Overview
Emerging markets for clinical research, particularly in countries like India, Brazil, and Colombia, present significant opportunities for pharmaceutical firms due to their economic growth, large populations, and improving healthcare systems, allowing for cost-effective studies. However, challenges such as regulatory hurdles, cultural differences, and logistical issues must be navigated to fully leverage these markets, as highlighted by the article's emphasis on the need for effective management and local knowledge to ensure successful research outcomes.
Introduction
The landscape of clinical research is undergoing a seismic shift as emerging markets like India, Brazil, and Colombia rise to prominence, driven by their burgeoning economies and improved healthcare infrastructures. These regions are not only offering pharmaceutical companies a cost-effective alternative for conducting clinical trials but also providing access to diverse patient populations that can enhance the validity of research outcomes.
Colombia, in particular, is making waves with innovative studies, such as ReGelTec's Early Feasibility Study on HYDRAFIL™ for chronic low back pain, showcasing the potential of these markets. However, this burgeoning opportunity comes with its own set of challenges, from navigating complex regulatory environments to overcoming cultural barriers in participant recruitment.
As the demand for inclusive and representative clinical research grows, understanding the intricacies of conducting trials in these regions becomes essential for stakeholders aiming to harness the full potential of emerging markets in shaping the future of healthcare.
The Rise of Emerging Markets in Clinical Research
Countries like India, Brazil, and Colombia are becoming emerging markets for clinical research, rapidly transforming into prime sites for medical studies due to their strong economic expansion, large populations, and improving healthcare systems. For pharmaceutical firms and research institutions, emerging markets for clinical research provide the opportunity to conduct studies at notably reduced expenses while accessing varied patient groups. Significantly, Colombia is improving its status as a premier location for research studies, illustrated by ReGelTec's Early Feasibility Study on HYDRAFIL™ for addressing chronic low back pain, which successfully enrolled patients in Barranquilla with remote proctoring.
However, US Medtech companies face several challenges in this landscape, including regulatory hurdles, language barriers, and fragmented resources, which can impede effective collaboration with local hospitals. As of 2024, the environment of research studies has transformed, especially in the emerging markets for clinical research, with the overall count of studies displaying significant growth. According to WHO data released in 2022, the total number of clinical studies carried out in 2021 rose by 11.7% compared to the prior year, indicating a rising need for comprehensive studies that accurately represent global demographics.
Additionally, recent data indicates that lower middle-income countries are considered emerging markets for clinical research, having experienced a remarkable 36% rise in newly recruiting studies from 2020 to 2023. This trend emphasizes the urgent need for studies that address the diverse health needs of these populations. The International Clinical Trials Registry Platform (ICTRP) observed that the yearly count of registered studies in high-income nations rose from 21,028 in 2010 to 29,538 in 2020, reflecting a rising global dedication to promoting medical investigations.
As emerging markets for clinical research continue to progress, especially in Latin America, they are positioned to play a vital role in influencing the future of medical research, fueled by the need for innovative solutions and partnerships, such as those between Greenlight Guru and bioaccess™, to effectively close gaps in research and execution.
