Introduction
Clinical evaluation reports (CERs) play a crucial role in the lifecycle of medical devices, providing a comprehensive assessment of clinical data to ensure both safety and effectiveness. Essential for regulatory submissions and compliance with international standards such as ISO 14155 and the Medical Device Regulation (MDR), these reports must be meticulously maintained and updated to meet the ever-evolving regulatory landscape. As manufacturers often face challenges with internal resources for exhaustive clinical evaluations, CER consultants become indispensable, bringing their expertise in analyzing complex clinical data and ensuring compliance with current regulations.
A detailed definition of intended use, as mandated by the EU MDR, involves describing the device's purpose, users, patient population, medical conditions addressed, and operational principles. This alignment is necessary for the relevance and feasibility of outcome parameters. Additionally, significant regulatory changes, such as the FDA's plan to regulate laboratory-developed tests (LDTs) as medical devices, highlight the dynamic nature of the regulatory environment.
Manufacturers increasingly adopt digital solutions and outsourcing to manage workload and ensure compliance.
A well-structured CER not only facilitates market access but also plays a critical role in post-market surveillance, maintaining the device's safety and efficacy. As the regulatory landscape continues to evolve, staying informed and compliant becomes more crucial than ever for success in the medical device sector.
Understanding the Importance of Clinical Evaluation Reports
Reports on medical assessments are crucial in the lifecycle of medical devices, acting as a thorough review of medical information to guarantee safety and effectiveness. These documents are essential for regulatory submissions and compliance with international standards like ISO 14155 and the Medical Device Regulation (MDR). The evolving regulatory landscape, with higher expectations from Notified Bodies, necessitates that Cars are meticulously maintained and updated.
Producers frequently face challenges with the internal assets needed for comprehensive assessments, making the role of CER advisors essential. These experts bring significant experience in analyzing complex clinical information, identifying gaps, and suggesting remediation strategies, ensuring that the clinical evaluation process aligns with current regulations. 'Their expertise is indispensable in navigating the intricate balance between an item's intended use and the performance data required to demonstrate safety and effectiveness.'.
The intended use, as defined by the EU MDR, involves a detailed description of the object's purpose, users, patient population, medical conditions addressed, and operational principles. This definition must align with the apparatus's description, indications, and functionalities. The process, though straightforward in concept, can be complex in practice, requiring collaboration among clinicians, patients, and subject matter experts to ensure the relevance and feasibility of outcome parameters.
Moreover, substantial regulatory modifications are approaching, including the FDA’s intention to oversee laboratory-developed tests (LDTs) as medical products, which highlights the ever-changing nature of the regulatory landscape. Keeping pace with these changes is vital for manufacturers, who are increasingly adopting digital solutions and outsourcing to manage the rising workload and ensure compliance.
A well-structured CER not only facilitates market access by demonstrating that a product meets safety and performance standards but also plays a critical role in post-market surveillance, ensuring the item's ongoing safety and efficacy. As the regulatory landscape continues to evolve, staying informed and compliant is more crucial than ever for success in the medical equipment sector.
