliance Checks
Control processes are crucial in the development and manufacturing of medical products, ensuring that items meet both regulatory standards and the intended inputs. These processes encompass a series of rigorous evaluations, engaging cross-functional teams to meticulously examine each design aspect for potential issues and alignment with industry best practices.
By utilizing industry examples, we comprehend that the objective is to attain a shared language and set of expectations among all stakeholders—from the FDA to end-users—which simplifies proactive troubleshooting and improves performance reliability. This is akin to obtaining certifications like UL, which, contrary to popular belief, is not exclusive to large corporations but is also attainable by smaller organizations dedicated to quality and safety.
The recent regulatory revisions, informed by firsthand experiences and case studies, offer comprehensive guidance on managing lifecycles of items, particularly for drug-device combinations and co-packaged medical items. This includes detailed protocols for labelling, consultations for apparatus with ancillary medicinal substances, and companion diagnostics. These insights into regulatory expectations are crucial for maintaining compliance throughout a product's lifecycle.
Data indicates that only a limited amount of AI/ML-enabled healthcare equipment have obtained approval, emphasizing the significance of comprehensive clinical trials to evaluate the effectiveness and safety of these technologies. These trials must consider diverse patient demographics to ensure external validity and inform regulatory bodies, healthcare professionals, and the public accurately.
The healthcare device industry's heartbeat is its commitment to innovation while navigating project and patient health risks. With a variety of design methodologies—from waterfall to agile—companies like Ketryx leverage structured, systematic approaches to software development. This not only promotes a culture of rigor but also ensures improved documentation, risk management, and ultimately a compliant product that aligns with the high-quality standards required in the healthcare field.
In the context of global patent filings and grants, companies like SomaLogic lead in Healthtech and nanomedicine innovations, with a significant portion of their patents granted in Canada. This shows the significance of intellectual property in advancing technology and ensuring these advancements are protected and acknowledged within the sector.
Design Verification: Testing and Analysis to Confirm Design Outputs
Design verification is a critical step in ensuring medical instruments meet the necessary design outputs and function as intended. It includes a variety of tests, such as performance, safety, and reliability testing to confirm that the item complies with all specified requirements. For example, in the realm of Electromagnetic Compatibility (EMC), objects are exposed to thorough emissions (EMI) and immunity (EMS) testing. These tests ensure that products operate normally without causing or being affected by interference, a crucial factor as electronic products are frequently used in close proximity to each other. Moreover, the aspect of 'Design for Testability' is often overlooked but holds immense value, particularly in software components of a device. It involves considering testability throughout the process, leading to improved engineering effectiveness, additional testing capabilities, and ultimately, time and cost savings during validation tasks.
The significance of comprehensive verification is further emphasized by the rapid technological advancements in industries like telecommunications, where EMC tests have become a global industry requirement. Additionally, embracing modern statistical approaches such as Bayesian statistics has been beneficial for healthcare companies in regulatory submissions, offering flexibility and valuable insights for decision-making in complex clinical trials.
In the end, the process of confirming the correctness is not only about complying with regulatory requirements but also about guaranteeing the safety and dependability of equipment used in healthcare, which is crucial for the well-being of patients and achieving success in the market. It is a testament to the industry's commitment to excellence and continuous improvement in the face of evolving technological landscapes and regulatory frameworks.
Design Validation: Ensuring Devices Meet User Needs and Intended Use
At the heart of development for healthcare instruments, design validation is the crucial process that verifies a tool meets the requirements of users and is suitable for its intended purpose. This procedure examines the performance of the equipment in its actual service setting, guaranteeing that it functions properly under anticipated circumstances. It is a comprehensive approach, often encompassing clinical evaluations to ascertain therapeutic effectiveness, user studies to verify ease of use and acceptance, and real-world testing to validate consistent performance outside of controlled environments. For instance, in a recent deployment, a new medical technology initially created for the US Marine Corps was subjected to rigorous real-world testing, including field use by the US Army Aeromedical Research Laboratory and the Air Force Medical Evaluation Support Activity, to confirm its adaptability and operation with various ventilator types.