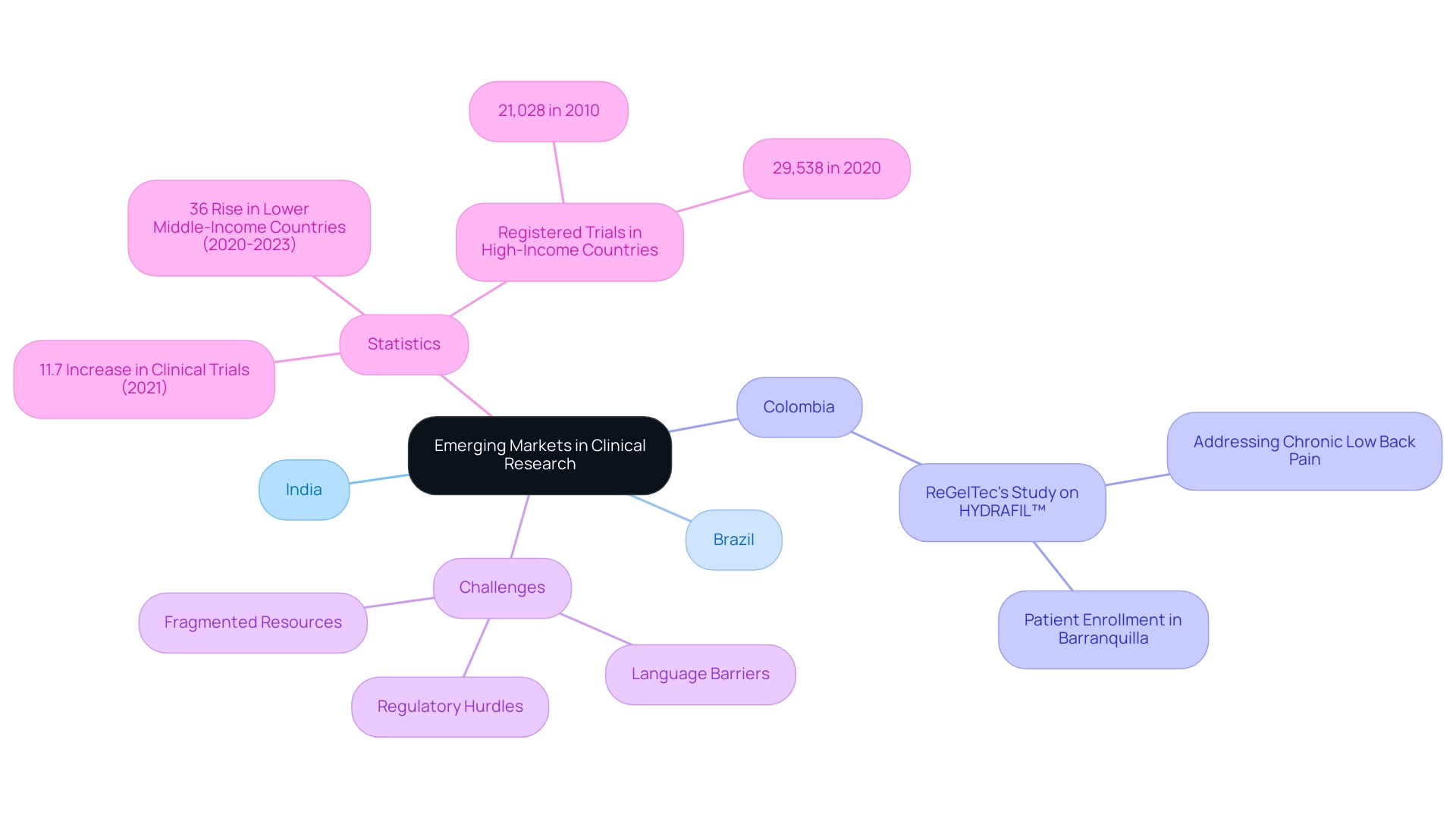
Key Advantages of Conducting Clinical Trials in Emerging Markets
Developing economies, particularly in Latin America, are considered emerging markets for clinical research that offer numerous attractive benefits for carrying out medical studies. Our extensive research management services guarantee smooth execution, including:
- Feasibility studies
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
These capabilities significantly expedite the approval process, supported by favorable regulatory environments, including the oversight provided by INVIMA, Colombia's National Food and Drug Surveillance Institute, which plays a crucial role in ensuring compliance with local regulations.
For example, as of 2023, China has recorded 4,300 clinical studies, representing a 26.1% increase from 2022, highlighting the rising trend in these sectors. Furthermore, emerging markets for clinical research often boast large treatment-naive populations, granting researchers access to participants who have not previously received treatments. This aspect enhances the reliability of study results by minimizing confounding variables associated with prior exposure to therapies.
Furthermore, the reduced operational expenses linked to conducting studies in these areas can generate significant savings for sponsors, rendering them financially appealing choices. According to Murray Aitken, 'The composite success rate for the pipeline surged in 2023 to 10.8% across all therapy areas after dropping to a 10-year low in 2022,' which highlights the growing success in medical studies. Our reporting processes encompass detailed study status updates, inventory management, and documentation of serious and non-serious adverse events, ensuring comprehensive oversight throughout the study.