Key Components of a Clinical Evaluation Report
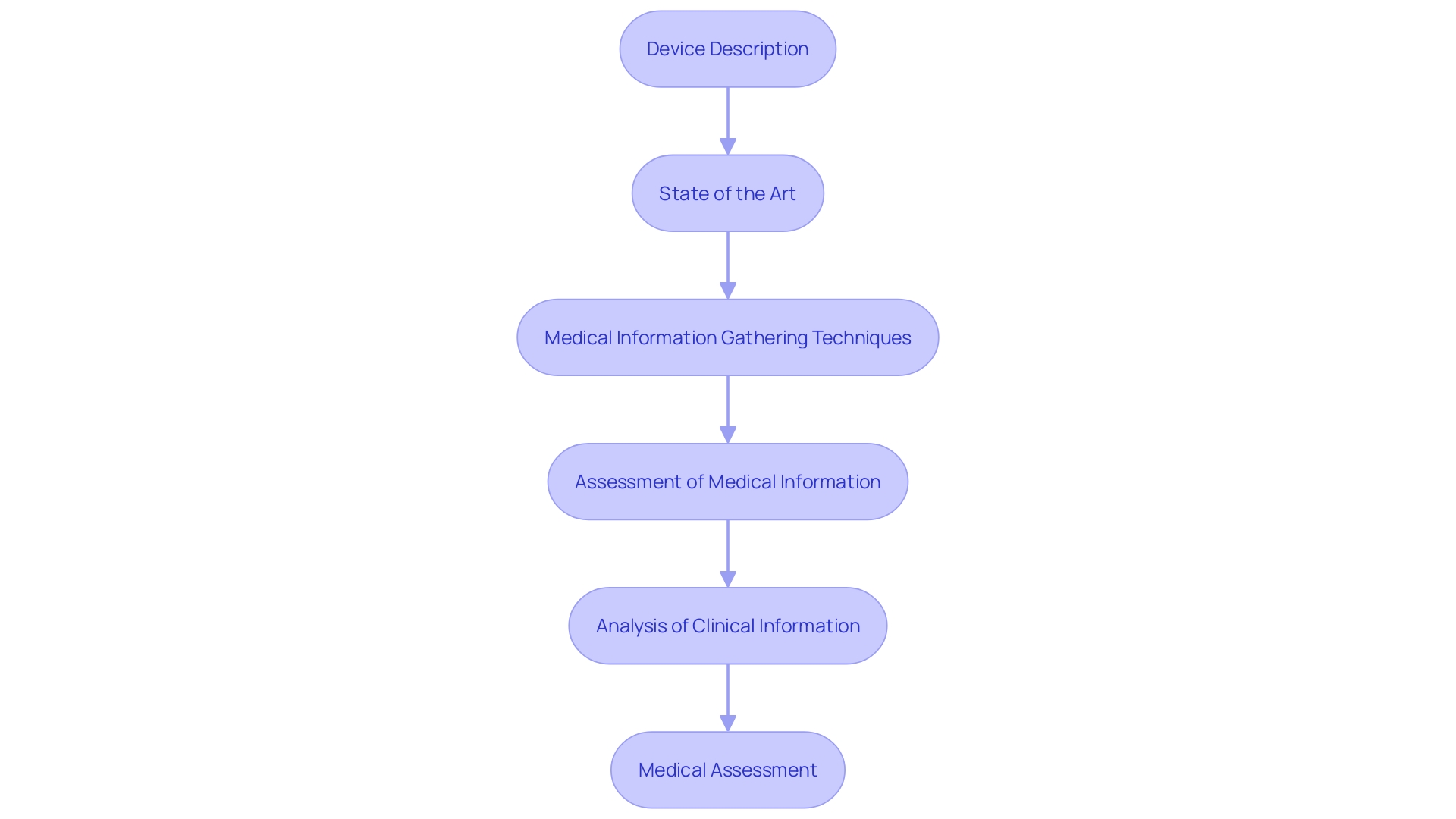
A comprehensive clinical evaluation report includes essential components, each contributing significantly to the overall understanding of a medical instrument's performance and safety. Key sections include:
- Device Description: Detailed information about the device's design, intended use, and operational principles forms the foundation of the report.
- State of the Art: This involves a thorough literature review and analysis of current medical practices, guidelines, and peer-reviewed studies to demonstrate the device's alignment with existing standards and its comparative safety and performance.
- Medical Information Gathering Techniques: Clear documentation of the methodologies used to collect medical information is crucial. This includes descriptions of study designs, patient demographics, and information collection protocols.
- Assessment of Medical Information: Thorough examination of the gathered medical information is crucial. This step often highlights the gap between the intended medical objectives and the actual outcomes, as well as the need for accurate and well-defined endpoints.
- Analysis of Clinical Information: Detailed and coherent presentation of information analysis is critical to avoid discrepancies between conclusions and actual findings. Uniformity across different documents such as the assessment plan, evaluation report, and risk management files is essential for coherence in information collection and analysis approaches.