The significance of integrating validation of the blueprint throughout the complete life cycle of the equipment is emphasized by the belief that 'quality system procedures anticipate regular changes in the merchandise.' This is particularly pertinent for devices containing software where upgrades are anticipated, or for devices that must interface with other evolving systems. A strategic approach to design validation means keeping the user at the center of the design process. Drafting a Human Factors Engineering and Usability Engineering Plan is crucial, prompting developers to continually ask who the users are, what the intended uses of the product are, and in which environments it will be deployed.
Quality assurance in medical product development does not solely focus on meeting regulatory requirements; it's about ensuring that products consistently deliver safe and effective outcomes. This multifaceted approach includes Process Validation, ensuring manufacturing consistency, and Design Control, addressing potential risks in the product's structure. The stakes of quality assurance are high, as any shortfall could lead to catastrophic outcomes. In the MedTech sector, companies encounter the difficulties of strict regulations, fast technological advancements, intricate global supply chains, and fierce market competition, all of which emphasize the necessity for thorough validation to uphold quality assurance throughout the product's lifespan.
Design Transfer: Transitioning from Development to Production
Design transfer is more than just a handoff from the development team to manufacturing; it's a crucial stage in medical device production that ensures the device can be produced with the same rigor and precision as it was designed. This procedure includes carefully moving all the documentation, specifications, and manufacturing processes. It's a phase where the focus shifts from the 'shiny' aspects of creation — the sleek engineering and attractive aesthetics that make an item stand out — to the less glamorous, yet vitally important 'Design for Testability'. This idea centers on incorporating testability into the process from the beginning, guaranteeing that every element is reachable and testable, which is not just vital for regulatory compliance but also for the effectiveness of the engineering team and the performance of the end product.
Recent advancements in medical technology underscore the importance of this phase. As an example, the launch of the Vscan Air SL by GE Healthcare is a testament to successful design transfer, where the equipment not only meets the expectations but also delivers clear cardiac and vascular images in a clinical setting. Such innovations highlight the smooth transition from concept to clinic, which is the aim of transfer. It's a strategic process that requires clear objectives, realistic goals, and a phased implementation plan to ensure every aspect of the product's development is ready for production and meets the standards that patients and healthcare providers expect.
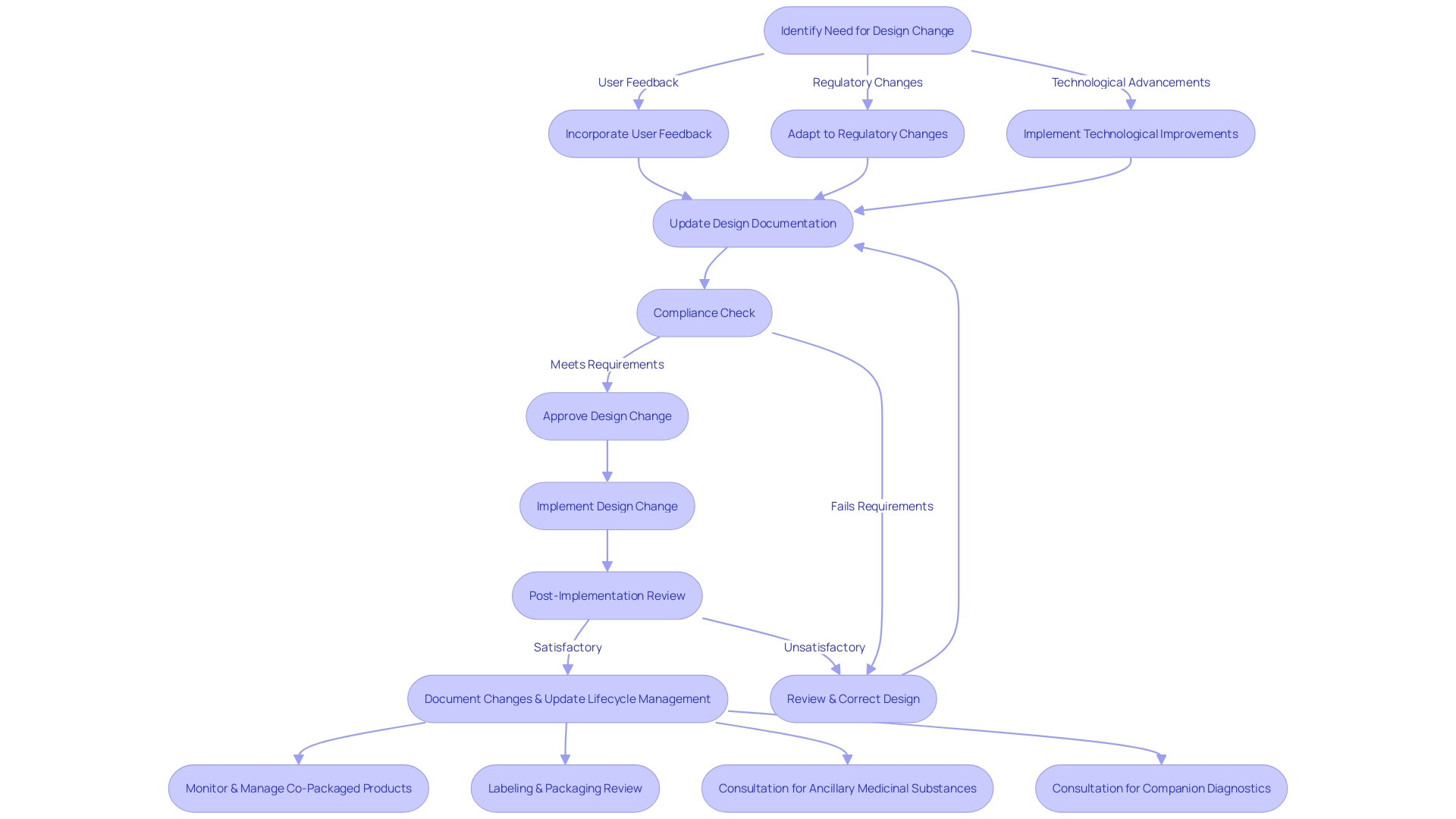