Together, these elements place emerging markets for clinical research as strategic options to conventional study sites, particularly in an environment where the need for efficient and effective trials keeps increasing. A significant illustration of this potential is observed in the increasing interest in psychedelics for treating central nervous system disorders, with almost 200 trials presently registered, highlighting innovative studies aimed at addressing unmet medical requirements.
Challenges in Navigating Clinical Research in Emerging Markets
Carrying out research in emerging markets for clinical research, particularly in Latin America, poses a variety of unique challenges that participants must manage. Regulatory hurdles can vary dramatically across different countries, complicating compliance and approval processes. For instance, the INVIMA in Colombia plays a critical role as a Level 4 health authority, overseeing medical device regulation and classification, which is essential knowledge for effective market access.
Moreover, in 2024, expectations indicate that:
- 83% of non-US respondents anticipate significant impacts from the EU's Corporate Sustainability Reporting Directive on their strategic approaches, highlighting the growing importance of regulatory frameworks that emphasize sustainability.
- 77% of US respondents anticipate greater regulatory focus on sustainability in 2025, highlighting the rising examination that will impact research operations.
Furthermore, participant recruitment poses substantial difficulties due to cultural differences, varying levels of healthcare literacy, and pre-existing trust issues within local communities.
Expert insights reveal that these factors can significantly affect the ability to enroll diverse and representative study populations. Logistical difficulties, such as insufficient infrastructure and ineffective data management systems, further complicate the implementation of medical studies. A comprehensive management service for research, like those offered by bioaccess®, can effectively tackle issues in emerging markets for clinical research by providing:
- Feasibility studies
- Site selection
- Compliance reviews
- Project setup
- Import permits
- Strong project management
For instance, bioaccess® illustrates how a prominent CRO supports medical device research studies in Latin America, ultimately contributing to job creation, economic growth, healthcare enhancement, and promoting international collaboration. Grasping these challenges and utilizing local knowledge is crucial for stakeholders seeking to effectively implement and oversee research in these intricate environments. Moreover, research has indicated that medical experiments can result in substantial advancements in local healthcare systems and generate many employment opportunities, further highlighting the significance of these studies in the area.
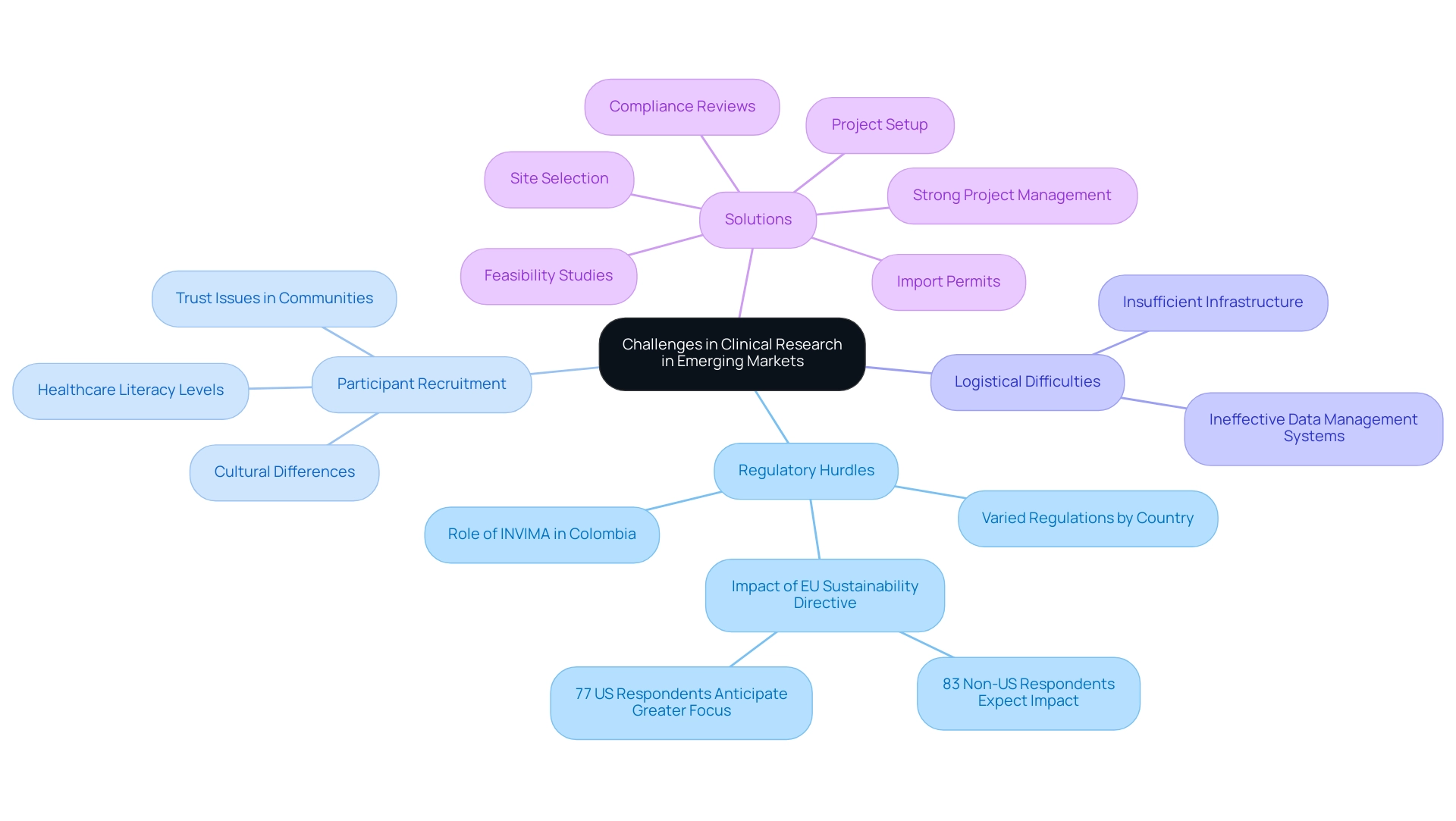
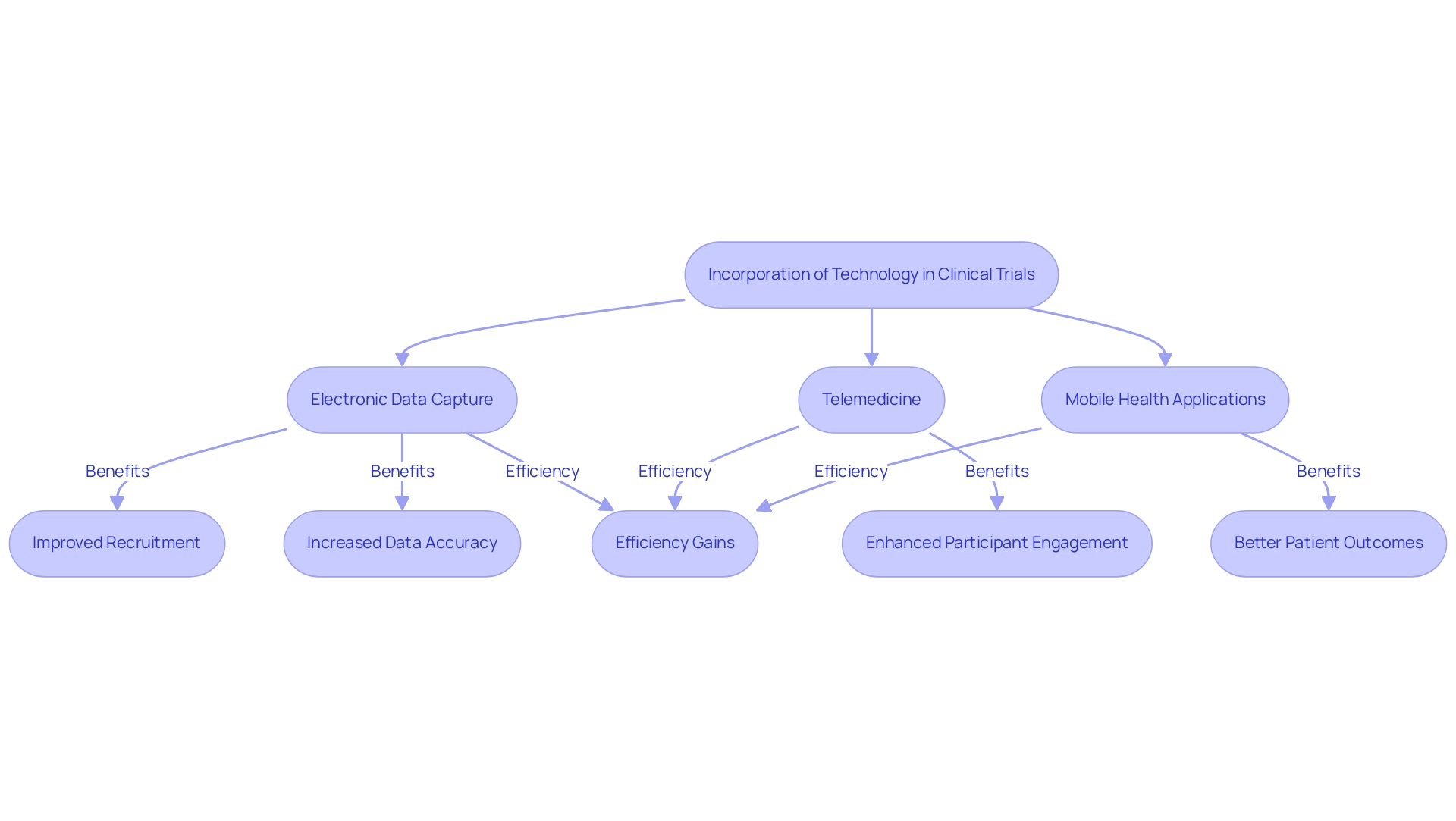
Leveraging Technology to Enhance Clinical Trials in Emerging Markets
The incorporation of technology is transforming research in emerging markets, serving as a catalyst for improved efficiency and participant involvement. Innovations such as electronic data capture, telemedicine, and mobile health applications are streamlining recruitment and data collection processes. Telemedicine, in particular, allows for remote participant monitoring, minimizing the need for frequent site visits and significantly increasing accessibility for potential participants.