- Conclusion on Medical Assessment: Summarizing the medical evidence, including a meta-summary of overall findings, and providing a conclusion on the apparatus's ability to meet its intended medical purpose is the final step. This should include both pre-market and post-market information, ensuring a thorough evaluation of the product's safety and performance.
Addressing these components effectively ensures regulatory compliance and facilitates ongoing post-market surveillance, ultimately contributing to improved patient outcomes and advancing medical knowledge.
Challenges and Best Practices in Clinical Evaluation Reports
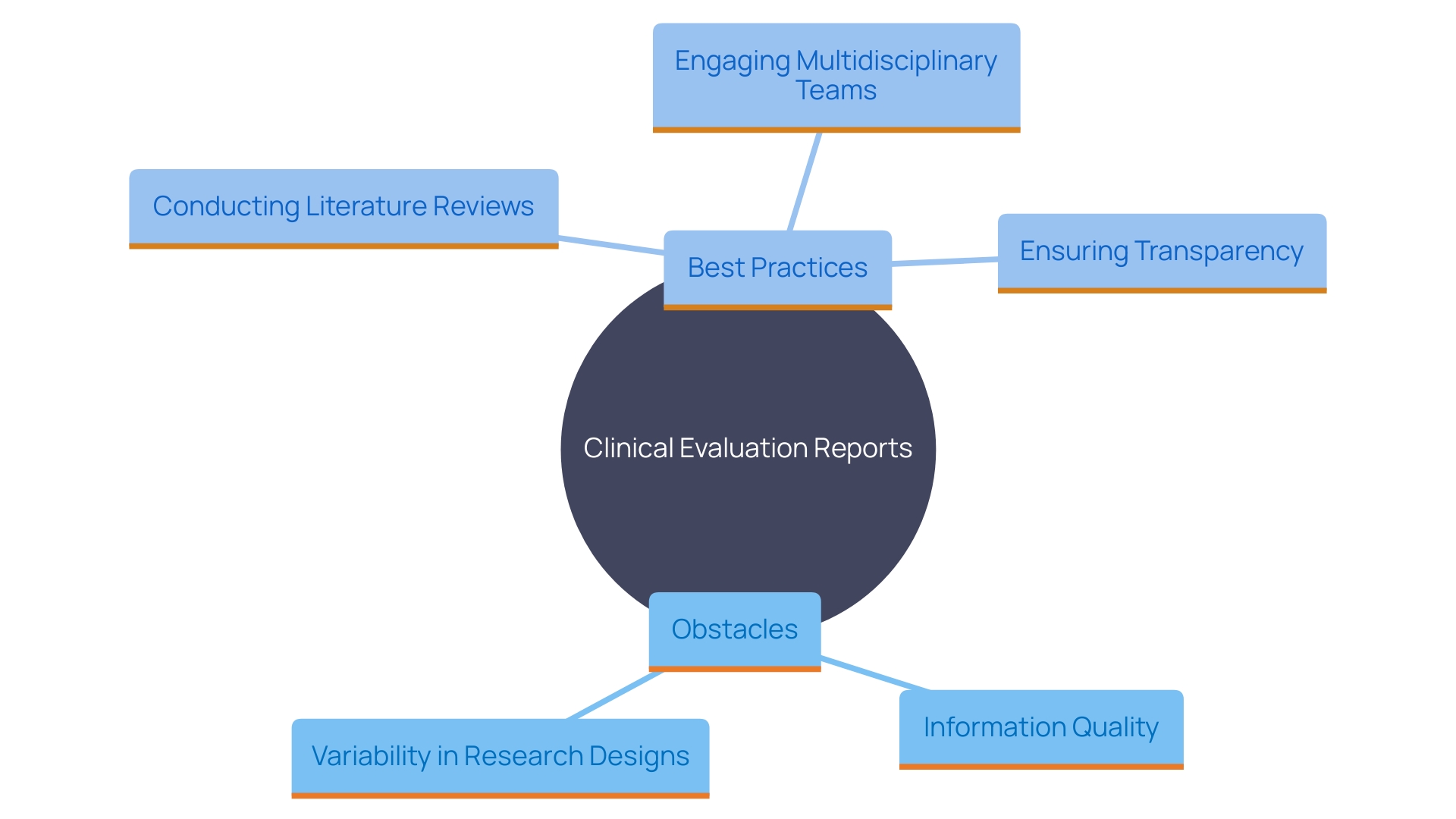
Creating impactful assessment reports (CERs) entails addressing multiple obstacles, such as guaranteeing information quality, handling variability in research designs, and acquiring pertinent medical information. A strong CER should include a thorough description of the apparatus, an extensive assessment plan, a complete literature review, and the incorporation of information from both the examined apparatus and similar marketed products.