Design Changes: Managing and Documenting Post-Production Modifications
Changes to a medical equipment's blueprint after manufacturing are a crucial part of its life cycle management. These design changes can stem from various sources, including user feedback, evolving regulatory landscapes, and technological advancements. It is crucial that such changes are managed with a meticulous approach to uphold the safety and efficacy of the equipment. As emphasized by the most recent regulatory updates, the incorporation of healthcare instruments with medicines, such as pre-filled syringes, requires a thorough comprehension of both pharmaceutical and equipment frameworks. The regulatory guidance underscores the importance of maintaining detailed documentation for integral drug-device combinations and their lifecycle. In the context of wireless medical equipment, compliance with national regulatory wireless requirements is non-negotiable, given their dual nature as both medical and radio instruments. These scenarios illustrate the continuous requirement for strong control processes that anticipate regular updates, especially in the realm of capital equipment where upgrades are frequent. By engaging in meticulous preparation and following the seven crucial components of control over the creation process, which encompass recorded outcomes, developers can guarantee that their products not only satisfy initial design prerequisites but also adjust effectively to subsequent enhancements or alterations in regulations.
Integrating Risk Management into Design Controls
The combination of risk management and control measures is a pivotal strategy in the medical equipment industry, particularly as it pertains to mitigating potential risks throughout the product's lifespan. A thorough strategy for risk management necessitates manufacturers to proactively recognize, evaluate, and handle risks linked to the product's structure, production, and operational utilization. This integrated process is not only about safeguarding patient safety but also about adhering to the regulatory expectations. As an example, the FDA's focus on integrating cybersecurity into every stage of the software lifecycle highlights the crucial importance of risk management. This implies that from the design phase through development, testing, monitoring, and maintenance, manufacturers must demonstrate through meticulous documentation how cybersecurity risks are being managed, responding to the complexity of modern threats, and ensuring the product's security throughout its total product life cycle. As healthcare equipment become more and more interconnected and dependent on software, the capability to anticipate and navigate potential cyber threats through systematic risk management becomes indispensable. The sector's shift towards all-encompassing hazard management frameworks demonstrates a wider dedication to the safety, efficiency, and security of healthcare equipment in a time where both automation and AI play substantial roles in equipment functionality and patient care.