This technology not only fosters greater engagement but also enhances adherence to protocols, ultimately improving patient outcomes. Electronic data capture systems further play a vital role by increasing data accuracy and expediting the processes of data entry and analysis. Notably, a European biotech firm collaborating with a CRO saw their time-to-offer reduced from over 50 days to just 36 days, underscoring the efficiency gains from technology integration.
According to Thermo Fisher Scientific Inc., this firm successfully collaborated with a CRO utilizing a Functional Service Provider (FSP) model, demonstrating the effectiveness of technology-driven approaches. Additionally, with bioaccess®'s 20+ years of expertise in Medtech and extensive research management services, including Early-Feasibility, First-In-Human, and Post-Market Follow-Up Studies, the incorporation of advanced technologies is crucial for addressing logistical obstacles such as patient recruitment difficulties and data management inefficiencies, thus enhancing study outcomes. The partnership between bioaccess™ and Caribbean Health Group, backed by Colombia's Minister of Health, seeks to establish Barranquilla as a premier location for medical research in Latin America, demonstrating the beneficial influence of Medtech studies on local economies.
As the sector for medical research technology continues to grow, utilizing advancements in emerging markets for clinical research will be essential for attaining improved recruitment efficiency and participant retention, as demonstrated by GlobalCare Clinical Trials' collaboration with bioaccess™, which achieved over 50% reduction in recruitment time and 95% retention rates. The 2024 environment of medical investigation will certainly witness a larger focus on these technological solutions, altering the dynamics of participant involvement and data management.