To address these challenges, conducting thorough literature reviews is crucial. This systematic approach should include a state-of-the-art report that outlines current best practices and demonstrates the device's comparability to others on the market. Utilizing standardized templates ensures consistency and clarity in reporting, which is essential for regulatory compliance and decision-making.
Engaging a multidisciplinary team is another best practice. This method permits various viewpoints and knowledge, guaranteeing a thorough assessment of medical information. Moreover, essential evaluation of all medical information is needed to recognize and reduce possible biases. Transparency in methods and limitations, as well as detailed statistical analysis, are also integral to the credibility of CERs.
Adopting these strategies not only enhances the quality and reliability of CERs but also supports better decision-making in regulatory processes. As the demand for scientifically rigorous research evidence increases, it is imperative to shift towards reusable and multipurpose information collection methods. This updated infrastructure will assist in the creation of thorough evidence, bridging gaps in medical data and supporting the development of dependable practice guidelines.
Case Study: Developing a Clinical Evaluation Report
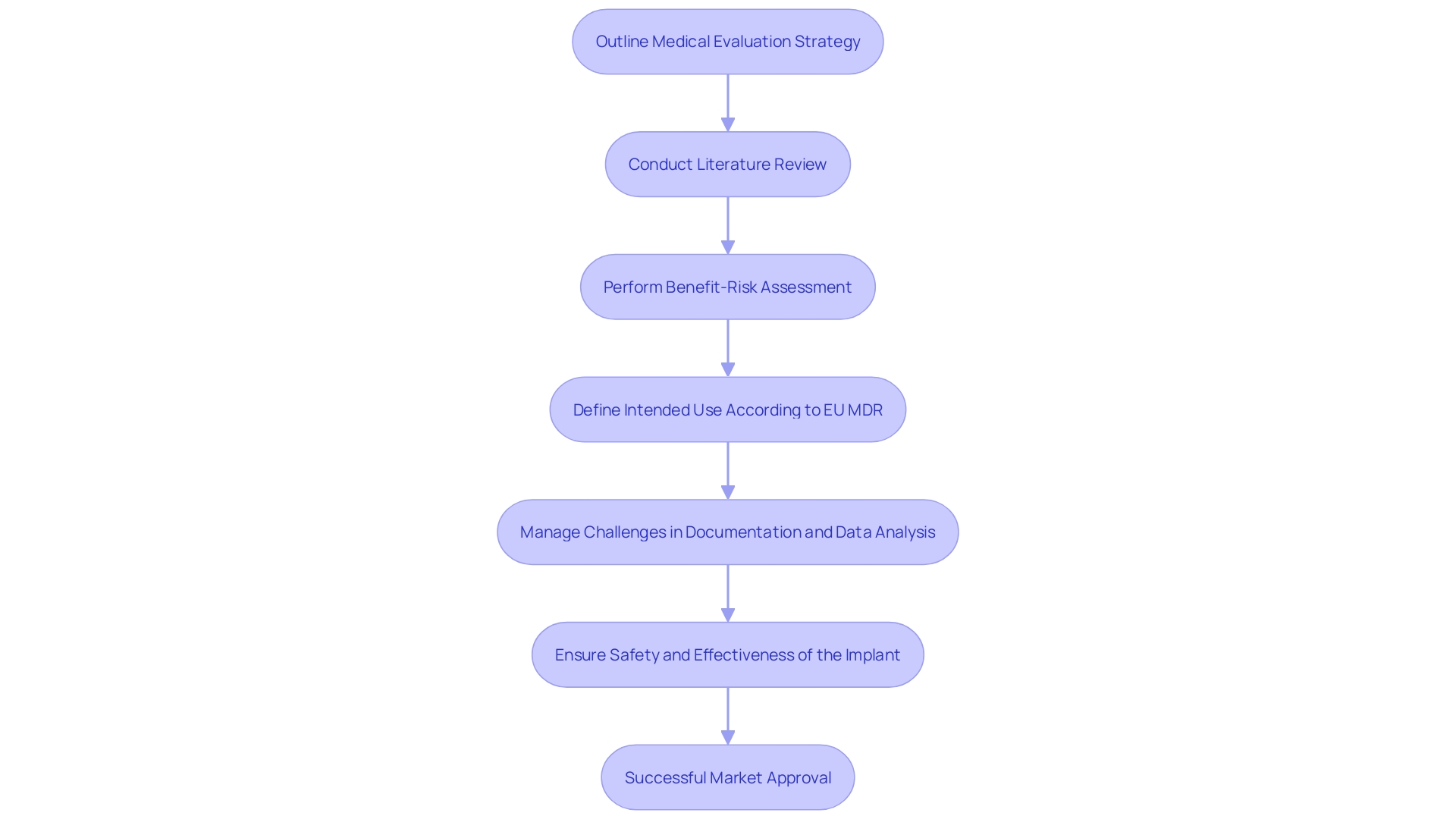
This case study explores a hypothetical medical firm that recently created an innovative orthopedic implant. The company began developing a medical assessment report (MAR) by first outlining a detailed medical evaluation strategy specifically tailored for their product. A thorough literature review was conducted to collect existing medical information, pinpointing significant studies that provided insights into the safety and effectiveness of comparable instruments.
The data collected underwent a rigorous benefit-risk assessment, ensuring that the advantages of the implant outweighed any potential risks, a crucial step in both the design and development process. This assessment also plays a pivotal role in the clinical evaluation and post-market surveillance, helping to ensure the product's safety and effectiveness throughout its lifecycle.