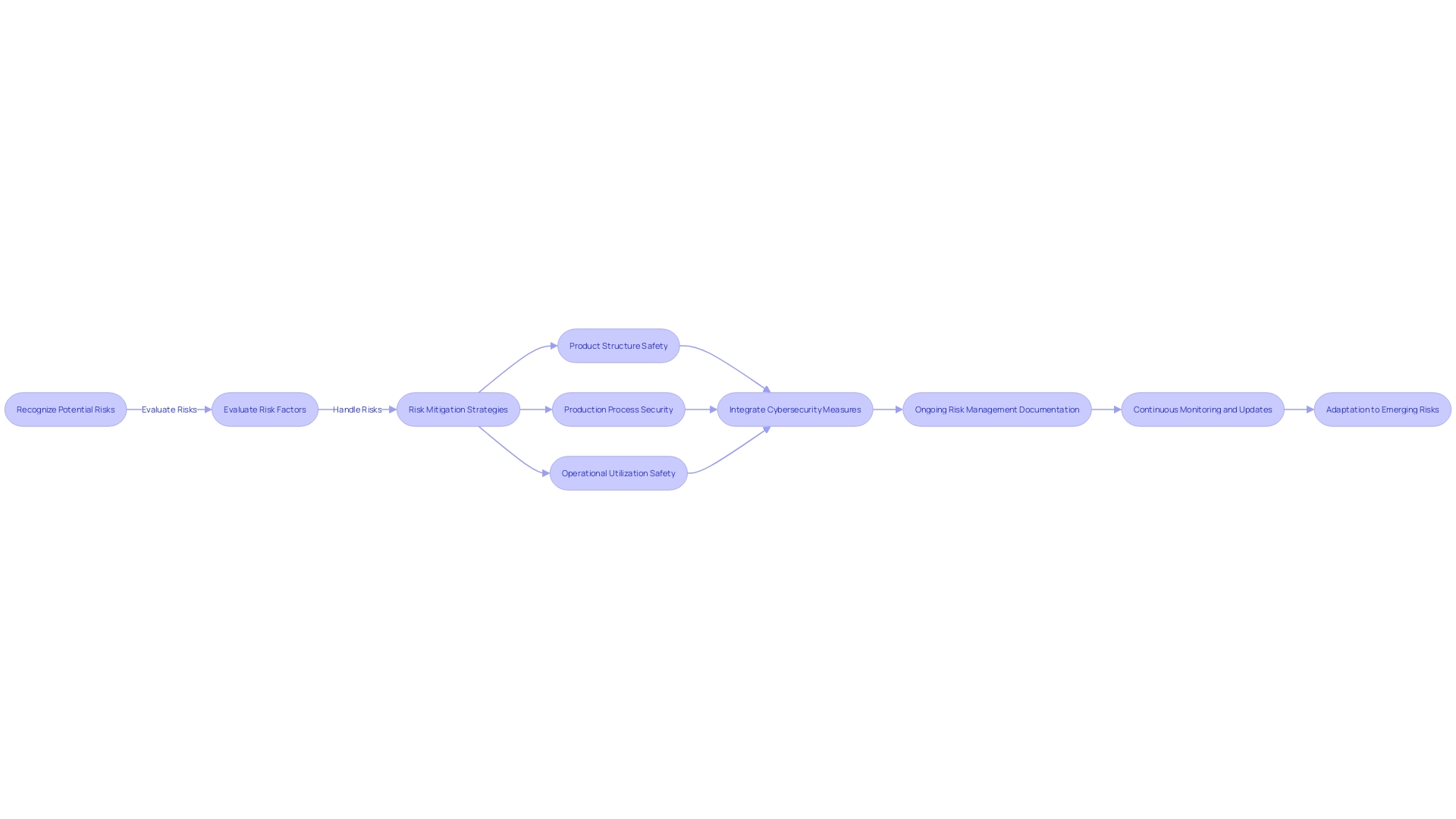
Compliance with FDA 21 CFR 820 and ISO 13485:2016
Ensuring that medical equipment meets strict safety and effectiveness standards is a fundamental aspect of regulatory compliance, with requirements such as the FDA's 21 CFR 820 and ISO 13485:2016 at the forefront. These standards require thorough controls over the structure, covering risk management, documentation, traceability, and a strong quality management system. A crucial update by the FDA has further emphasized the integration of cybersecurity into the entirety of a product's lifecycle, highlighting the need for secure software practices from initial development to post-market maintenance. This includes not just the 'shiny' aspects of an object, such as advanced technical features or aesthetic appeal, but also the foundational elements like design for testability, which although less glamorous, are vital for regulatory approval.