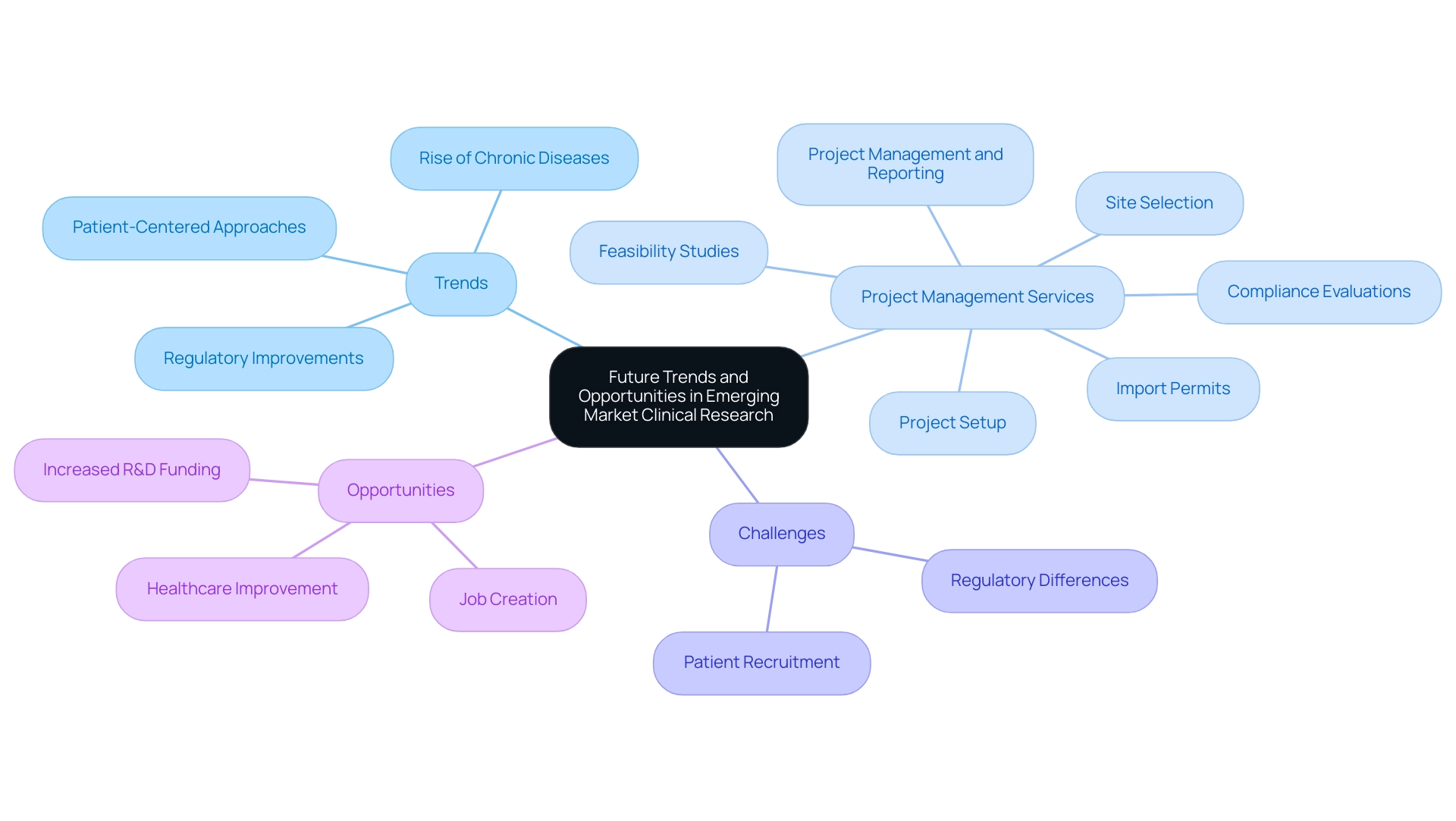
Future Trends and Opportunities in Emerging Market Clinical Research
The future environment of medical research in emerging markets for clinical research seems very encouraging, marked by several essential trends that are poised to stimulate both expansion and funding. As regulatory agencies such as INVIMA in Colombia, designated as a Level 4 health authority by PAHO/WHO, develop and optimize their procedures, the conventional obstacles that obstruct the implementation of research studies are expected to lessen considerably. This is particularly critical as the prevalence of chronic diseases continues to rise, increasing the demand for innovative treatments and positioning emerging markets as vital hubs for pharmaceutical development.
Our extensive research project management services include:
- Feasibility studies
- Careful site selection
- Compliance evaluations of study documents
- Project setup
- Import permits for investigational devices
- Strong project management and reporting
The compliance review process ensures that all study documents adhere to local regulations, which is essential for obtaining necessary approvals. Moreover, our reporting services encompass regular updates on study status and comprehensive documentation of significant and non-significant adverse events, which are essential for ensuring transparency and safety in research.