Furthermore, the intended use of the equipment was clearly defined, adhering to EU MDR standards, which include the purpose, users, patient population, medical conditions to be addressed, and the operational principles of the equipment. This precise definition aligns with the device's description, indications, and functionalities, ensuring a coherent narrative across all documentation.
Challenges such as detailed information analysis and consistent documentation were effectively managed. The clinical data was meticulously analyzed and coherently presented, ensuring alignment across the clinical evaluation plan, CER, and risk management files. This thorough process resulted in a well-documented CER that met all regulatory requirements, leading to a timely market approval.
Conclusion
The significance of clinical evaluation reports (CERs) in the medical device lifecycle cannot be overstated. These reports serve as comprehensive assessments of clinical data, ensuring that devices meet safety and efficacy standards essential for regulatory submissions. With the evolving regulatory landscape and increasing demands from Notified Bodies, the meticulous maintenance and updating of CERs have become imperative for manufacturers.
The role of CER consultants is crucial in this regard, as they bring expertise to navigate complex clinical data and ensure compliance with current regulations.
Key components of a well-structured CER, including device descriptions, clinical data collection methods, and rigorous data appraisal, contribute significantly to demonstrating a device's alignment with safety and performance standards. The importance of thorough literature reviews and the inclusion of comparative data from similar devices further strengthen the credibility of the evaluation. Engaging multidisciplinary teams enhances the quality of the CER, enabling a comprehensive understanding of clinical data and mitigating potential biases.
Addressing the challenges associated with CER development through best practices not only facilitates regulatory compliance but also supports ongoing post-market surveillance. The case study of a hypothetical orthopedic implant company illustrates the necessity of a well-defined intended use and a robust clinical evaluation plan. By adhering to regulatory standards and managing challenges effectively, manufacturers can ensure timely market access while maintaining the safety and efficacy of their devices.
As the regulatory environment continues to evolve, the commitment to thorough clinical evaluations remains vital for success in the medical device sector.