Furthermore, the notion of Electromagnetic Compatibility (EMC) is becoming more and more significant in a world where electronic devices are omnipresent. EMC refers to the capability of a piece of equipment to operate properly in its electromagnetic surroundings without emitting levels of electromagnetic interference that could impact other devices. Regulatory bodies, recognizing the growing complexity of telecommunication products and their susceptibility to electromagnetic disturbance, require thorough EMC testing to prevent interference issues.
In the realm of medical instruments, every step from design to market is thoroughly examined. The FDA classifies equipment into three categories based on patient risk, with each category requiring a specific regulatory pathway—Premarket Notification (510(k)), Premarket Approval (PMA), or De Novo process—to legally market an item in the U.S. Understanding the significance of establishment registration and item listing with the FDA is crucial for professionals navigating the approval or clearance process.
This comprehensive approach to control and lifecycle management ensures that healthcare equipment not only meet current regulatory standards but are also prepared for future challenges, safeguarding both public health and a manufacturer's ability to innovate and compete in the global market.
Best Practices for Implementing Design Controls
For medical device manufacturers, the implementation of controls is not just a regulatory requirement; it is a strategic approach to ensure product quality and safety. To optimize this process, it is essential to create a cross-functional team encompassing various disciplines. This team should work cohesively, with clearly delineated roles and responsibilities, to foster a culture of accountability and transparency. Thorough documentation is essential, recording every decision and activity within the control framework to uphold a strong and traceable development history.
Engaging in open communication with all stakeholders, including regulatory authorities and end-users, is crucial. This conversation ensures that the control process remains aligned with the latest industry standards and regulatory expectations, as highlighted by the Association for the Advancement of Medical Instrumentation (AAMI), which sets the consensus standards for the field.
Undertaking thorough risk assessments at every phase of the development process is essential. As Bijan Elahi, an expert with extensive experience in safety risk management for medical devices, suggests, this is imperative to minimize potential hazards associated with the use of the device. His work emphasizes that risk management is an ongoing process that demands continuous education and vigilance.
Furthermore, the efficiency of controls for the creation process must be constantly monitored and assessed. This proactive approach is similar to how companies such as Xbox, in their dedication to environmental responsibility, have seamlessly incorporated energy efficiency enhancements into their offerings, without compromising the user experience. Likewise, manufacturers of healthcare equipment can incorporate control measures in a way that boosts the advancement of goods without hindering creativity.
Staying abreast of the latest regulatory requirements is also crucial. For instance, the FDA's recent final rule on direct-to-consumer prescription drug advertisements underscores the importance of clear, conspicuous, and neutral presentation of information. This principle is equally applicable to the medical device sector, where clarity and compliance in communication can significantly impact the success and safety of the medical device.
As the industry evolves, so too must the approach to control of creation. The dynamic nature of regulations and the introduction of novel product combinations, such as drug-device integrals, necessitate an adaptive and informed strategy. It is through such diligence and dedication to best practices that manufacturers can achieve excellence in design control processes, ensuring the delivery of safe, effective, and high-quality medical devices.
Conclusion
In conclusion, design control processes are essential in medical device development, ensuring products meet quality standards, comply with regulations, and deliver safe and effective results. These processes involve a systematic approach to managing the design and development life cycle, mitigating risks, and upholding the highest quality.
Real-world applications of design controls, like technologies developed for critical situations, demonstrate their importance. Incorporating design controls early can also influence cost-effectiveness and market competitiveness. Quality assurance in MedTech extends across the entire product lifecycle, emphasizing the significance of design control and process validation.
The versatility of electronic height gages and the relevance of design controls in Agile methodologies are noteworthy. The integration of electronic height gages in the design phase can lead to high-quality outcomes in medical device manufacturing. Design controls can also be compatible with Agile methodologies when used properly, meeting regulatory compliance.
Experts advocate for a pragmatic approach to engineering and quality assurance, highlighting the significance of design controls in medical device production. Design control processes are crucial for successful, compliant, and beneficial medical device manufacturing.
To ensure success, it is important to have a cross-functional team, rigorous documentation, and open communication with stakeholders. Risk assessments should be conducted at each stage, and the effectiveness of design controls should be continually monitored. Staying informed about the latest regulatory requirements and adapting to the evolving industry landscape are also key.
By implementing best practices, manufacturers can achieve excellence in design control processes, delivering safe, effective, and high-quality medical devices. The commitment to design controls is essential for regulatory compliance, patient safety, and competitiveness in the market.