In fact, the integration of study software has been demonstrated to lower overall study expenses by approximately 30%, emphasizing the potential for enhanced efficiency in procedures. However, stakeholders must also navigate challenges such as patient recruitment and retention, along with regulatory differences, which pose hurdles to growth. Moreover, the increasing focus on patient-centered approaches—where patient input and preferences are actively incorporated—will not only improve the overall quality of trials but also guarantee that the resulting therapies are more closely aligned with patient needs.
As R&D funding levels reset in 2023 after a steep decline from peak levels in 2020-21, it is crucial for stakeholders to capitalize on the vast opportunities that these emerging markets present for advancing clinical research, while also recognizing the significant impact of Medtech clinical studies on local economies through job creation and healthcare improvement.
Conclusion
The rise of emerging markets such as India, Brazil, and Colombia in the field of clinical research signifies a transformative shift, driven by improved economic conditions and healthcare infrastructure. These regions provide pharmaceutical companies with cost-effective options and access to diverse patient populations, enhancing the validity of research outcomes.
- Colombia, in particular, has showcased its potential with innovative studies like ReGelTec's Early Feasibility Study, highlighting the opportunities available in these markets.
- However, stakeholders must navigate complex regulatory landscapes and cultural barriers to fully leverage these advantages.
The benefits of conducting clinical trials in emerging markets are substantial, including:
- Lower operational costs
- Access to treatment-naive populations
These factors not only improve the reliability of trial results but also promise significant savings for sponsors. The increasing success rates in clinical research further underscore the growing importance of these regions as strategic alternatives to traditional research locations. Additionally, the integration of technology in clinical trials enhances efficiency and participant engagement, addressing logistical challenges and improving patient outcomes.
As the landscape of clinical research continues to evolve, emerging markets are poised to play a crucial role in addressing global health needs. By understanding the unique challenges and opportunities that these regions present, stakeholders can contribute to a future where clinical research is more inclusive and representative.
The call for innovative solutions and collaborations is stronger than ever, making it imperative for the clinical research community to embrace the potential of these burgeoning markets to drive advancements in healthcare for diverse populations worldwide.
Frequently Asked Questions
What countries are emerging markets for clinical research?
Countries like India, Brazil, and Colombia are becoming emerging markets for clinical research due to their strong economic growth, large populations, and improving healthcare systems.
What advantages do emerging markets offer for clinical research?
Emerging markets provide opportunities to conduct studies at reduced expenses while accessing diverse patient groups, which can enhance the reliability of study results.
What is significant about Colombia in the context of clinical research?
Colombia is improving its status as a premier location for research studies, as demonstrated by successful trials like ReGelTec's Early Feasibility Study on HYDRAFIL™ for chronic low back pain.
What challenges do US Medtech companies face in emerging markets?
US Medtech companies encounter challenges such as regulatory hurdles, language barriers, and fragmented resources, which can hinder effective collaboration with local hospitals.
How has the landscape of clinical research studies changed by 2024?
By 2024, the environment for research studies in emerging markets has transformed, with a notable increase in the overall count of studies, indicating a rising need for comprehensive studies that represent global demographics.
What recent trends have been observed in lower middle-income countries regarding clinical research?
Lower middle-income countries have experienced a 36% rise in newly recruiting studies from 2020 to 2023, emphasizing the need for studies that address diverse health needs.
How has the number of registered studies in high-income nations changed over the years?
The yearly count of registered studies in high-income nations increased from 21,028 in 2010 to 29,538 in 2020, reflecting a growing global commitment to medical investigations.
What role do regulatory bodies play in facilitating clinical research in emerging markets?
Regulatory bodies like INVIMA in Colombia ensure compliance with local regulations, which helps expedite the approval process for clinical studies.
Why are large treatment-naive populations important for clinical research in emerging markets?
Large treatment-naive populations enhance the reliability of study results by minimizing confounding variables associated with prior exposure to therapies.
What are the financial benefits of conducting studies in emerging markets?
The reduced operational expenses in emerging markets can generate significant savings for sponsors, making these locations financially appealing for clinical research.
What is the significance of the rise in interest in psychedelics for clinical trials?
The increasing interest in psychedelics for treating central nervous system disorders is reflected in almost 200 registered trials, highlighting innovative approaches to addressing unmet medical needs.

